Abstract
Environmental change challenges local and global survival of populations and species. In a species-poor environment like the Baltic Sea this is particularly critical as major ecosystem functions may be upheld by single species. A complex interplay between demographic and genetic characteristics of species and populations determines risks of local extinction, chances of re-establishment of lost populations, and tolerance to environmental changes by evolution of new adaptations. Recent studies show that Baltic populations of dominant marine species are locally adapted, have lost genetic variation and are relatively isolated. In addition, some have evolved unusually high degrees of clonality and others are representatives of endemic (unique) evolutionary lineages. We here suggest that a consequence of local adaptation, isolation and genetic endemism is an increased risk of failure in restoring extinct Baltic populations. Additionally, restricted availability of genetic variation owing to lost variation and isolation may negatively impact the potential for evolutionary rescue following environmental change.
Keywords: Biocomplexity, Endemic lineages, Evolution of tolerance to contamination, Genetic variation, Marginal environment
Introduction
Today human activities impact all major ecosystems resulting in rapid and large-scale environmental changes. The oceans, earlier protected by their enormous sizes, are undergoing dramatic alterations that fundamentally affect most marine organisms. The Millenium Ecosystem Assessment (2005) points out that certain species or species communities will be more prone to extinction due to environmental changes than others and that vulnerable species often have one or more of the following features: limited climatic ranges, restricted habitat requirements, reduced mobility, low genetic diversity, or isolated and/or small populations.
The ecological (short term, within generation) effect of large-scale environmental change results in changes in population sizes or in patterns of distribution of species that may be hard to predict on forehand. When trying to predict long-term (over generations) consequences of environmental change, including evolutionary changes of species traits resulting in new inherited characteristics of organisms due to new selection regimes, we add substantial uncertainty as complexity increases several orders of magnitudes. However, in all evolutionary change, a basic principle is that by constantly providing alternatives or new possibilities, genetically based trait-variation allow populations to adapt under changed or changing environments by natural selection, and such an evolutionary change may potentially rescue populations and species from local or global extinction (Bell and Gonzalez 2009). In other words, the more genetic variation available to a population, either as already present or as available through gene flow from other populations, the greater the chance that the population may cope with a new environment through evolutionary change and adaptation.
In the marine environment, the beheld view has been that long distance dispersal by ocean currents, lack of physical barriers to dispersal, and long-lived planktonic propagules result in large and widely distributed and genetically homogeneous populations of most marine species. However, recent studies of genetic structure of species of marine fishes, invertebrates, algae, and plants have changed this paradigm and show numerous examples of structured species (reviewed in Hellberg 2009) in which populations are locally adapted (Hemmer-Hansen et al. 2007; Larsen et al. 2008; Andersen et al. 2009; Larmuseau et al. 2010). An important consequence of species being divided into locally adapted populations that may have key roles in their local ecosystem is that an extinct local population may not easily be replaced by recruits from other areas. Indirect support for such a conclusion is found in species where extensive mixing of populations occurs during some life stage without break down of the discrete population structure—a phenomenon sometimes referred to as biocomplexity (Ruzzante et al. 2006; Schindler et al. 2010).
Basically, a population will, as a consequence of a change in its local environment, either survive by adaptation or disappear from the local ecosystem (that is, move or become extinct). Phenotypic plasticity allows populations to adjust within the time-scale of a generation to a minor environmental change, while the presence of genetic variation constitutes the basis for evolutionary changes resulting in differentiation among populations after a minimum of some generations. In some cases evolutionary change may even result in formation of new species, and a pertinent example of this in the Baltic Sea is the new species Fucus radicans formed within the last few thousands of years (Pereyra et al. 2009).
The Baltic Sea is a young and evolving environment established as a marine (brackish) ecosystem about 6500–9800 year BP (Zillén et al. 2008). Surface salinity was initially much higher than today (about 10–15 psu compared to today 7–8 in the Baltic Proper) (Zillén et al. 2008). Climate data scenarios combined with oceanographic modeling predicts that the salinity will decrease further (and more rapidly) as a result of global warming during coming decades (Meier 2006; Neumann 2010). Critical issues are: (1) what will happen to the Baltic Sea flora and fauna under continued environmental changes, and (2) will locally extinct Baltic Sea populations be replaced by recruitment of individuals from outside the Baltic? In addition, one may raise the issue if there will be sub-lethal effects from losses in genetic diversity of Baltic Sea organisms. Here, we review the genetic characteristics of Baltic Sea populations and discuss their potential of evolving new adaptations in response of future environmental changes. In addition, we consider the potential to restore locally adapted Baltic populations that go extinct. Finally, we briefly survey sub-lethal effects of loss of genetic variation and how these may impact ecosystem functions.
The Baltic Sea Ecosystem
The Baltic Sea (here we include for biological reasons the Danish Belts and Öresund, but not Kattegat, which is more similar to a fully marine area) is a large post-glacial and brackish water estuary. Being atidal, the Baltic Sea has a notably stable salinity gradient spanning 1–10 PSU in surface waters and slightly higher salinities in bottom waters. This, together with the extremely young age of the environment makes the Baltic Sea a globally unique environment. In addition, the low salinity and the young age make this environment ecologically marginal and most of the species inhabiting the Baltic Sea have either a marine or a freshwater origin, although some recently introduced species have a remote brackish water origin (Leppäkoski and Olenin 2000). The sea is physically isolated with narrow connections to other marine areas, and strong out-flowing currents that are likely to remove rather than add passively transported propagules and rafting organisms, although deeper counter-currents may to some extent bring passively transported larvae into the Baltic.
The Baltic Sea has notably low species diversity, and only a handful of species dominate the ecosystem in biomass and numbers (Fig. 1). Still the Baltic Sea is as productive as the adjacent North Sea that has about ten times more species (Elmgren and Hill 1997). Consequently, important ecosystem functions are essentially upheld by single or a few species, such as the blue mussels (Mytilus edulis species complex), which are dominant benthic filter-feeders present in the Baltic proper that link pelagic and benthic production (Kautsky and Evans 1987), and the macrophytes Zostera marina, Fucus vesiculosus, and F. radicans that are habitat-forming species supporting biodiversity with food and shelter (Williams and Heck 2001; Wikström and Kautsky 2007). The isopods Monoporeia affinis and Saduria entomon, and the bivalves Macoma balthica and Mya arenaria are main benthic detritus-feeders and predators dominating the biomass of benthic macrofauna north of Åland. Important pelagic predators are herring, sprat, cod (in the south), and salmonids (in the north). A few species of seals and birds are the only natural top predators of the Baltic Sea ecosystem.
Fig. 1.
