Abstract
Policy and research issues in the framing and qualities of uncertainties in risks are analyzed, based on the assessments of dioxin-like compounds (DLCs) and other ingredients in Baltic Sea fish, a high-profile case of governance. Risks are framed broadly, to then focus on dioxins and beneficial fatty acids, fish consumption, human health, and science-management links. Hierarchies of uncertainty (data, model, decision rule, and epistemic) and ambiguity (of values) are used to identify issues of scientific and policy contestation and opportunities for resolving them. The associated complexity of risks is illustrated by risk–benefit analyses of fish consumption and by evaluations of guideline values, highlighting value contents and policy factors in presumably scientific decision criteria, and arguments used in multi-dimensional risk and benefit comparisons. These comparisons pose challenges to narrow assessments centered, for e.g., on toxicants or on food benefits, and to more many-sided and balanced risk communication and management. It is shown that structured and contextualized treatment of uncertainties and ambiguities in a reflexive approach can inform balances between wide and narrow focus, detail and generality, and evidence and precaution.
Keywords: Food contamination, Guideline values, Health, Integrated assessment, Risk–benefit, Uncertainty
Introduction
Persistent and bio-accumulating toxic (PBT) substances constitute a high-profile case of risk governance in environmental management, health care and food safety, and related fields such as fisheries management. PBTs exert pressures on policy-makers, regulators and others, and none more than dioxins and PCBs (polychlorinated biphenyl compounds) in food, especially fish. Political pressures have been caused by the continuous high levels, though much reduced, of such compounds in fatty fish from the Baltic Sea (Assmuth and Jalonen 2005). Pressures are also due to crises with dioxins in other areas, notably in feed in Belgium in the 1990s, prompting EU regulations (CEC 2001, 2002). However, there is increasing concern for health benefits of fish too, especially from polyunsaturated fatty acids (PUFAs), and for losing benefits by reducing human fish intakes (SPCFC 2005). Dioxins and Baltic Sea fish thus illustrate crucial scientific and policy issues of uncertainty and complexity in risk–benefit comparisons, in integration of sectors and issues, and in balancing precaution and evidence.
A large body of research exists on dioxins, dioxin-like PCBs (dlPCBs) and other PCBs, other dioxin-like compounds (DLCs) and related PBTs both in the Baltic Sea fish and in other contexts, including the >1000 references in Assmuth and Jalonen (2005). Human health is the key issue partly as putative eco-toxicological effects of PBTs in non-human fish-consuming animal populations have subsided. Many studies have addressed effects on human health from dioxins, based on epidemiological or non-human animal evidence, mechanistic studies and human exposure information; many have also focused on the Baltic Sea region and high consumers of its fish. Research in the health benefits of fish has constituted a separate, even larger body of evidence. Recently, these fields have drawn closer to relate the risks and benefits (Axmon et al. 2004; Hites et al. 2004a; Tuomisto et al. 2004; Cohen et al. 2005; Foran et al. 2006; Domingo et al. 2007, Genuis 2008; Mozaffarian 2009).
Many assessments have been made of human health risks from dioxins and PCBs, influencing and drawing on inquiries into them in the Baltic Sea. These assessments are based on extrapolations to other dioxins from the most studied TCDD and to humans from other animals, especially laboratory rodents, and on supportive such as mechanistic information. Established assessment procedures involve (often non-explicated) value judgments. The inference is more indirect and thus more uncertain than that based on epidemiological evidence, usually prioritized in human risk assessment. The assessments have omitted risk–benefit considerations, with few exceptions (Joas et al. 2001; IOM 2003; SACN and COT 2004; SPCFC 2005). There is thus a possibility of biased and incoherent assessments and advice for risk (also risk–benefit) management.
Uncertainties have been often identified as an issue in the above research and assessments. However, most of them, also those on the Baltic, only implicitly or narrowly address uncertainties. Broad and unified analysis of uncertainties of various kinds in both risks and benefits is lacking. In the present case, these uncertainties emerge as the crucial science and policy issue.
Approach, Scope, and Concepts
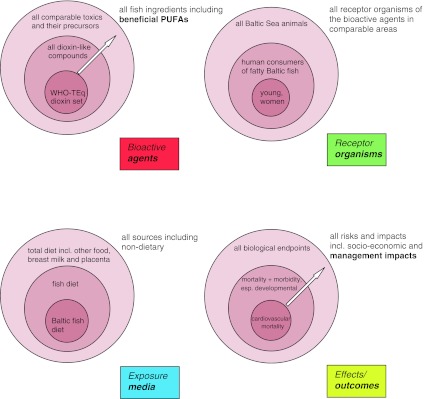
The present article investigates issues in research and policy and in their interactions that are associated with the framing and qualities of uncertainties in risks through the case of dioxin-like compounds (DLCs) in Baltic Sea fish. It draws on an extensive assessment by Assmuth and Jalonen (2005). Risks are initially framed broadly by considering (Fig. 1):
multiple agents, including 2,3,7,8-chloro-p-dioxins and -dibenzofurans, dioxin-like and other PCBs, other DLCs and PBTs; and vital long-chain omega-3 (n-3) PUFAs;
multiple exposure routes and settings (fish, other food items, and other intakes)
multiple exposed systems (ecosystems, human populations, and socio-economic systems);
multiple effects and consequences (including risks and benefits from fish);
multiple spatial and time scales (Baltic Sea vs. other regions; cumulative risks and benefits);
biological and social characteristics (constitution and relevance) of risks.
Fig. 1.
Focusing assessment within key dimensions of risks associated with dioxins and beneficial ingredients in Baltic Sea fish (modified from Assmuth and Jalonen 2005). Note the key shifts in focus of the present article, indicated by arrows; a shift toward management impacts in particular may entail reframing also of exposure media (including additional sources) and receptor organisms. WHO-TEq = TCDD toxicity equivalent quantities defined by WHO (1998) using mammalian toxicity equivalency factors; PUFAs polyunsaturated fatty acids
Based on the evidences, previous assessments and governance context, the focus is then put on key policy aspects of uncertainties in these risks: low-dose perinatal reproductive and developmental disorders; high-risk human groups; exposures from fatty fish; DLCs versus omega-3 PUFAs. Emphasis is placed on long-term risks, on consequences of management options and on relationships between evidence and precaution. The basis of quantitative risk assessment criteria (guideline values for intakes and fish levels) encapsulating salient points is scrutinized as a focal theme.
