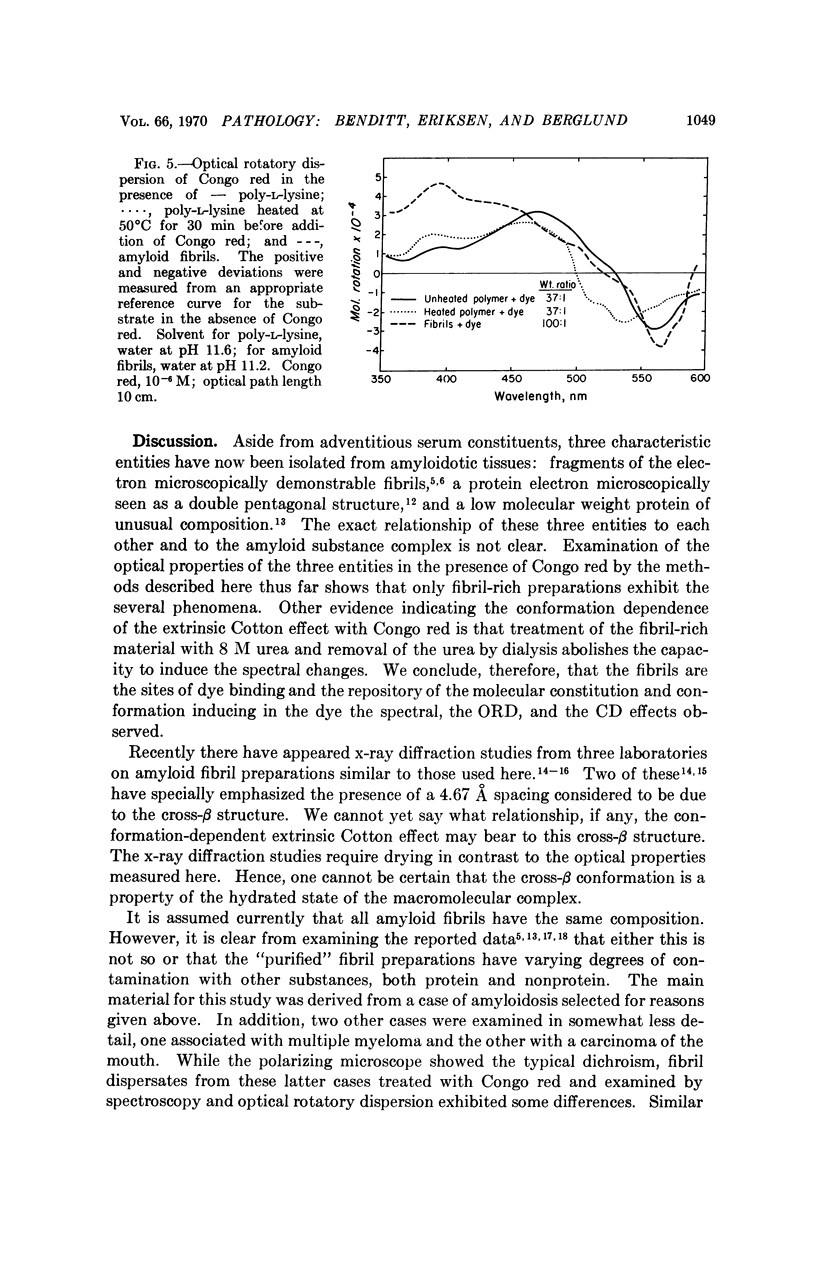
Abstract
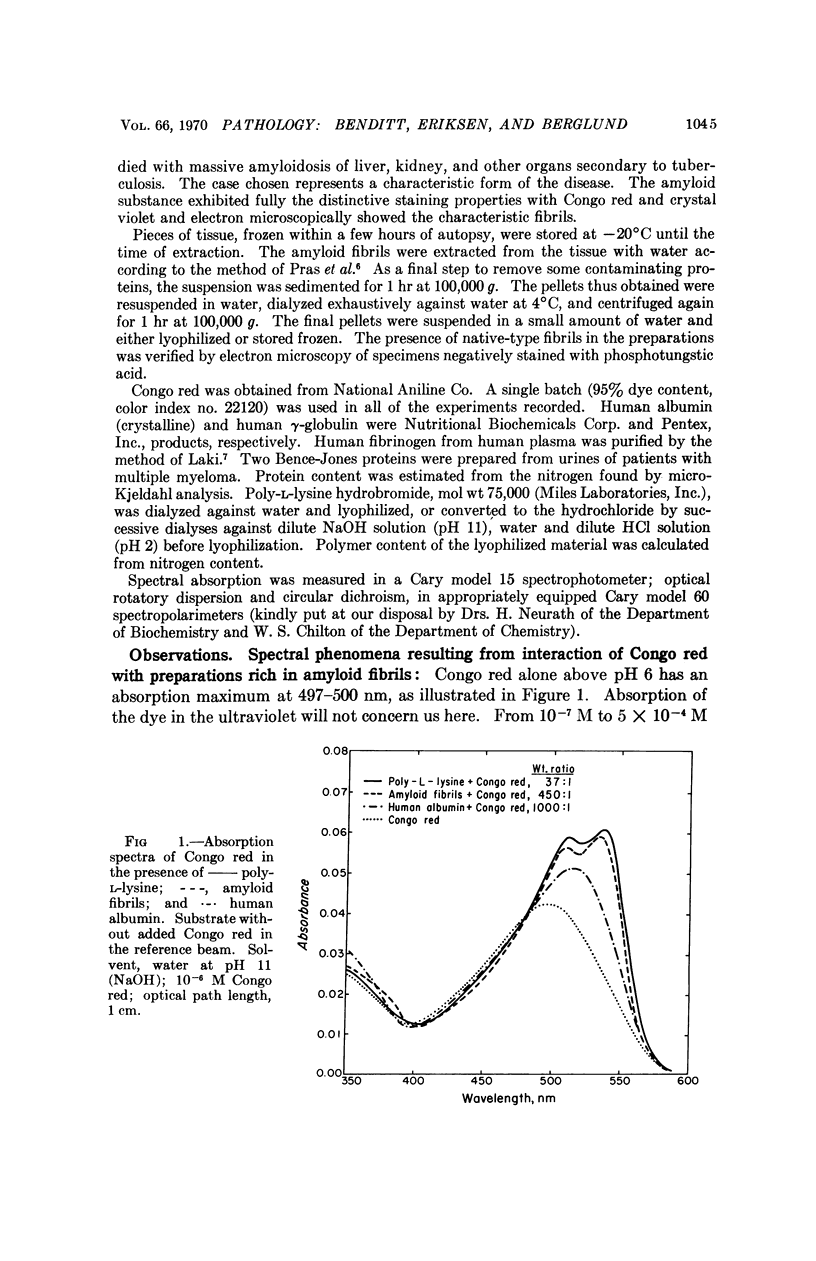
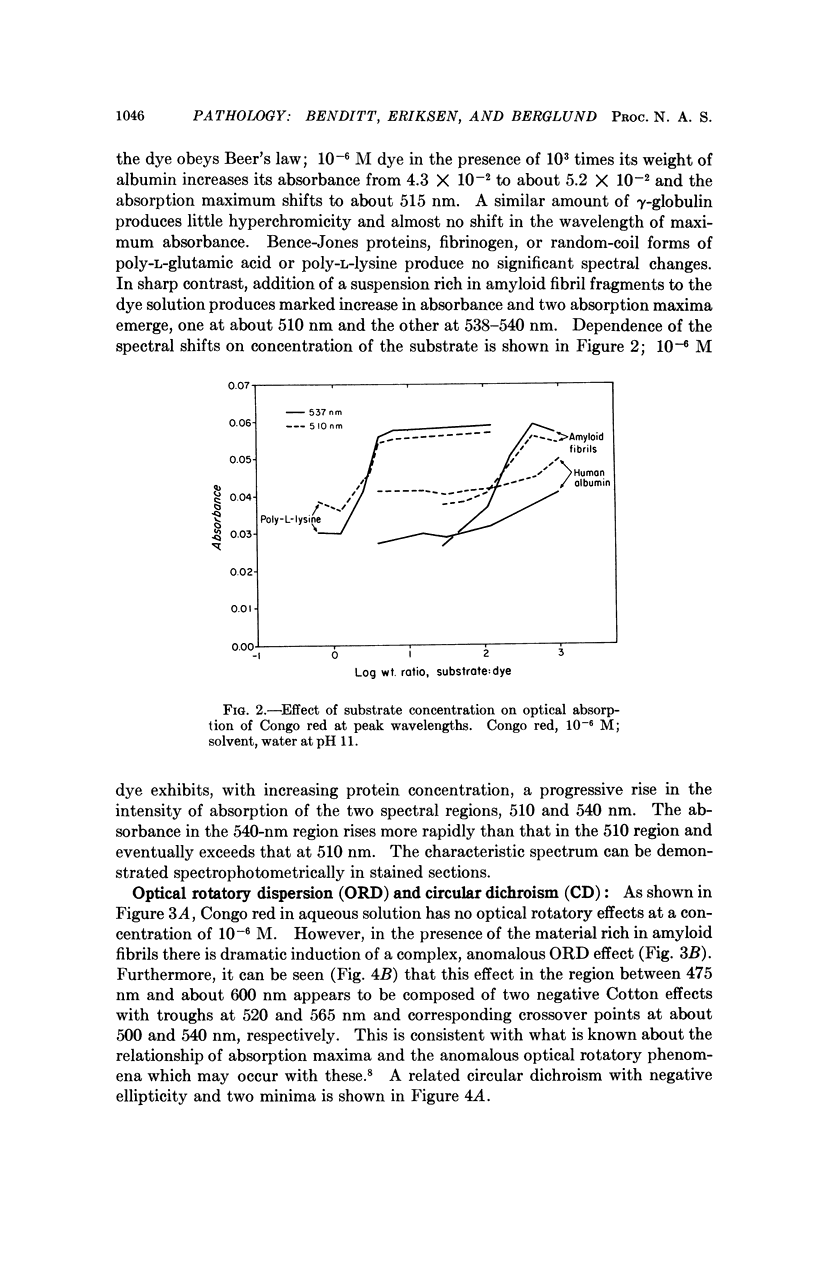
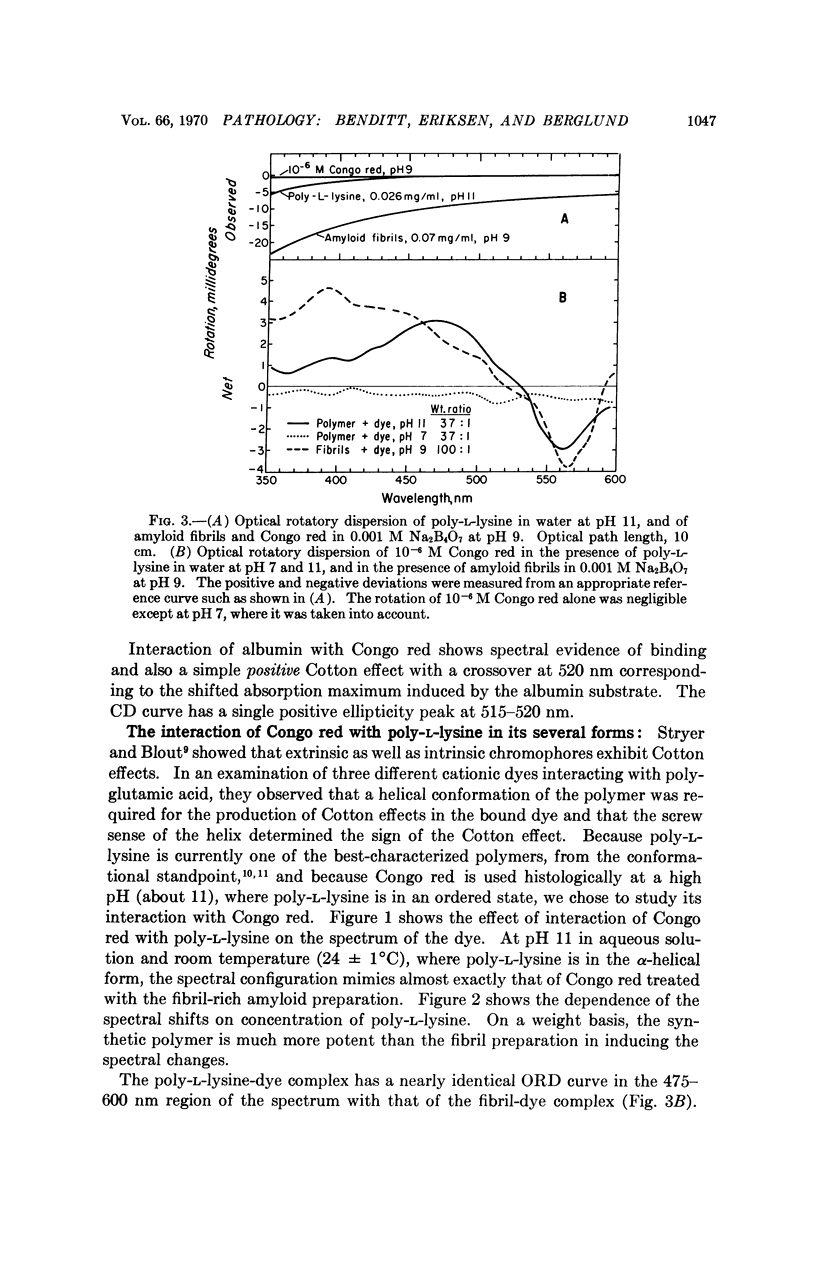
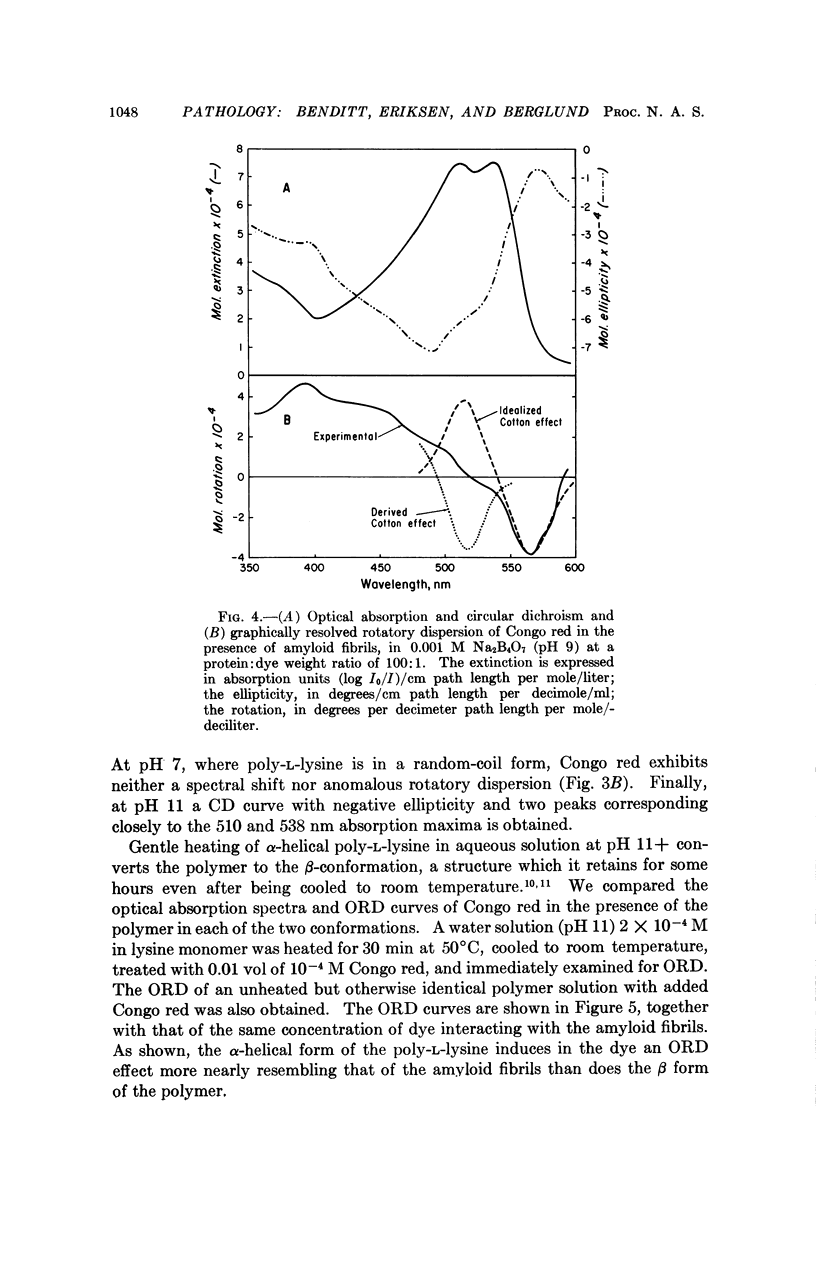
The spectral absorption, optical rotatory dispresion, and circular dichroism associated with interaction of Congo red dye with partly purified suspensions of amyloid fibril fragments were examined. A set of phenomena consistent with a Cotton effect was found. A nearly identical set of phenomena was obtained with poly-L-lysine in its α-helical form. The dichroism seen when