The Baltic Sea has a low diversity of marine species likely because it is a young (post-glacial) and ecologically marginal (brackish water) marine habitat (photo: Daniel Johansson)
On top of natural stress from low salinity, the Baltic Sea biota is exposed to high levels of anthropogenic contaminants (nutrients, oil, heavy metals, and toxins) (Jansson and Dahlberg 1999; Ducrotoy and Elliott 2008), and commercial fish species in particular are affected by intense fisheries and physical exploitation of migration routes and spawning sites (Nilsson et al. 2005). In addition, deep benthic habitats are fragmented owing to increasing occurrence of large oxygen-depleted areas (Conley et al. 2009). Finally, stress in the form of decreased salinity and increased temperature of the Baltic ecosystem, is expected as an effect of global warming (Meier 2006; Neumann 2010).
The Importance of Genetic Variation
Genetic variation may either be neutral or affect fitness of individuals, and in principle only genetic variants (alleles) improving fitness is expected to increase in frequency following environmental change. Hence, among neutral or slightly deleterious alleles present in a population a few may increase tolerance to a new environmental stressor (Bell and Collins 2008). These alleles are likely to be rare in the original population due to no or negative effect on fitness, but will increase in frequency under selection in the new environment. An illustrative example of what may happen during an environmental shift is the rare EDA allele present in oceanic populations of sticklebacks in a frequency of 1%. This allele is strongly favored in freshwater populations (frequency 100%) and is critical to the repeated development of locally adapted freshwater populations of sticklebacks (Colosimo et al. 2005). Hence, less common and rare alleles contribute to the standing genetic variation of a population and although the majority of genes may be neutral or even slightly deleterious under present selection regimes, this variation may be critical to the population during adaptation to a new environment (Barrett and Schluter 2008).
Population size is important for maintenance of genetic variation because stochastic loss of genetic variation each generation is much less in large populations compared to small populations. Moreover, large populations generate more new mutations each generation than small populations. In addition, immigrants to populations contribute new genetic variation if they originate in populations with different gene pools. Consequently, large and well-connected populations have accumulated more standing genetic variation than small and isolated populations. The level of genetic variation in a population is also affected by historical demographic changes. A population that earlier passed a bottleneck has lost genetic variation in proportion to the duration (in generations) of the bottleneck (Nei et al. 1975), and genetic variation will only be restored very slowly unless inflow of genes from other populations is large (Chakraborty and Nei 1977). Consequently, overall low levels of genetic variation are likely to have a negative impact on the capacity to adapt to a new environment. In particular small and isolated populations or populations that have passed through bottlenecks are all likely to have smaller pools of standing genetic variation compared to large and well-connected populations.
Genetic Variation and Differentiation of Baltic Populations
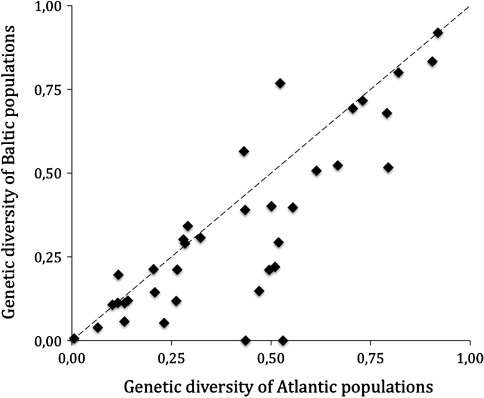
Genetic variation of wild populations is typically estimated from sets of random or neutral gene loci. Although these loci are mostly not themselves under selection (but see Larsson et al. 2007), the overall level of genetic variation of a population generated from these estimates can be used as a proxy for the level of standing genetic variation, and hence in assessing the potential to evolve new adaptations during environmental change (Bell and Collins 2008). Screening of population genetic variation using various genetic markers (allozymes, microsatellites, mtDNA) have hitherto generated estimates of genetic variation for about 30 of the most common Baltic Sea species. These data show that most populations living inside the Baltic Sea are less genetically variable than populations living in the North Sea and adjacent areas (Fig. 2). A likely explanation for the lower levels of genetic variation in most Baltic Sea populations is that they are smaller and more isolated than populations outside the Baltic Sea, and in addition, at least some of them may have passed through one or several bottlenecks (Härkönen et al. 2005).
Fig. 2.
Comparing genetic diversity in populations of the same species from the Atlantic and the Baltic Sea. Each point represents a separate species and points above the line are species in which Baltic populations are more genetically variable than Atlantic populations, and vice versa (redrawn from Johannesson and André 2006)
One exception, the Baltic clam (Macoma baltica), is more genetically variable inside than outside the Baltic Sea (Johannesson and André 2006). The reason for this is that the Baltic Sea hosts two genetically distinct lineages of this species and through hybridization they share a larger gene pool than each separate lineage (Nikula et al. 2008). In this way, hybridization may increase the potential for evolutionary rescue in this species. However, hybridization may also have negative consequences (so called outbreeding depression) during which introduced alleles destroy genetic complexes promoting local adaptation (Edmands 1999).
Despite lower genetic variation than elsewhere, there is hitherto no direct evidence of loss of fitness of Baltic populations owing to inbreeding depression. For example, species with currently small and isolated Baltic populations like harbor seal and harbor porpoise (Härkönen et al. 2005; Wiemann et al. 2010) seem to do well at the moment despite very small population sizes (harbor seal N < 50 during 1970s, and at present <400, Härkönen et al. 2005; harbor porpoise N < 100, Berggren et al. 2004). Even if they have lost only minor proportions of their original genetic variation, which can be explained by long generation times and much larger population sizes earlier in history, genetic variation is expected to decrease during coming generations if low population sizes are maintained.
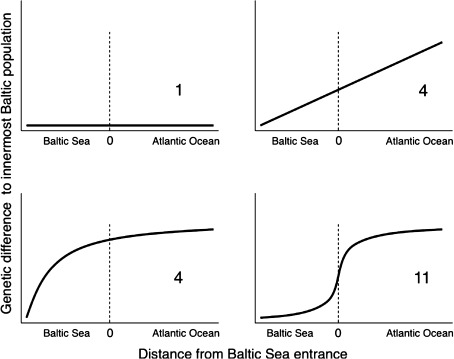
Eleven out of twenty marine species representing algae, plants, invertebrates and vertebrates reviewed by Johannesson and André (2006) showed evidence of genetic shifts (steep genetic clines) over the Baltic Sea–North Sea border zone (Öresund and the Danish Belts) (Fig. 3). Recent studies have supported this pattern with more data (Hemmer-Hansen et al. 2007; Florin and Höglund 2008; Gaggiotti et al. 2009) and for additional species (sand goby—Larmuseau et al. 2010; harbor porpoise—Wiemann et al. 2010; sprat—Limborg et al. 2009, but not for plaice—Was et al. 2010). The clines are explained either as a consequence of differential selection on individual gene loci, favoring one type of genes inside the Baltic Sea and another outside, or lack of gene exchange (migration) between Baltic and North Sea populations, or both. Evidence of selection for particular gene variants inside the Baltic Sea is particularly strong in blue mussels (Riginos and Cunningham 2005), Baltic clam (Luttikhuizen et al., unpublished data), herring (Larsson et al. 2007; André et al. 2010), and cod (Andersen et al. 2009).