Distinction is made between uncertainties in different levels: in data, models, and decision rules, including epistemic and non-epistemic (value-related) ambiguity (Finkel 1996). These hierarchies and qualities of uncertainty are used as hermeneutic devices to identify key issues of scientific and policy contestation, and opportunities for resolving uncertainties and ambiguities.
Results and Discussion
Uncertainties Surrounding Health Risks from Dioxins in Fish
DLCs exert a variety of adverse effects in many species, in patterns that have much resemblance. However, the effects are multi-faceted, and so is knowledge of them. Some effects such as those by TCDD on reproductive development have been extensively characterized (USEPA 2000), but there are uncertainties of their real-world occurrence, variations, factors, and implications. The understanding of neurological, immune, hormonal, carcinogenic and metabolic effects, and of other DLCs is poorer. The mechanistic (Ah-receptor) basis adds to the plausibility of effects also in humans but does not allow certainty in explanation and prediction. The dose–response relationships are as yet very insufficiently quantifiable.
Various adverse effects have been reproduced in rodents at low exposures corresponding to doses in humans near background levels. However, alternative benchmark doses demonstrate great variation in estimates of effective exposure levels also within a type of effect and biological system. The most critical effects occur after perinatal exposure on reproductive and, more inconsistently, neurobehavioral development (Table 1).
Table 1.
Characterizations of the treatment of some key uncertainties in relevant authoritative assessments of human health risks from dioxins and in proposals for human tolerable daily intakes
| Assessment | Pivotal effects | Uncertainty factors (UF) used in deriving risk criteria | TDI (TWI)a pg g bw−1 day−1 |
|---|---|---|---|
| WHO (1998)b | Rhesus monkey endometriosis and neurobehaviour; rat male offspring reproduction and immune effects | Mainly pharmacokinetic differences accounted for but not very extensively or explicitly | 1–4 |
| EU/SCF (2000)c | Approximately as above (but with different body burden estimation and dose conversion) | 1–3 (7–21 TWI) | |
| EU/SCF (2001)d | Rat male offspring reproduction; rhesus monkey and rat immune effects omitted) | 10 overall, unspecified | 2 (14 TWI) |
| USEPA (2000a)e | Including carcinogenicity in humans, not a critical endpoint(s); assessment is debatable | Various | 0.06 |
| UK 2001 (2004)f | Rat reproductive development; tumors | LOAEL–NOAELi extrapolation 3 × inter-individual variation in toxicokinetics 3.2 (+half order-of-magnitude due to TEFsj and uncertainties in rat kinetics etc.) | 2, reproduction/development (8, cancer) |
| Germany (2002)g (unofficial) |
More endpoints, species, dosing ages/periods and dioxin-like agents requested | More (empirical) UFs required for pharmacokinetics, LOAEL–NOAEL, dose and animal-human extrapolation, individual variation, additional DLCsk | 1 |
| Japan (2004)h | Rat female reproductive development | 10 LOAEL–NOAEL extrapolation | 4 |
aTDI/TWI tolerable daily or weekly intake, b WHO (1998), based on Gehrs et al. Toxicology 122;3(1997):229–40; Gray et al. Toxicol Appl Pharmacol 146;1(1997a):11–20; Hurst et al. Toxicol Sci 53;2(2000) and 57;2(2000):275–83; Mably et al. Toxicol Appl Pharmacol 114;1(1992):97–107, 108–17, 118–26; Rier et al. Fundam Appl Toxicol 21;4(1993):433–41; Schantz & Bowman, Neurotoxicol Teratol 11;1(1989):13, see also Gaylor and Aylward, Regulat Toxicol Pharmacol 40;1(2004):9–17, cSCF (2000), d SCF (2001), based also on Faqi et al. Toxicol Appl Pharmacol 150;2(1998):383–92; Ohsako et al. Toxicol Sci 66;2(2002):283-92; note that the lowest-dose effects on sperm count of offspring have been found non-reproducible (Foster et al. Environ Health Perspect 118;4(2010):458–64), eUSEPA 2000a, based on Kociba et al. Toxicol Appl Pharmacol 46;2(1978):279–303; fSACN and COT (2004), gUBA unpublished 2002; Gies et al. (2007) (see references), see also Hojo et al. Environ Health Perspect 110;3(2002):247–54 on lowest-dose neurobehavioral effects, and sources in Assmuth and Jalonen (2005) on other putative low-dose effects in humans, hSumida et al. Organohalogen Compds 2005: 2537–9, based also on Gray et al. Toxicol Appl Pharmacol 146;2(1997b):237–44, see also Arima et al. Reproduction 28;4(2009):495–502, iLowest Observed Adverse Effect Level–No Observed Adverse Effect Level, jTCDD (2,3,7.8-Tetrachloro-p-dioxin) Equivalency Factors, Van den Berg et al. (1998) (see references), kDLCs Dioxin-Like Compounds
Epidemiological studies of links between dioxins or PCBs and health conditions in human populations have also in the Baltic Sea region been constrained by the small sample size and low specificity of measures of exposures and effects, as reviewed by Assmuth and Jalonen (2005). This may lead to over- or underestimation of risks, and is compounded by the complex mixtures of substances including DLCs and non-dioxin-like PCBs and DDT metabolites. The human studies in fish consumers provide inconclusive evidence of anomalies and their causes, specifically of the role of dioxins. These studies exemplify the multiple uncertainties of scientific evidence, especially regarding the nature, magnitude, and causal attribution of multi-dimensional and multi-factorial risks.
Uncertainties Surrounding Health Benefits from Fish Consumption
SPCFC (2005) summarized the evidence for cardiovascular health benefits from dietary consumption of fish, fish oil, and PUFAs based, for e.g., on reviews by Wang et al. (2004), He et al. (2004), Hooper et al. (2004), and SACN and COT (2004). In general, these key health impacts of fish ingredients have been studied more extensively and reliably than the adverse health effects of DLCs in fish, using large cohorts, randomization, and long follow-ups (Balk et al. 2004).