Congo red binds to amyloid substance in tissue sections can also be interpreted as a Cotton effect. This suggests that some special conformation, presumably in protein, is present in a major constituent of amyloid. This conformation is not present in gamma globulin, Bence-Jones protein, albumin, fibrinogen, or other proteins tested so far. These and other optical properties of amyloid substance can be used to compare amyloid deposits in different human cases and in different species. Extension of the use of polarization microscopy with other dyes that bind to other substances in tissue sections should permit more exquisite probing of the conformation of important macromolecules in situ in cells and tissues than has hitherto been possible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth W. F., Glenner G. G., Waldmann T. A., Zelis R. F. Primary amyloidosis. Ann Intern Med. 1968 Oct;69(4):787–805. doi: 10.7326/0003-4819-69-4-787. [DOI] [PubMed] [Google Scholar]
- Bonar L., Cohen A. S., Skinner M. M. Characterization of the amyloid fibril as a cross-beta protein. Proc Soc Exp Biol Med. 1969 Sep;131(4):1373–1375. doi: 10.3181/00379727-131-34110. [DOI] [PubMed] [Google Scholar]
- Davidson B., Fasman G. D. The conformational transitions of uncharged poly-L-lysine. Alpha helix-random coil-beta structure. Biochemistry. 1967 Jun;6(6):1616–1629. doi: 10.1021/bi00858a008. [DOI] [PubMed] [Google Scholar]
- Eanes E. D., Glenner G. G. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968 Nov;16(11):673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- LAKI K. The polymerization of proteins; the action of thrombin on fibrinogen. Arch Biochem Biophys. 1951 Jul;32(2):317–324. doi: 10.1016/0003-9861(51)90277-9. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M. Metachromatic properties of amyloid in solution. J Histochem Cytochem. 1969 Apr;17(4):258–265. doi: 10.1177/17.4.258. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P. K., Doty P. The optical rotatory properties of the beta-configuration in polypeptides and proteins. Proc Natl Acad Sci U S A. 1966 Apr;55(4):981–989. doi: 10.1073/pnas.55.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli U., Gafni J., Sohar E., Ashkenazi Y. An x-ray study of amyloid. J Mol Biol. 1969 Apr;41(2):309–311. doi: 10.1016/0022-2836(69)90397-0. [DOI] [PubMed] [Google Scholar]