Fig. 3.
Generalized patterns of genetic differentiation over the North Sea–Baltic Sea environmental gradient for a total of 20 species of marine organisms (data from Johannesson and André 2006)
Both divergent natural selection and genetic isolation indicate local adaptation of Baltic populations to the special conditions of the Baltic Sea. Baltic cod, for example, has a genetic variant of hemoglobin with two mutations that differ from hemoglobin of North Sea cod (Andersen et al. 2009) (Fig. 4). This seems likely an adaptation to both colder water temperatures and low oxygen conditions in the Baltic Sea compared to the shallow North Sea. Furthermore, Baltic cod spawns in summer instead of early spring, and produce eggs that are buoyant at lower salinities than North Sea cod eggs (Nissling and Westin 1997). In sand goby, a visual pigment has evolved by local adaptation to the stable but locally divergent light conditions in different parts of the Baltic Sea (Larmuseau et al. 2010). Baltic populations of fucoid macroalgae have evolved clonality and decreased resistance to desiccation and temperature stress (Tatarenkov et al. 2005; Pearson et al. 2000; Lago-Leston et al. 2010). Even regularly migrating species like herring show evidence of strong local adaptation among Baltic stocks (Gaggiotti et al. 2009).
Fig. 4.
Cods have the capacity to migrate over large distances, yet the species is divided into more or less discrete local populations distinguished by different genetic set ups. Most probably, a large part of these differences reflects adaptation to local environmental conditions (photo: Bo Johannesson)
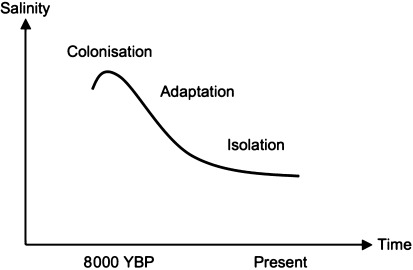
Limited gene flow contributes to divergence of Baltic populations from North Sea populations and is also likely to promote local adaptation. The flip-side of the coin is that the Baltic populations will also be isolated from alleles that are part of the standing genetic variation available in the NE Atlantic populations, and that potentially may be useful for Baltic Sea populations in future adaptations. If completely isolated, a Baltic population would be left with its present standing genetic variation in addition to new mutations that arise locally, while alleles present or arising outside the Baltic Sea would be inaccessible. Data available suggests that many of the Baltic populations have restricted access to the standing genetic variation available outside the Baltic Sea (Johannesson and André 2006). Upon invading the Baltic Sea, ca 8,000 years ago, the situation must have been very different. Early Baltic Sea invaders were likely in good connection with populations outside the newly formed sea since the environmental conditions (i.e., salinity) were more similar during the initial phase of the marine period (the Littorina Sea). Consequently, populations were not yet genetically differentiated by selection and not genetically isolated. Following local adaptation to a stronger environmental gradient, Baltic populations became increasingly isolated, and the current situation for many of them is that they seem to be trapped in the local conditions of the Baltic Sea (Fig. 5). As discussed below, this is likely to affect their potential of evolutionary rescue.
Fig. 5.
Stages of separation of Baltic Sea populations from ancestral North Sea populations. Initially salinity differences were small and populations presumably strongly connected. Following decreasing salinity, there was a phase of local adaptation (or for many species extinction from the Baltic Sea). Presently, most Baltic Sea populations left seem to be genetically isolated from North Sea populations
Clonality is Common in Baltic Sea Populations
Several marine plant and algal species have increased asexual recruitment inside the Baltic Sea. Such trends of increased asexual reproduction is common toward marginal habitats and may be a consequence of problems with sexual reproduction under extreme conditions, or an evolutionary strategy to avoid breaking up favorable genetic associations in genotypes adapted to marginal conditions (Peck et al. 1998; Silvertown 2008). Strict clonality comes with an increased cost in the form of a slower evolutionary response to environmental change limited by the lack of recombination of new genotypes. If populations switch between clonal lines and sexual reproduction, recombination of genes starts to become possible and favorable mutations appearing in one clone may be transmitted to other clones (Bengtsson 2003).
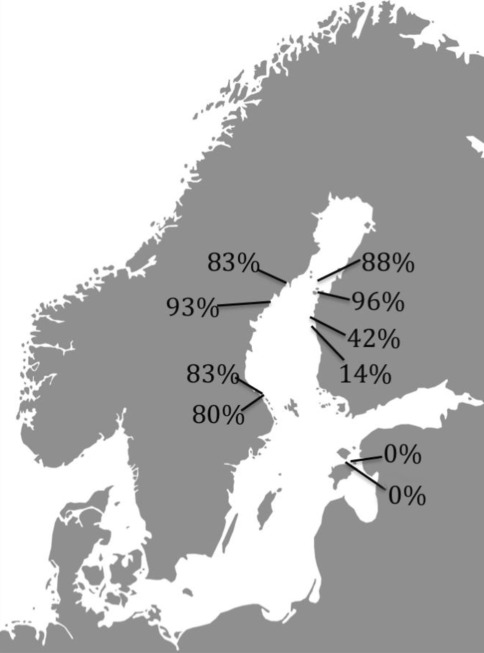
Reproductive strategies of a handful of Baltic macrophytes have been examined in detailed using genetic markers. Eelgrass, Z. marina, for example, has substantially higher levels of asexual recruitment inside the Baltic Sea (60–90% of the individuals of a seagrass meadow is asexually recruited in sea grass meadows in the northern Baltic Sea, compared to 0–10% in North Sea populations, Reusch et al. 2000). Bladderwrack (F. vesiculosus) recruits new attached individuals both sexually and asexually inside the Baltic Sea, while exclusively sexually outside (Tatarenkov et al. 2005). A most intriguing example is the newly discovered macroalgae endemic to the Baltic Sea, F. radicans (Bergström et al. 2005) that shows a highly variable strategy of recruitment, from complete sexual recruitment in some populations to over 90% clonality in others (Figs. 6, 7). Effects of high clonality is observed as a 25% decrease in the level of genetic variation within the populations dominated by asexual recruitment (Johannesson et al., unpublished). A notable feature in both F. radicans and Z. marina is the presence of old and widespread clones. A several thousand year old eelgrass clone has been identified in the Åland archipelago, and on the Swedish and Finnish coasts of the Bothnian Bay a single female clone of F. radicans is widespread and dominates several populations over a geographical area of 550 × 100 km (Johannesson et al., unpublished).