There is substantial evidence that fish consumption, preferably of fatty fish and alternatively of fish oil or isolated LC omega-3 PUFAs, benefits the cardiovascular system and is suited for secondary prevention in manifest coronary artery disease, decreasing mortality in patients who suffered a myocardial infarction (Ruxton et al. 2004). By some estimates, fish consumption of 30–50 g day−1 is associated with a cardiovascular mortality risk reduction of 50% (Din et al. 2004; Konig et al. 2005; cf. Studer et al. 2005).
The consensus on these benefits is yet not as clear as is often claimed or implied. Some effects are attributable to omega-3 PUFAs, but also other fatty fish ingredients play a role. The effects may vary depending on population, for e.g., its age (greater benefits among elderly) and condition (cardiovascular status); the traits of fish (oil) consumed; the level and duration of consumption. Some large and well-designed studies could not demonstrate such benefits (Ascherio et al. 1995; Kris-Etherton et al. 2003), and some of the above reviews arrived at different conclusions, depending on study inclusion and evaluation criteria. SPCFC (2005) stressed that few studies have considered the simultaneous effects of contaminants.
As to health benefits from Baltic fish consumption, Svensson et al. (1995) presented some evidence of lowered mortality risk from cardiovascular diseases in Swedish fishermen (12% on East coast). Axmon et al. (2004) noted that health benefits of fish consumptions may have compensated for adverse effects of PBTs in fish also among the wives of these fishermen.
There are other potential benefits of fatty fish ingredients besides those on cardiovascular health, but the evidence for these is not as strong (Table 2).
Table 2.
Evidence on health benefits from fatty fish consumption, with emphasizing uncertainties
| Benefit | Factors | Significance | Evidence |
|---|---|---|---|
| Cardiovascular health | LC PUFAs | Prevention (esp. secondary) and prevention of infarcts | Very strong; quantitative and specifieda |
| Bone development | Vitamin D supply in infants | Reduced consumption may increase risk if vitamin D is not supplied by other means | Strongb |
| Reproductive health and development | Fetus depends on mother for PUFAs | Maternal LC n-3 PUFA intake may benefit lower birth weight populations | Tentativec |
| Neurodevelopment (visual function, IQ) | LC n-3 PUFA supply in prenatal stage | PUFAs may counteract neurodevelopmental effects of dioxins in fish | Tentative; evidence in some but not all studiesd |
| Metabolism (especially lipid) | LC n-3 PUFAs | Decrease in serum trigycerides, a risk factor for myocardial infarction | Considerablee |
| Anti-inflammatory (rheumatoid arthritis) | Dietary fish oils | Also possible synergism with cardiovascular benefits | In some studies but not in others in patientsf |
| Immune state and development | PUFA supplement in pregnancy | Immune suppression may benefit atherosclerotic diseases, impair pathogen defense | Inconsistentg |
aFor references, see text and Kromhout et al. New England Journal of Medicine 312;19(1985):1205–9, Marckmann and Gronbaek, Eur J Clin Nutr 53(1999):585; Harper and Jacobson, Arch Int Med 161;18(2001):2185–92, b Molgaard & Michaelsen, Proc Nutr Soc 62;4(2003):823–8, c Lewin et al. AHRQ Publ. 05-E025-2, www.ahrq.gov; SACN and COT (2004) (see references), d Helland et al. Pediatrics 111;1(2003):e39-44, cf. Gustafsson et al. Acta Paediatrica 93(2004):1280–7, Williams et al. Am J Clin Nutr 73(2001):316–22, cf. Hodge et al. AHRQ Publ. 05-E008-2, MacLean et al. AHRQ Publ. 05- E011-2, e Shekelle et al. AHRQ Publ. 04-E012-2. www.ahrq.gov, f Cleland et al. Drugs 63;9(2003):845–53, g SPCFC 2005 (see references)
Excessive intake of fatty fish and the fatty acids may lead to adverse effects, for instance by lowered blood coagulation and increases in cholesterol (SPCFC 2005).
Uncertainties and Ambiguities Surrounding Comparisons of Risks and Benefits
Following the assessment of health risks from consumption of farmed salmon by Hites et al. (2004a), replies pointed out health benefits of fatty fish consumption and the dangers of reducing it (Lund et al. 2004; Tuomisto et al. 2004; Hites et al. 2004b). Little attention has been paid to some other key weaknesses in this assessment, such as the limitation of dioxin risks to cancer. Tuomisto et al. (2004) estimated the (maximal) excess yearly cancer mortality due to pollutants in farmed salmon at ca. 200 cases, and 40 yearly cancer deaths preventable by restricting consumption. Considering cardiovascular deaths, restricted consumption would cause yearly ca. 5000 deaths, i.e., 100-fold more. It was stated that none of the “scientific uncertainties” considered changed the result. In a similar vein, Leino et al. (2005) analyzed the health risks and benefits in Finland from fish dioxins and omega-3 PUFAs comparing cancer risks with avoided coronary heart disease mortality. The mean benefit/risk ratio varied from ca. 20 to 400 among the fish species consumed. It was concluded that banning commercial fishing of salmon and herring would save <4 cancer deaths but cause ca. 90 deaths from coronary heart disease per year, decreasing the net benefit of consuming all domestic fish from 410 to 320 avoided deaths per year.
However, not all uncertainties were considered in these analyses. They have significant limitations also beyond uncertainties quantified by probability distributions (Smith et al. 2002; Leino et al. 2005). First, the relevance and commensurability of effects and thus the validity of the net risk estimates may be questioned. Analysis should include morbidity, also from developmental effects of DLCs (Table 3). Second, depending on risk and benefit distributions, their ratio may greatly deviate from mean value (Finkel 1996; Ponce et al. 2000). It is likely that a larger population experiences net benefits than net risks from fatty fish, as cardiovascular disease is common; but in some groups the risk/benefit ratio is much greater. Third, some important uncertainties are not captured by the probability distribution functions of risk and benefit variables. All such additional uncertainties cannot be dismissed as “political” (Tuomisto et al. 2004); they can be associated with real impacts and have also a scientific dimension. They include uncertainties of framing and of models underlying risk–benefit comparisons. For instance, Tuomisto et al. (2004) mentioned that care was taken not to exaggerate benefits, but their estimate was based only on some studies such as Din et al. (2004), not on more extensive evaluations (see above) that had much reservations in the certainty and generalizability of dose–benefit relationships.