Fig. 6.
Fucus radicans is an important habitat-forming species in the northern and eastern Baltic Sea. Genetic variation is in some areas low owing to the dominance of a widespread female clone (photo: Lena Bergström)
Fig. 7.
Proportions of asexually recruited (clonal) individuals of the Baltic Sea endemic species Fucus radicans (data from Johannesson et al., unpublished data)
Rapid Evolution of Baltic Sea Populations
The post-glacial history of the Baltic Sea is characterized by dramatic environmental changes from marine conditions to fresh-water and then again to more or less marine and finally approaching brackish water conditions. Initially, the Littorina Sea contained more marine species than today’s Baltic Sea but as a consequence of successively decreasing salinity many of them went extinct (e.g., the periwinkle Littorina littorea that lends its name to this stage of the Baltic Sea). The species that remain today were able to either tolerate decreasing salinity or adapt by evolutionary changes. Notably, a number of species have thus been able to evolve local adaptations over only a few thousand years, and in one exceptional case, the rapid evolution has even resulted in the formation of a new and endemic species, F. radicans. This species originates from Baltic Sea populations of F. vesiculosus and was formed no more than a few thousand years ago (Pereyra et al. 2009).
For some species, the Baltic–North Sea gradient unveil intriguing evolutionary interactions, such as between the two sibling or semi-species M. edulis and M. trossulus where a complex pattern of hybridization and introgression is evident at the entrance and in the southern parts of the Baltic Sea (Riginos and Cunningham 2005). A recent analysis of both nuclear and mitochondrial genetic markers shows strong clinal shape differences among markers but weak genome-related incompatibilities in reproductive isolation, high-lighting the role of genetic drift and hybrid zone movement in addition to selection in formation of hybrid zone genetic structures in blue mussels (Stuckas et al. 2009).
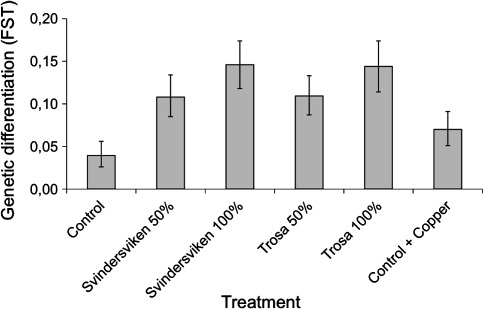
High concentrations of pollutants in the Baltic Sea water and sediment drive evolution of resistance to contamination. Also in this case genetic variation available in gene-loci involved in resistance is critical to the rate of evolution and magnitude of the resistance. When populations of the small crustacean species Attheyella crassa were exposed to contaminated coastal Baltic Sea sediment over 120 days (4-5 generations) mortality increased, levels of genetic variation decreased, and experimental populations diverged genetically (Gardeström et al. 2008). These experiments underline the complicated interactions between selection and stochastic processes such as random genetic drift. Indeed, both factors are potentially influenced by environmental change either directly or indirectly through effects on population demography. In exposed populations of this experiment, the population sizes were, for example, significantly smaller in the treatment replicates compared to the controls (Gardeström et al. 2008). Smaller populations are more inbred and generally more susceptible to genetic drift, which tends to genetically differentiate populations at random loci. Nevertheless, the reduction in number of individuals in the exposed populations, leading to an increased genetic divergence, was likely caused by selective losses of less tolerant genotypes (Fig. 8). Evolution of increased tolerance to contaminants is also demonstrated in natural populations of coastal waters (Wirgin and Waldman 2004; Eriksson et al. 2009). Moreover, populations of marine organisms sometimes show lower genetic variation at contaminated sites compared to control sites (Ma et al. 2000; Bickham et al. 2000).
Fig. 8.
Amount of genetic divergence (FST) in AFLP genetic markers over 4–5 generations between replicate populations (six per treatment) of Attheyella crassa exposed to contaminated Baltic Sea sediments. Vertical bars indicate 95% credible intervals (data from Gardeström et al. 2008)
These examples show that there is a potential for genetic changes and adaptations to new conditions within a few hundred generations or less. Except for availability of genetic variation, the more specific requirements for this to happen are complex and largely unclear. It is also important to underline that many species established in the Baltic Sea during the Littorina Sea period did not survive the environmental transition to a low-saline habitat, and still more marine species have a distribution that indicate that they are not (for the moment) able to extend their distribution into the Baltic Sea.
Effects of Genetic Variation on Ecosystem Function
Loss of an ecosystem key-species such as a filter-feeding invertebrate, a dominating macrophyte, or a large fish predator may dramatically affect the Baltic Sea ecosystem. However, less obvious is the effects of a changing genetic set-up following an environmental change. Although adaptation to a contaminated or altered environment may prevent extinction of a population, a secondary consequence of the adaptation may be that the ecosystem function of the population is altered (or even lost). For example, experimental studies of CO2 absorption by ocean phytoplankton showed that under increased levels of CO2, the phytoplankton adapted in the way that their capacity to buffer against high levels of CO2 by increased uptake was lost, compared to unselected strains (Bell and Collins 2008).
Additional effects of lost genetic variation is the sub-lethal effects on ecosystem function that have been documented most comprehensively in cultured plants (Hughes et al. 2008) but is observed also in populations of marine species. For example, decreased genetic variation in populations of barnacle larvae had a negative impact on larval recruitment (Gamfeldt et al. 2005). In seagrass meadows, it has been shown that stands with less genetic variation are less productive, have lower diversity of associated flora and fauna, and are more susceptible toward extreme climate conditions (Reusch et al. 2005). Direct effects on ecosystem services (fisheries harvest) were strongly related to the existence of genetically distinct populations of Sockeye salmon in the Pacific (Schindler et al. 2010), and it seems possible that Baltic Sea salmon fishery and possibly other fisheries like the cod fishery may be benefitting in a similar way owing to genetically distinct local stocks (Nilsson et al. 2001; Sterner 2007).
Evolutionary Rescue or Local Extinction?
In general terms, whether a population will collapse under a changing environment or not is a race between demography and adaptive evolution (Maynard Smith 1989). If environmental variation breaks the limits of the normal range of variation that a population experience, mortality increases and individuals with genotypes that resist the new environment are selectively favored. If there is no genotype tolerant to the new environment, the population will collapse. If very few individuals survive, the population risks collapse owing to stochastic events, allee effects or a combination of these. Under a changing environment input of new genetic variation will be essential, either in the form of new mutations arising in the population or inflow of new genes from other populations. The amount of available genetic variation in a population (standing genetic variation) is critical to the evolution of new adaptations. Also alleles with no or even slightly negative effects on individual fitness may become important under new selective regimes. New mutations may be important for adaptation to a new environment, but long generation time and small population size limit the number of new mutations. Sometimes, however, only very few mutations are needed for an essential adaptation to take place (Larmuseau et al. 2010). Finally, the possibility of combining new genotypes each generation is critical, that is, a species that is predominantly reproducing by cloning will not be able to generate as many new genotypes as a sexually reproducing species.
As intuitively expected, earlier adaptation to similar but less severe conditions supports later adaptation to a more severe stress of the same kind, and successive adaptation is more likely to be successful than a single abrupt change (Samani and Bell 2010). Baltic species that are already exposed to low salinity are thus probably more likely to evolve resistance to decreased salinity compared to species living outside the Baltic Sea. The introduction of new genes and genotypes from hybridization with native species is shown to be important for colonization of new terrestrial plant species (Ellstrand and Schierenback 2000). However, whether addition of new genotypes that may enrich genetic variation and facilitating adaptation in isolated populations is always positive is hard to judge, because local adaptation may be swamped instead of improved by immigrant genotypes (van Doorslaer et al. 2009).
In general, populations will have a higher chance of evolutionary rescue the more genetic variation is available as standing genetic variation, or will become available by new mutations or immigration. Consequently, larger and less isolated populations with short generation time are likely to evolve new adaptations more easily than small, isolated populations with long generation time. In addition, populations that already have high levels of genetic variation in critical traits owing to variable selection pressure during earlier periods of time would be more likely to survive environmental changes than populations lacking genetic variation in key traits. Finally, any population may simply be lucky enough to carry genes that may be critical for survival in a new environment.
What is the Future of the Baltic Sea Ecosystem?
The settings for evolutionary trends and processes that will impact the future are in part framed by our current management principles and measures. Although predicting the future risk of extinction of local populations is virtually impossible without a much more detailed understanding of evolutionary mechanisms and processes involved, we need to use present day data to try inferring likely scenarios. Given existing theoretical and empirical knowledge backgrounds, we here make an attempt to make an educated guess about the future for the Baltic Sea populations.
The physical environment of the Baltic Sea is changing rapidly due to both global processes such as climate change (e.g., Neumann 2010) and to regional processes such as the Baltic drainage area being heavily populated, industrialized and farmed (e.g., Ducrotoy and Elliott 2008). The long water residence time creates a complex mix of contaminants in addition to effects of temperature increase, salinity decrease and increased effects of ocean acidification. The current Baltic populations are evolutionary tailored to the marginal marine habitat, and these populations are critical to continued maintenance of the Baltic ecosystem functions, and thereby ecosystem functions and services. That is, if a Baltic population of a species goes extinct, the empty niche will likely not be filled up again by spontaneous migration of individuals from conspecific populations outside the Baltic Sea. Also restoration attempts transplanting individuals from other populations into the Baltic Sea is likely to fail, owing to the local adaptation needed to survive the Baltic Sea environment. Possibly, an emptied niche will instead be taken over by another local species or invaded by an alien species, deliberately or not, introduced to the Baltic Sea. A parallel and illustrative example is the extinction of genetically distinct populations of cod in fjords along the Swedish west coast that have failed to re-establish despite the fact that large numbers of young cods from the North Sea visit these fjords each summer (Stenseth et al. 2006). Hence, if major populations of key species fail to adapt to ongoing environmental changes, the Baltic Sea ecosystem will undergo a dramatic shift. Alien species, in particularly those with a brackish water origin, will continue to become established in the sea. Lower salinity will favor establishment and spread of more freshwater species than today, and we would also expect an increased evolution of tolerance to low salinities in some of the organisms already present. One such candidate is the blue mussel that has a large potential of evolutionary rescue in its enormous population size (1013) and in having genes from two hybridizing evolutionary lineages already available in the Baltic Sea (M. edulis and M. trossulus, see Riginos and Cunningham 2005). Likewise, the Baltic clam (M. balthica) is a likely candidate as it has a very large population size, a distribution almost over the complete Baltic Sea (Väinölä and Varvio 1989) and a population that is a mix of two hybridizing lineages, resulting in a substantial increase in the standing genetic variation compared to populations outside the Baltic Sea (Nikula et al. 2008). An additional species with large population size is herring (1010–1011) that also has increased genetic variation generated by population differentiation also inside the Baltic Sea (Larsson et al. 2010). Although cod has a relatively large population size, it only spawns in one site in the Baltic (Bleil et al. 2009), and the recruitment is challenged by stochastic inflow of salt-water required for successful spawning (MacKenzie et al. 2007), hence there is an increased risk of local extinction of this species unless fishery management allow for large enough population size and earlier spawning sites can be re-established.
Clonality in macrophyte species such as Fucus vesiculosus, F. radicans, and Z. marina gives these species an immediate advantage to survive conditions benign for sexual reproduction, but at the same time lower their resistant to environmental stress (Reusch et al. 2005) and give them a lower potential for evolutionary change compared to fully sexual species. It seems likely that decreased salinity will further reduce their level of sexual reproduction, and in the long run this may be problematic.
Evolution of new adaptations in the marine mammals of the Baltic Sea will likely be hampered by long generation times. Grey seal and ringed seal are, in addition, isolated from other populations of the same species, while both harbor seal and harbor porpoise have large populations outside the Baltic Sea that may contribute genetic variation into the Baltic populations but at the moment seems not to be in connection with the Baltic populations (Berggren et al. 2004; Härkönen et al. 2005). For these species, small population size is likely to be a main issue for their chances to survive.
From current data we know that many populations and species inhabiting the Baltic Sea have rapidly evolved local adaptation and genetic differentiation from ancestral populations outside the Baltic Sea. Hence, these populations are unlikely to be rescued by migrants from neighboring North Sea populations. This is probably the most serious threat for the current Baltic Sea ecosystem and a main challenge for nature conservation and management.
Acknowledgments
We are very grateful to Michael Gilek and the organizers of the conference “Coping with Uncertainty” and to two anonymous reviewers that indicated weaknesses in an earlier version. This work was in part performed at the Linnaeus Centre for Marine Evolutionary Biology (www.cemeb.science.gu.se) supported by the Swedish Research Councils VR and Formas. The work was in addition funded by the Foundation for Baltic and East European Studies (to MG and KS) and by the EU BONUS programs BaltGene and RISKGOV through EC and Formas funding.