Table 3.
Semi-quantitative comparison of human health impacts of ingredients in fatty Baltic Sea fish, including beneficial effects
| Effect type | Effect direction and strength in human populationsa | Notes on risks (R) and benefits (B) | ||||
|---|---|---|---|---|---|---|
| DLCsb | Other OXsc | MeHgd | n-3 PUFAse | Other ingredients | ||
| Cardiovascular | (−) Tentative | – | ++ | Selenium may modify | Subtle or inconclusive high-dose Rs (lower dose myocardiac fibrosis in monkey), strong clear Bs (in infarct patients) | |
| Neurological | −(−) | − (CBsf) | (−) | +(+) | Vitamins | Potential Rs from DLCs and non-dlPCBsh; potential Bs of PUFAs to young and ageing; MeHg important locally (freshwater fish) |
| Developmental | −(−) | +(+) | Vitamin D | Tooth/bone and potential birth weight etc. Rs from DLCs; offsetting Bs from vitamin D and PUFAs | ||
| Reproduction | −(−) | (−) (DDEg) | +(+), (−) | Inconclusive Rs in fish-eating cohorts; Bs from PUFAs in other cohorts | ||
| Immune | −(−) | (+) | Unclear Rs from DLCs; complex effects; some persons allergic to fish | |||
| Metabolism | − | + | Vitamin benefits | R and B in diabetes/lipid metabolism | ||
| Tumors | (−) | (−) | +(+) | Selenium may modify | Rs vary by tumor type; inconclusive Bs | |
| Hormonal | − | − (DDE) | + | Rs of thyroid and possibly sex hormone effects from DLCs/PBTs; Bs vary | ||
a− = adverse, + = beneficial, (−)/(+) = weak, −(−)/+ (+) = moderate, −−/++ = strong effect; those of lowest uncertainty are shown in gray, b Dioxin-like compounds, c Organohalides, d Methyl mercury, for risk–benefit analyses, see Ponce et al. (2000) in references and Ginsberg and Toal, Environ Health Perspect 117 (2): 267–75, e Omega-3 Polyunsaturated Fatty Acids; f Chlorobenzenes, g p,p′-DDE, 1,1-dichloro-2,2-bis(chlorophenyl)-ethylene (metabolite of DDT), h Non-dioxinlike polychlorinated biphenyls
In particular, risk–benefit comparisons often omit alternatives to fatty fish consumption, as pointed out by Hites et al. (2004a) as well as (Tuomisto et al. 2004). Straightforward juxtapositions of risks and benefits from fatty fish often presuppose that people automatically shift from fish to more unhealthy diets; yet dietary habits may be influenced (Verbeke et al. 2008).
It seems overall that both the certainty and the magnitude of the beneficial health effects of fish consumption, primarily for cardiovascular health, exceed those of risks from fish contaminants. At issue are the limits and specifications of this general comparison, especially for fish and consumer sub-populations and, more broadly, for the different decision alternatives, including dietary, fisheries, and other choices. Conflicting assessments (compare e.g., Cohen et al. 2005 and Leino et al. 2005 with Foran et al. 2005a, 2005b, 2006 and Domingo 2007) suggest differing framings and valuations of risks, benefits, and associated certainties.
Much of the health benefits from fatty fish consumption are accrued to the elderly who are at particular risk for cardiovascular diseases (He et al. 2004). On the other hand, particularly great risks from dioxins are caused to early developmental stages (Maruyama et al. 2004). Ponce et al. (2000) showed that while the overall population may receive health gains from fish consumption, younger may experience a net health deficit. In the case of dioxins, this must be put in relation to the greatly decreased exposures. This allows greater protection to the young now than to the young at peak exposures 30–40 years ago. The consumption of fatty (Baltic) fish by children may now be even too small, considering both the loss of benefits for their healthy development and the loss of habit of consuming fish that may result (Lund et al. 2004).
Melanson et al. (2005) proposed that clean fish oil instead of whole fish be consumed to both ensure health benefits and avoid risks from contaminants. Still, the substitution of fatty fish with cleaner and healthy foods is problematic (Bell et al. 2005; Foran et al. 2005a, b). Many fish oil products contain appreciable dioxins (FSAI 2002). Clean omega-3 PUFA products based on either fish or other sources can be used as a surrogate to fish as such (Fig. 2). Jacobs et al. (2002) pointed out that vegetable oil-based feeds have also other advantages, and could offer economically high-energy feeds while reducing contamination of food chains. However, alternative feeds have limitations in fat composition and other qualities (SPCFC 2005; Genuis 2008). Thus, also the continued use of fish-based even relatively dioxin-rich feeds (such as those from Baltic Sea fish) may be an option.
Fig. 2.
Due to their health benefits, omega-3 polyunsaturated fatty acids are increasingly supplemented in dietary fats. However, balancing is also required between different dietary items including various types of fish, between their beneficial and harmful ingredients, between alternative intervention measures—and between uncertainties of these. (Photo: Timo Assmuth)
Case: Quantitative Risk Criteria for Dioxins and Beneficial Ingredients in Food
Criteria for Dioxin-Like Compounds
Tolerable Daily Intakes
In the derivation of tolerable daily intakes (TDIs) also for dioxins based on toxicological assessment, safety factors are a decisive element. The importance of uncertainties is illustrated by the fact that in the definition of TDIs for dioxins they have varied by orders of magnitude, for e.g., from 200 in Ahlborg et al. (1989) to 3.2–10 in WHO (1998) and SCF (2001). The reasoning is that by safety factors and focusing on “reasonable” worst cases and lowest-dose endpoints, protection will be ensured from risks overall. However, this assumption and the specific foundations of the safety factors can be questioned.
SCF (2001) concluded that no safety factors are needed due to inter-individual variation in susceptibility, without further justification. This is remarkable considering the standard guidance for safety factors (ECETOC 1995; ATSDR 1998) and the evidence on variation in susceptibility (Masten et al. 1998) and vulnerability (Wallin et al. 2003) also in humans. The decision of SCF (2001) to drop a safety factor based on inter-individual variation (SCF 2000) was criticized by UBA (Gies et al. 2007, Table 1).