Biographies
Kerstin Johannesson
is Professor at the University of Gothenburg and the Department of Marine Ecology—Tjärnö. Her research focuses on conservation genetics and evolutionary ecology of marine invertebrates and algae and she coordinates the Linnaeus Centre of Marine Evolutionary Research (www.cemeb.science.gu.se), and the BONUS research programme BaltGene.
Katarzyna Smolarz
is a PhD in marine biology and toxicology at the Institute of Oceanography and Geography at Gdansk University (Gdansk, Poland). Presently she is a postdoc at the Centre for the Baltic and East European Studies at Södertörns University. Her focus is on environmental risk assessment in the Baltic Sea, in particular impact of deposited contaminants on marine invertebrates.
Mats Grahn
is a Senior Lecturer in molecular ecology at Södertörn University and does research on genetic variation and adaptation in relation to environmental variation.
Carl André
is Professor at University of Gothenburg and the Department of Marine Ecology—Tjärnö (www.marecol.gu.se). He has an interest in understanding the processes that drive genetic structure in marine organisms.
Contributor Information
Kerstin Johannesson, Email: Kerstin.Johannesson@marecol.gu.se.
Katarzyna Smolarz, Email: katarzyna.smolarz@sh.se.
Mats Grahn, Email: mats.grahn@sh.se.
Carl André, Email: Carl.Andre@marecol.gu.se.
References
- Andersen Ø, Wetten OF, Rosa MC, Andre C, Alinovi CC, Colafranceschi M, Brix O, Colosimo A. Haemoglobin polymorphisms affect the oxygen-binding properties in Atlantic cod populations. Proceedings of the Royal Society of London B. 2009;276:833–841. doi: 10.1098/rspb.2008.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André, C., L.C. Larsson, L. Laikre, D. Bekkevold, J. Brigham, G.R. Carvalho, T.G. Dahlgren, and W.F. Hutchinson et al. 2010. Detecting population structure in a high gene-flow species, Atlantic herring (Clupea harengus): Direct, simultaneous evaluation of neutral versus putatively selected loci. Heredity doi:10.1038/hdy.2010.71. [DOI] [PMC free article] [PubMed]
- Barrett RDH, Schluter D. Adaptation from standing genetic variation. Trends in Ecology & Evolution. 2008;23:38–44. doi: 10.1016/j.tree.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Bell G, Collins S. Adaptation, extinction and global change. Evolutionary Applications. 2008;1:3–16. doi: 10.1111/j.1752-4571.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Gonzalez A. Evolutionary rescue can prevent extinction following environmental change. Ecology Letters. 2009;12:942–948. doi: 10.1111/j.1461-0248.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- Bengtsson BO. Genetic variation in organisms with sexual and asexual reproduction. Journal of Evolutionary Biology. 2003;16:189–199. doi: 10.1046/j.1420-9101.2003.00523.x. [DOI] [PubMed] [Google Scholar]
- Berggren, P., L. Hiby, P. Lovell, and M. Scheidat. 2004. Abundance of harbour porpoises in the Baltic Sea from aerial surveys conducted in summer 2002. Paper SC/56/SM7. International Whaling Commission, Cambridge, UK.
- Bergström L, Tatarenkov A, Johannesson K, Jönsson RB, Kautsky L. Genetic and morphological identification of Fucus radicans sp. nov. (Fucales, Phaeophyceae) in the brackish Baltic Sea. Journal of Phycology. 2005;41:1025–1038. doi: 10.1111/j.1529-8817.2005.00125.x. [DOI] [Google Scholar]
- Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R. Effects of chemical contaminants on genetic diversity in natural populations: Implications for biomonitoring and ecotoxicology. Mutational Research. 2000;463:33–51. doi: 10.1016/S1383-5742(00)00004-1. [DOI] [PubMed] [Google Scholar]
- Bleil M, Oeberst R, Urrutia P. Seasonal maturity development of Baltic cod in different spawning areas: Importance of the Arkona Sea for the summer spawning stock. Journal of Applied Ichthyology. 2009;25:10–17. doi: 10.1111/j.1439-0426.2008.01172.x. [DOI] [Google Scholar]
- Chakraborty R, Nei M. Bottleneck effects on average heterozygosity and genetic distance with stepwise mutation model. Evolution. 1977;31:347–356. doi: 10.2307/2407757. [DOI] [PubMed] [Google Scholar]
- Colosimo PF, Hosemann KE, Balabhadra S, Villarreal G, Jr, Dickson M, Grimwood J, Schmutz J, Myers RM, et al. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science. 2005;37:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- Conley DJ, Björck S, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hietanen S, Kortekaas M, et al. Hypoxia related processes in the Baltic Sea. Environmental Science and Technology. 2009;43:3412–3420. doi: 10.1021/es802762a. [DOI] [PubMed] [Google Scholar]
- Ducrotoy J-P, Elliott M. The science and management of the North Sea and the Baltic Sea: Natural history, present threats and future challenges. Marine Pollution Bulletin. 2008;57:8–21. doi: 10.1016/j.marpolbul.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Edmands S. Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution. 1999;53:1757–1768. doi: 10.2307/2640438. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC, Schierenback K. Hybridization as a stimulus for the evolution of invasiveness in plants? Proceedings of the National Academy of Sciences USA. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmgren R, Hill C. Ecosystem function at low biodiversity—the Baltic example. In: Ormond RFG, Gage JD, Angel MV, editors. Marine biodiversity patterns and processes. Cambridge: Cambridge University Press; 1997. pp. 319–336. [Google Scholar]