Many arguments concerning safety factors are related to fundamental epistemic and policy principles, i.e. uncertainties and ambiguities at a high level. Some of the UBA claims are justified; e.g., other species than rats are desirable and primates are more human-like. However, some arguments seem selective or overdone: there are both similarities and differences between species (Peterson et al. 1993), and epidemiological studies, while generally desirable as checks, confirm neither non-effect nor effect, and yet may add weight to more stringent or more lenient risk criteria. Thus, in both defenses and criticism of TDIs, facts and values mix and certainty is exaggerated.
A key limitation of TDIs for dioxins, even if including other “sufficiently” dioxin-like compounds such as dlPCBs (involving order-of-magnitude uncertainties often ignored, cf. Van den Berg et al. 1998), is that other contaminants are omitted. In Baltic Sea fish, these prominently include non-dioxinlike PCBs and DDT. This is mirrored by the difficulty of ascertaining the effects and assessing the risks of mixtures and their components. Although such other contaminants give added cause for limiting risks from dioxins, if the adverse effects are mainly due to other components there is a danger of misdirected risk management. Analyses then should address tradeoffs and synergisms between such agents in a more multi-dimensional manner.
Contaminant Levels in Fish and Other Foods and Feeds
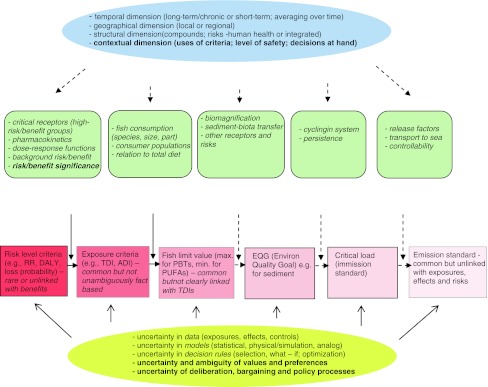
No clear and detailed methodology has been published in connection with the CEC (2002) recommendations for defining how TDIs are related to allowable (maximum, action and target) levels of dioxins in fish and fish products. These levels are not affected only by the assumptions and judgments in deriving TDIs, many of which involve great uncertainties. They additionally depend on assumptions and judgments concerning many other factors previous and subsequent to intakes in the risk chain (Fig. 3). Importantly, the limit values in fish depend on fish consumption and its relation to exposure from other sources. As such, the guidelines are influenced by political factors to still higher degree than the TDIs are.
Fig. 3.
Simplified risk/benefit based process of deriving quantitative human health management criteria for dioxins and PUFAs (polyunsaturated fatty acids) in fish (modified from Assmuth and Jalonen 2005), emphasizing multi-level influencing factors and uncertainties. Note that the different levels and types of uncertainty overlap. Those factors and criteria related only to PBTs have been shown by hatched lines. RR risk ratio (in epidemiological studies), DALYs disability-adjusted life years, TDI tolerable daily intake; ADI advisable daily intake
Key factors and framing issues in setting quantitative targets for fish dioxins that have not been sufficiently addressed include: fish species (wild and farmed) and fish qualities, for e.g., sizes (Fig. 4); frequency and quantities consumed; consumption directly or by non-human animals, and transfer models to humans (Kruse and Meng 2005); consumption of other dietary items, including fish from other waters. The inclusion of guidelines for beneficial ingredients in fish complicates this inference, but is essential for balanced policies. Additional guideline values or benchmarks are defined “upstream” the risk chain, e.g., for emissions, but are as yet not linked directly or well with guidelines for intakes and food levels (Fig. 3).
Fig. 4.
The silver of the Baltic, herring. The concentrations of fat-soluble toxic substances have a strong positive correlation with the size and thus the age of the fish. Also the use of herring for human consumption is related to fish size, due partly to technical requirements for filleting. (Photo: courtesy of Finnish Environment Institute)
All these factors vary between population segments that may thus need to be specified. Specifically, children have a body weight several fold lower and potentially greater susceptibility than adults (KEMI and IEM 2003). In principle, allowable levels of fish consumption (and dioxin intakes) might be derived also for breast-fed children and fetuses. However, these would have to be tied to body burdens and consumption levels of mothers, which add to uncertainties due to the complex toxicokinetics.
A fixed allowable fish concentration supposes time-averaged fish consumption. However, those consuming seldom and little may during those short periods safely eat fish containing even orders of magnitude higher levels (SACN and COT 2004); conversely, regular high consumers may need to avoid fish containing even lower dioxin levels, at least if health benefits from fish are ignored. With PBTs, the long-term average exposure is crucial. The time dimension is also associated with the need to take the various age groups into account.
With strict levels of allowable fish concentrations counter-intuitive effects may result. It can be difficult for some to understand that a fish is risky under some circumstances and risk-free under others, and that the limits are not as uncertain as TDIs, but also include, or disregard, many additional factors of variation and data and model uncertainty in consumption and in the consumers. Thus, while on scientific grounds specified and variable risk criteria are justifiable, for political reasons more uniformity and permanence is called for. In any case, transparency of the assumptions, rationales, and valuations is needed, and, therefore, also acknowledgment of the multiple uncertainties and ambiguities surrounding the criteria.
Guideline Values for Beneficial Fish Ingredients
There are recommendations for sufficient intake of fish in general and fish rich in PUFAs, especially during pregnancy (SPCFC 2005). Few quantitative guidelines have been given for minimum intakes. Dietary content of PUFAs is recommended by WHO (2003) in the range 6–10% of daily energy intake (E%). IMNA (2002) further recommended that 10% be represented by LC PUFAs. A lower recommended omega-3 PUFA intake of 0.3–0.5 E% was proposed by the Belgian Federal Health Council that also specified contributions of different fatty acids (also plant-derived).
Antonijevic et al. (2007) provided a quantitative analysis of the relation between the Belgian guideline and those for dioxins and dlPCBs in fish, accounting for some uncertainties by Monte Carlo simulation. The analysis highlighted the importance of what fraction of fats and their beneficial and toxic ingredients are obtained from fish and what from other sources: if all PUFAs supplied in diet would come from fish, guideline levels for tolerable dioxin intakes would be exceeded by a majority of the population. Similarly, Van der Voet et al. (2007) simulated risk and benefit distributions to derive optimal points of consuming fatty fish based on dioxin and PUFA health advisories in a general population with less frequent excess of guideline values.