- Eriksson KM, Clarke AK, Franzen L-G, Kuylenstierna M, Martinez K, Blanck H. Community-level analysis of psbA gene sequences and irgarol tolerance in marine periphyton. Applied and Environmental Microbiology. 2009;75:897–906. doi: 10.1128/AEM.01830-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin AB, Höglund J. Population structure of flounder (Platichthys flesus) in the Baltic Sea: Differences among demersal and pelagic spawners. Heredity. 2008;101:27–38. doi: 10.1038/hdy.2008.22. [DOI] [PubMed] [Google Scholar]
- Gaggiotti OE, Bekkevold D, Jørgensen HBH, Foll M, Carvalho GR, André C, Ruzzante DE. Disentangling the effects of evolutionary, demographic, and environmental factors influencing genetic structure of natural populations: Atlantic herring as a case study. Evolution. 2009;63:2939–2951. doi: 10.1111/j.1558-5646.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- Gamfeldt L, Wallén J, Jonsson PR, Berntsson KK, Havenhand J. Intraspecific diversity enhances settling success in a marine invertebrate. Ecology. 2005;86:3219–3224. doi: 10.1890/05-0377. [DOI] [Google Scholar]
- Gardeström J, Dahl U, Kotsalainen O, Maxson A, Elfwing T, Grahn M, Bengtsson BE, Breitholtz M. Evidence of population genetic effects of long-term exposure to contaminated sediments—a multi-endpoint study with copepods. Aquatic Toxicology. 2008;86:426–436. doi: 10.1016/j.aquatox.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Härkönen T, Harding KJ, Goodman SJ, Johannesson K. Colonization history of the Baltic harbor seals: integrating archaeological, behavioral and genetic data. Marine Mammal Science. 2005;21:695–716. doi: 10.1111/j.1748-7692.2005.tb01260.x. [DOI] [Google Scholar]
- Hellberg ME. Gene flow and isolation among populations of marine animals. Annual Review of Ecology, Evolution, and Systematics. 2009;40:291–310. doi: 10.1146/annurev.ecolsys.110308.120223. [DOI] [Google Scholar]
- Hemmer-Hansen J, Nielsen EE, Frydenberg J, Löeschcke V. Adaptive divergence in a high gene flow environment: Hsc70 variation in the European flounder (Platicthys flesus L.) Heredity. 2007;99:592–600. doi: 10.1038/sj.hdy.6801055. [DOI] [PubMed] [Google Scholar]
- Hughes AR, Inouye BD, Johnson MTJ, Underwood N, Vellend M. Ecological consequences of genetic diversity. Ecology Letters. 2008;11:609–623. doi: 10.1111/j.1461-0248.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- Jansson BO, Dahlberg KK. The environmental status of the Baltic Sea in the 1940s, today and in the future. AMBIO. 1999;28:312–319. [Google Scholar]
- Johannesson K, André C. Life on the margin: genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Molecular Ecology. 2006;15:2013–2029. doi: 10.1111/j.1365-294X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- Kautsky N, Evans S. Role of biodeposition by Mytilus edulis in the circulation of matter and nutrients in a Baltic coastal ecosystem. Marine Ecology Progress Series. 1987;38:201–212. doi: 10.3354/meps038201. [DOI] [Google Scholar]
- Lago-Leston A, Mota C, Kautsky L, Pearson GA. Functional divergence in heat shock response following rapid speciation of Fucus spp. in the Baltic Sea. Marine Biology. 2010;157:683–688. doi: 10.1007/s00227-009-1348-1. [DOI] [Google Scholar]
- Larmuseau MHD, Vancampenhout K, Raeymaekers JAM, Houdt JKJ, Volchaert FAM. Differential modes of selection on the rhodopsin gene in coastal Baltic and North Sea populations of the sand goby, Pomatoschistus minutus. Molecular Ecology. 2010;19:2256–2268. doi: 10.1111/j.1365-294X.2010.04643.x. [DOI] [PubMed] [Google Scholar]
- Larsen PF, Nielsen EE, Williams TD, Loeschcke V. Intraspecific variation in expression of candidate genes for osmoregulation, heme biosynthesis and stress resistance suggests local adaptation in European flounder (Platichthys flesus) Heredity. 2008;101:247–259. doi: 10.1038/hdy.2008.54. [DOI] [PubMed] [Google Scholar]
- Larsson LC, Laikre L, Palm S, André C, Carvalho GR, Ryman N. Concordance of allozyme and microsatellite differentiation in a marine fish, but evidence of selection at a microsatellite locus. Molecular Ecology. 2007;16:1135–1147. doi: 10.1111/j.1365-294X.2006.03217.x. [DOI] [PubMed] [Google Scholar]
- Larsson LC, Laikre L, André C, Dahlgren TG, Ryman N. Temporally stable genetic structure of heavily exploited Atlantic herring (Clupea harengus) in Swedish waters. Heredity. 2010;104:40–51. doi: 10.1038/hdy.2009.98. [DOI] [PubMed] [Google Scholar]
- Leppäkoski E, Olenin S. Non-native species and rates of spread: lessons from the brackish Baltic Sea. Biological Invasions. 2000;2:152–163. doi: 10.1023/A:1010052809567. [DOI] [Google Scholar]
- Limborg MT, Pedersen JS, Hemmer-Hansen J, Tomkiewicz J, Bekkevold D. Genetic population structure of European sprat Sprattus sprattus: Differentiation across a steep environmental gradient in a small pelagic fish. Marine Ecology Progress Series. 2009;379:213–224. doi: 10.3354/meps07889. [DOI] [Google Scholar]
- Ma XL, Cowles DL, Carter RL. Effects of pollution on genetic diversity in the bay mussel Mytilus galloprovincialis and the acorn barnacle Balanus glandula. Marine Environmental Research. 2000;50:559–563. doi: 10.1016/S0141-1136(00)00109-4. [DOI] [PubMed] [Google Scholar]
- MacKenzie BR, Gislason H, Mollmann C, Koster FW. Impact of 21st century climate change on the Baltic Sea fish community and fisheries. Global Change Biology. 2007;13:1348–1367. doi: 10.1111/j.1365-2486.2007.01369.x. [DOI] [Google Scholar]
- Meier HEM. Baltic Sea climate in the late twenty-first century: a dynamical downscaling approach using two global models and two emission scenarios. Climate Dynamics. 2006;27:39–68. doi: 10.1007/s00382-006-0124-x. [DOI] [Google Scholar]
- Millenium Ecosystem Assessment. 2005. Ecosystems and Human Well-being: Biodiversity Synthesis. World Resources Institute, Washington, DC.