Such methods offer ways to strike balances between risks and benefits from fish, especially when coupled with analyses of consequences of alternative actions. Thus, also dietary guidelines could become better balanced, as acknowledged by SPCFC (2005). However, both the level and the qualities of uncertainties (Table 3) and the interaction of epistemic and non-epistemic (value-based) uncertainties or ambiguities (Fig. 3) should be taken into consideration in these comparisons and advisories. Processes of reflexive deliberation and discourse are, therefore, essential.
Conclusions
The limited quantitative analyses suggest that, in general, the benefits to human health from fatty Baltic fish exceed the corresponding health risks, at least measured by mortality and quite likely also by morbidity, depending on weighting. Deviations from this may be significant in some population segments, as suggested by Antonijevic et al. (2007). However, dioxin and PCB levels in Baltic Sea fish have decreased to a fraction of peak levels (Assmuth and Jalonen 2005) and, after lags, also effects in high-risk groups are below peak levels.
The distributions and qualities of risks and benefits and of uncertainties in them need to be better accounted for. The interaction of DLCs, other PBTs including emerging toxicants and other fish ingredients is complex and depends on the case and the endpoint (SPCFC 2005). Indirect risks and benefits also require attention, and the relative benefits and risks from various alternatives need to be considered, also those of scaring people away from healthy fish diets. Risk–benefit analysis thus requires more many-sided and careful framing and scrutiny of effects, causes, co-factors, and target populations, often also options, and of associated uncertainties. As such, risk–benefit considerations, communication on their uncertainties (Verbeke et al. 2008), and associated reframing of problems and solutions constitute an important part of more integrated inter-sector governance taking into account multiple points of view.
SACN and COT (2004) and SPCFC (2005) considered the evidence and methods insufficient for quantitative risk–benefit analysis of oily fish consumption. However, sufficiency depends on the requirements and thus the context. Particular groups of high or low risks or benefits may be focused on to simplify analysis. This may represent a “reasonable worst case” approach and could also be extended to weight-of-evidence schemes for attribution of complex and uncertain risks, coupled with evaluation of uncertainties.
Despite the complexity of multiple risks, benefits and consequences, including conflicting premises and values, a structured treatment of uncertainties that combines quantitative and qualitative aspects in a reflexive approach can help strike a balance between wide and narrow focus and detail and generality. Tensions between flexibility and consistency in policy and assessment should, however, be acknowledged, also in guideline values and in balancing the requirement for evidence with precautionary action.
With complex risk and risk–benefit issues such as those of Baltic Sea fish, a narrow and rigid assessment and management approach based on illusory certainty and on sector governance style needs to be complemented by a broader, more flexible deliberative, and evolutionary approach. Such an approach could also help refocusing on issues found to be salient, such as distributions and qualities of risk–benefit comparisons, and strategic and policy alternatives (IOM 2003). This would often require that uncertainties are explicated for negotiations on risk management.
Acknowledgments
This work has been aided by grants from Nordic Council of Ministers, Finnish Ministry of the Environment; the EU’s 6th Framework Programme for R&D (contract No 003956), and Finnish Fulbright Center. Support from Finnish Environment Institute and University of Helsinki is also gratefully acknowledged. Thanks go to colleagues for their contributions, and to the anonymous reviewers. The author is responsible for the opinions expressed.
Timo Assmuth
is a senior researcher at Finnish Environment Institute (SYKE) and docent at University of Helsinki. At SYKE he leads R&D in waste, soil contamination and risk assessment, endocrine disrupters, environmental health and chemicals policy. His interests include trans-disciplinary aspects of environmental risks from psychological, historical, philosophical, and science studies perspectives.
References
- Ahlborg, U., H. Håkansson, F. Wærn, and A. Hanberg. 1989. Nordic dioxin risk assessment. Miljørapport 1989:7. 129 pp. + app. Copenhagen: Nordic Council of Ministers. (in Swedish, English summary).
- Antonijevic, B., C. Matthys, I. Sioen, M. Bilau, J. Van Camp, J.L. Willems, and S. De Henauw. 2007. Simulated impact of a fish based shift in the population n-3 fatty acids intake on exposure to dioxins and dioxin-like compounds. Food Chemistry and Toxicology 45: 2279–2286. http://dx.doi.org/10.1016/j.fct.2007.06.003. [DOI] [PubMed]
- Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake and the risk of coronary disease among men. New England Journal of Medicine. 1995;332:977–982. doi: 10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- Assmuth, T. and P. Jalonen. 2005. Risks and management of dioxin-like compounds in Baltic Sea fish: An integrated assessment. Copenhagen, Nordic Council of Ministers. TemaNord 2005: 568. 376 p. http://www.norden.org/da/publikationer/publikationer/2005-568/at_download/publicationfile.
- ATSDR. 1998. Toxicological profile for polychlorinated biphenyls. Agency Toxic Substances Disease Registry, U.S. Dept Health Human Services, Atlanta, Georgia, Dec 1998.
- Axmon A, Rylander L, Strömberg U, Jonsson B, Nilsson-Ehle P, Hagmar L. Polychlorinated biphenyls in serum and time to pregnancy. Environmental Research. 2004;96:186–195. doi: 10.1016/j.envres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Balk, E., M. Chung, A. Lichtenstein, P. Chew, B. Kupelnick, A. Lawrence, D. DeVine, and J. Lau. 2004. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Agency of Healthcare Research Quality, Rockville, MD, US, Mar 2004. AHRQ Publ. 04-E010-2. [PMC free article] [PubMed]
- Bell JG, McGhee F, Dick JR, Tocher DR. Dioxin and dioxin-like PCBs in Scottish farmed salmon: effects of replacement of dietary marine fish oil with vegetable oils. Aquaculture. 2005;243:305–314. doi: 10.1016/j.aquaculture.2004.10.016. [DOI] [Google Scholar]
- CEC. 2001. Community strategy for dioxins, furans and polychlorinated biphenyls. Communication from the Commission to the Council, the European Parliament and the Economic and Social Committee. COM(2001)593, Final. Official Journal of European Commission 17.11.2001, C322/2-18.
- CEC. 2002. Commission recommendation of 4 March 2002 on the reduction of the presence of dioxins, furans and PCBs in feedingstuffs and foodstuffs. Notified under number C(2002a) 836. Official Journal of European Commission 9.3.2002 L 67/69 (2002/201/EC).
- Cohen JT, Bellinger DC, Connor WE, Shaywitz BA. A quantitative risk benefit analysis of changes in population fish consumption. American Journal of Preventive Medicine. 2005;29:325–334. doi: 10.1016/j.amepre.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Din, J.N., D.E. Newby, and A.D. Flagan. 2004. Omega 3 fatty acids and cardiovascular disease—fishing for a natural treatment. British Medical Journal 328: 30–35. Comments and discussion, British Medical Journal 3288: 406–407. [DOI] [PMC free article] [PubMed]
- Domingo, J.L., A. Bocio, R. Martí-Cid, and J.M. Llobet. 2007. Benefits and risks of fish consumption: Part II. RIBEPEIX, a computer program to optimize the balance between the intake of omega-3 fatty acids and chemical contaminants. Toxicology 230: 227–233. http://dx.doi.org/10.1016/j.tox.2006.11.059. [DOI] [PubMed]
- Domingo, J.L. 2007. Omega-3 fatty acids and the benefits of fish consumption: Is all that glitters gold? Environment International 33: 993–998. http://dx.doi.org/10.1016/j.envint.2007.05.001. [DOI] [PubMed]
- ECETOC. 1995. Assessment factors in human health risk assessment. Brussels: European Centre for Ecotoxicology and Toxicology of Chemicals, 57. ECETOC Publ. 68
- Finkel AM. Comparing risks thoughtfully. Risk-Health Safety Environment. 1996;7:325–346. [Google Scholar]
- Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Risk-based consumption advice for farmed Atlantic and wild Pacific salmon contaminated with dioxins and dioxin-like compounds. Environmental Health Perspectives. 2005;113:552–556. doi: 10.1289/ehp.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran JA, Good DH, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Quantitative analysis of the benefits and risks of consuming farmed and wild salmon. Journal of Nutrition. 2005;135:2639–2643. doi: 10.1093/jn/135.11.2639. [DOI] [PubMed] [Google Scholar]
- Foran JA, Carpenter DO, Good DH, Hamilton MC, Hites RA, Knuth BA, Schwager SJ. Risks and benefits of seafood consumption. American Journal of Preventive Medicine. 2006;30:438–439. doi: 10.1016/j.amepre.2006.01.002. [DOI] [PubMed] [Google Scholar]
- FSAI. 2002. Summary of investigation of dioxins, furans and PCBs in farmed salmon, wild salmon, farmed trout and fish oil capsules. Food Safety Authority of Ireland, March 2002. 9 p. http://www.fsai.ie/industry/Dioxins3.htm.
- Genuis SJ. To sea or not to sea: Benefits and risks of gestational fish consumption. Reproductive Toxicology. 2008;26:81–85. doi: 10.1016/j.reprotox.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Gies A, Neumeier G, Rappolder M, Konietzka R. Risk assessment of dioxins and dioxin-like PCBs in food—Comments by the German Federal Environmental Agency. Chemosphere. 2007;67:S344–S349. doi: 10.1016/j.chemosphere.2006.05.128. [DOI] [PubMed] [Google Scholar]
- He K, Song Y, Daviglus ML, Liu K, Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Science. 2004;303:226–229. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hites, R.A., J.A. Foran, D.O. Carpenter, M.C. Hamilton, B.A. Knuth, and S.J. Schwager. 2004b. Reply. Science 305: 478.
- Hooper, L., R.L. Thompson, R.A. Harrison, C.D. Summerbell, H. Moore, H.V. Worthington, P.N. Durrington, A.R. Ness, et al. 2004. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. The Cochrane Database Systematic Rev. 4. Art. No.: CD00317. pub2. doi: 10.1002/14651858.CD00317.pub2. [DOI] [PMC free article] [PubMed]
- IMNA. 2002. Dietary Reference Intakes for Energy Carbohydrate, Fibre, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: Institute of Medicine of the National Academies, National Academies Press.
- IOM. 2003. Dioxins and dioxin-like compounds in the food supply: Strategies to decrease exposure. Committee on Implications of Dioxin in the Food Supply, Food and Nutrition Board, Institute for Medicine of the National Academies. Washington, DC: National Academies Press. [PubMed]
- Jacobs MN, Covaci A, Schepens P. Investigation of selected persistent organic pollutants in farmed Atlantic salmon (Salmo salar), salmon aquaculture feed, and fish oil components of the feed. Environmental Science and Technology. 2002;36:2797–2805. doi: 10.1021/es011287i. [DOI] [PubMed] [Google Scholar]
- Joas, R., A. Potrykus, and G. Chambers 2001. Effects on the fisheries industry of the Commission proposals (SANCO) on dioxin content of fish, fish oil and fish meal as part of animal feed regulation. D-G Research Working Paper STOA 101 EN, 10-2001. Scientific and Technological Options Assessment Series. Luxemburg: European Parliament.
- KEMI and IEM. 2003. Human health risk assessment. Proposals for the use of assessment (uncertainty) factors. Application to risk assessment for plant protection products, industrial chemicals and biocidal products within the European Union. Body for Competence in Methodological Development, National Chemicals Inspectorate and Institute for Environmental Medicine, Karolinska Institute. KEMI Rapport 1/03.
- Konig A, Bouzan C, Cohen JT, Connor WE, Kris-Etherton PM, Gray GM, Lawrence RS, Savitz DA, Teutsch SM. A quantitative analysis of fish consumption and coronary heart disease mortality. American Journal of Preventive Medicine. 2005;29:335–346. doi: 10.1016/j.amepre.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton, P.M., W.S. Harris, and L.J. Appel; for the AHA Nutrition Committee. 2003. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arteriosclerosis and Thrombosis in Vascular Biology 23: e20–e30 (Erratum: e31). Comment in: Arteriosclerosis and Thrombosis in Vascular Biology 23: 151–152. [DOI] [PubMed]
- Kruse, S. and W. Meng. 2005. Risk management of dioxins in feed by the German Federal Ministry of Consumer Protection. Organohalogen Compounds CD-ROM ID 362.
- Leino, O., S. Lensu, M. Niittynen, and J. Tuomisto. 2005. Risk-benefit analysis of fish in Finland: Dioxins and omega-3 fatty acids. Organohalogen Compounds (2005): 2476–2478. CD-ROM ID 1097.