- Nei M, Maruyama T, Chakraborty R. Bottleneck effect and genetic variability in populations. Evolution. 1975;29:1–10. doi: 10.2307/2407137. [DOI] [PubMed] [Google Scholar]
- Neumann T. Climate-change effects on the Baltic Sea ecosystem: A model study. Journal of Marine Systems. 2010;81:213–224. doi: 10.1016/j.jmarsys.2009.12.001. [DOI] [Google Scholar]
- Nikula R, Strelkov P, Väinölä R. A broad transition zone between an inner Baltic hybrid swarm and a pure North Sea subspecies of Macoma balthica (Mollusca, Bivalvia) Molecular Ecology. 2008;17:1505–1522. doi: 10.1111/j.1365-294X.2007.03688.x. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Gross R, Asplund T, Dove O, Jansson H, Kelloniemi J, Kohlmann L, Löytynoja A, et al. Matrilinear phylogeography of Atlantic salmon (Salmo salar L.) in Europe and postglacial colonization of the Baltic Sea area. Molecular Ecology. 2001;10:89–102. doi: 10.1046/j.1365-294X.2001.01168.x. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Lepori F, Malmqvist B, Tornlund E, Hjerdt N, Helfield JM, Palm D, Östergren J, et al. Forecasting environmental responses to restoration of rivers used as log floatways: An interdisciplinary challenge. Ecosystems. 2005;8:779–800. doi: 10.1007/s10021-005-0030-9. [DOI] [Google Scholar]
- Nissling A, Westin L. Salinity requirements for successful spawning of Baltic and Belt Sea cod and the potential for cod stock interactions in the Baltic Sea. Marine Ecology Progress Series. 1997;152:261–271. doi: 10.3354/meps152261. [DOI] [Google Scholar]
- Pearson G, Kautsky L, Serrao E. Recent evolution in Baltic Fucus vesiculosus: Reduced tolerance to emersion stresses compared to intertidal (North Sea) populations. Marine Ecology Progress Series. 2000;202:67–79. doi: 10.3354/meps202067. [DOI] [Google Scholar]
- Peck JR, Yearsley JM, Waxman D. Explaining the geographic distributions of sexual and asexual populations. Nature. 1998;391:889–892. doi: 10.1038/36099. [DOI] [Google Scholar]
- Pereyra R, Bergström L, Kautsky L, Johannesson K. Rapid speciation in a newly opened post-glacial marine environment, the Baltic Sea. BMC Evolutionary Biology. 2009;9:70. doi: 10.1186/1471-2148-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch TBH, Stam WT, Olsen JL. A microsatellite-based estimation of clonal diversity and population subdivision in Zostera marina, a marine flowering plant. Molecular Ecology. 2000;9:127–140. doi: 10.1046/j.1365-294x.2000.00839.x. [DOI] [PubMed] [Google Scholar]
- Reusch TBH, Ehlers A, Hämmerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences USA. 2005;102:2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riginos C, Cunningham CW. Local adaptation and species segregation in two mussel (Mytilus edulis × Mytilus trossulus) hybrid zones. Molecular Ecology. 2005;14:381–400. doi: 10.1111/j.1365-294X.2004.02379.x. [DOI] [PubMed] [Google Scholar]
- Ruzzante D, Mariani S, Bekkevold D, André C, Mosegaard H, Clausen L, Dahlgren T, Hutchinson W, et al. Biocomplexity in a highly migratory marine pelagic fish. Proceedings of the Royal Society London Series B. 2006;273:1459–1464. doi: 10.1098/rspb.2005.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani P, Bell G. Adaptation of experimental yeast populations to stressful conditions in relation to population size. Journal of Evolutionary Biology. 2010;23:791–796. doi: 10.1111/j.1420-9101.2010.01945.x. [DOI] [PubMed] [Google Scholar]
- Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS. Population diversity and the portfolio effect in an exploited species. Nature. 2010;465:609–613. doi: 10.1038/nature09060. [DOI] [PubMed] [Google Scholar]
- Silvertown J. The evolutionary maintenance of sexual reproduction: Evidence from the ecological distribution of asexual reproduction in clonal plants. International Journal of Plant Sciences. 2008;169:157–168. doi: 10.1086/523357. [DOI] [Google Scholar]
- Smith JM. The causes of extinction. Philosophical Transactions of the Royal Society B: Biological Sciences. 1989;325:241–252. doi: 10.1098/rstb.1989.0086. [DOI] [PubMed] [Google Scholar]
- Stenseth NC, Jorde PE, Chan KS, Hansen E, Knutsen H, André C, Skogen MD, Lekve K. Ecological and genetic impact of Atlantic cod larval drift in the Skagerrak. Proceedings of the Royal Society London B. 2006;273:1085–1092. doi: 10.1098/rspb.2005.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner T. Unobserved diversity, depletion and irreversibility: The importance of subpopulations for management of cod stocks. Ecological Economics. 2007;61:566–574. doi: 10.1016/j.ecolecon.2006.05.015. [DOI] [Google Scholar]
- Stuckas H, Stoof K, Quesada H, Tiedemann R. Evolutionary implications of discordant clines across the Baltic Mytilus hybrid zone (Mytilus edulis and Mytilus trossulus) Heredity. 2009;103:146–156. doi: 10.1038/hdy.2009.37. [DOI] [PubMed] [Google Scholar]
- Tatarenkov A, Bergström L, Jönsson RB, Serrao EA, Kautsky L, Johannesson K. Intriguing asexual life in marginal populations of the brown seaweed Fucus vesiculosus. Molecular Ecology. 2005;14:647–651. doi: 10.1111/j.1365-294X.2005.02425.x. [DOI] [PubMed] [Google Scholar]
- Väinölä R, Varvio SL. Biosystematics of Macoma balthica in north-western Europe. In: Ryland JS, Tyler A, editors. Reproduction, genetics and distribution of marine organisms. Fredensborg: Olsen & Olsen; 1989. pp. 309–316. [Google Scholar]
- Doorslaer W, Vanoverbeke J, Duvivier C, Rousseaux S, Jansen M, Jans B, Feuchtmayr H, Atkinson D, et al. Low adaptation to higher temperatures reduces immigration success of genotypes from a warmer region in the water flea Daphnia. Global Change Biology. 2009;15:3046–3055. doi: 10.1111/j.1365-2486.2009.01980.x. [DOI] [Google Scholar]
- Was A, Gosling E, Hoarau G. Microsatellite analysis of plaice (Pleuronectes platessa L.) in the NE Atlantic: Weak genetic structuring in a milieu of high gene flow. Marine Biology. 2010;157:447–462. doi: 10.1007/s00227-009-1331-x. [DOI] [Google Scholar]
- Wiemann A, Andersen LW, Berggren P, Siebert U, Benke H, Teilmann J, Lockyer C, Pawliczka I, et al. Mitochondrial control region and microsatellite analyses on harbour porpoise (Phocoena phocoena) unravel population differentiation in the Baltic Sea and adjacent waters. Conservation Genetics. 2010;11:195–211. doi: 10.1007/s10592-009-0023-x. [DOI] [Google Scholar]
- Wikström SA, Kautsky L. Structure and diversity of invertebrate communities in the presence and absence of canopy forming Fucus vesiculosus in the Baltic Sea. Estuarine, Coastal and Shelf Science. 2007;72:168–176. doi: 10.1016/j.ecss.2006.10.009. [DOI] [Google Scholar]
- Williams SL, Heck KL, et al. Seagrass community ecology. In: Bertness MD, et al., editors. Marine community ecology. Sunderland: Sinauer; 2001. pp. 317–338. [Google Scholar]
- Wirgin I, Waldman JR. Resistance to contaminants in North American fish populations. Mutation Research. 2004;552:73–100. doi: 10.1016/j.mrfmmm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Zillén L, Conley DJ, Andrén T, Andrén E, Björck S. Past occurrence of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth-Science Reviews. 2008;91:77–92. doi: 10.1016/j.earscirev.2008.10.001. [DOI] [Google Scholar]