- Lund E, Engeset D, Alsaker E, Skeie G, Hjartaker A, Lundebye A-K, Niebor W. Cancer risk and salmon intake. Science. 2004;305:477. doi: 10.1126/science.305.5683.477. [DOI] [PubMed] [Google Scholar]
- Maruyama W, Yoshida K, Aoki Y. Dioxin health risk to infants using simulated tissue concentrations. Environmental Toxicology and Pharmacology. 2004;18:21–37. doi: 10.1016/j.etap.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Masten SA, Grassman JA, Miller CR, Spencer DL, Walker NJ, Jung D, Edler L, Patterson DG, et al. Population-based studies of dioxin responsiveness: Individual variation in CYP1A1 levels and relationship to dioxin body burden. Organohalogen Compounds. 1998;38:13–16. [Google Scholar]
- Melanson SF, Lewandrowski EL, Flood JG, Lewandrowski KB. Measurement of organochlorines in commercial over-the-counter fish oil preparations: implications for dietary and therapeutic recommendations for omega-3 fatty acids and a review of the literature. Archives of Pathology and Laboratory Medicine. 2005;129:74–77. doi: 10.5858/2005-129-74-MOOICO. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. Fish, Mercury, Selenium and Cardiovascular Risk: Current Evidence and Unanswered Questions. International Journal of Environmental Research in Public Health. 2009;6(6):1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Critical Reviews in Toxicology. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Ponce RA, Bartell SM, Wong EY, LaFlamme D, Carrington C, Lee RC, Patrick DL, Faustman EM, Bolger M. Use of quality-adjusted life year weights with dose response models for public health decisions: a case study of the risks and benefits of fish consumption. Risk Analysis. 2000;20:529–542. doi: 10.1111/0272-4332.204050. [DOI] [PubMed] [Google Scholar]
- Ruxton CHS, Reed SC, Simpson MJA, Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. Journal of Human Nutrition and Diet. 2004;17:449–459. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- SACN and COT. 2004. Advice on fish consumption: benefits & risks. Sci Advisory Committee Nutr and Committee Toxicol. The Stationery Office, London. www.food.gov.uk/multimedia/pdfs/fishreport2004full.pdf.
- SCF. 2000. Opinion of the Scientific Committee for Food on the risk assessment of dioxins and dioxin-like PCBs in food. EC, Brussels. Adopted Nov 2000. www.europa.eu.int/comm./food/Fs/sc/scf/out78_en.pdf.
- SCF. 2001. Opinion of the Scientific Committee for Food on the risk assessment of dioxins and dioxin-like PCBs an food. Update based on new scientific information available. Adopted 30th May 2001. www.europa.eu.int/comm./food/Fs/sc/scf/out90_en.pdf.
- Smith GC, Hart AD, Rose MD, MacArthur R, Fernandes A, White S, Moore DR. Intake estimation of polychlorinated dibenzo-p-dioxins, dibenzofurans (PCDD/Fs) and polychlorinated biphenyls (PCBs) in salmon: the inclusion of uncertainty. Food Additives and Contaminants. 2002;19:770–778. doi: 10.1080/02652030210145045. [DOI] [PubMed] [Google Scholar]
- SPCFC. 2005. Opinion of the Scientific Committee on Contaminants in the Food Chain on a request from the European Parliament related to the safety assessment of wild and farmed fish. Question N EFSA-Q-2004-23. Adopted on Jun 2005. EFSA Journal 236:1.
- Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Archives of Internal Medicine. 2005;165:725–730. doi: 10.1001/archinte.165.7.725. [DOI] [PubMed] [Google Scholar]
- Svensson BG, Mikoczy Z, Strömberg U, Hagmar L. Mortality and cancer incidence among Swedish fishermen with a high dietary intake of persistent organochlorine compounds. Scandinavian Journal of Work and Environmental Health. 1995;21:106–115. doi: 10.5271/sjweh.17. [DOI] [PubMed] [Google Scholar]
- Tuomisto JT, Tuomisto J, Tainio M, Niittynen M. Risk-benefit analysis of eating farmed salmon. Science. 2004;305:478. doi: 10.1126/science.305.5683.476. [DOI] [PubMed] [Google Scholar]
- USEPA. 2000. Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Draft final. USEPA, Washington, DC. EPA/600/6-88/005Ca. www.epa.gov/ncea/dei.html.
- Berg M, Birnbaum L, Bosveld ATC, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environmental Health Perspectives. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet H, Mul A, Klaveren JD. A probabilistic model for simultaneous exposure to multiple compounds from food and its use for risk-benefit assessment. Food and Chemical Toxicology. 2007;45:1496–1506. doi: 10.1016/j.fct.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Verbeke W, Vanhonacker F, Frewer LJ, Sioen I, Henauw S, Camp J. Communicating risks and benefits from fish consumption: Impact on Belgian consumers’ perception and intention to eat fish. Risk Analysis. 2008;28:951–967. doi: 10.1111/j.1539-6924.2008.01075.x. [DOI] [PubMed] [Google Scholar]
- Wallin, E., L. Rylander, B. Jonsson, and L. Hagmar. 2003. Intra-individual variations over time for 2,2′,4,4′,5,5′-hexachlorobiphenyl (CB 153) in relation to consumption of fatty fish from the Baltic Sea. Organohalogen Compounds 60–65 (2003), CD-ROM Vol. 5, Section 1.
- Wang, C., M. Ching, E. Balm, B. Kupelnick, D. DeVine, A. Lawrence, A. Lichtenstein, and J. Lau. 2004. Effects of omega-3 fatty acids on cardiovascular disease. Agency of Healthcare Research Quality, Rockville, MD, USA, Mar 2004. AHRQ Publ. No 04-E009-2.
- WHO. 1998. Assessment of the health risk of dioxins: re-evaluation of the Tolerable Daily Intake (TDI), executive summary. WHO Consultation, May 25–29 1998, Geneva, Switzerland. Geneva: WHO. www.who.int/pcs/dioxin-exec-sum/exe-sum-final.doc.
- WHO. 2003. Joint WHO/FAO Expert Consultation. Diet, nutrition and the prevention of chronic diseases. Recommendations for preventing cardiovascular diseases, pp. 81–94.