Abstract
A re-survey of acid-sensitive lakes in Ireland (initial survey 1997) was carried out during spring 2007 (n = 60). Since 1997, atmospheric emissions of sulfur dioxide and deposition of non-marine sulfate (SO42−) in Ireland have decreased by ~63 and 36%, respectively. Comparison of water chemistry between surveys showed significant decreases in the concentration of SO42−, non-marine SO42−, and non-marine base cations. In concert, alkalinity increased significantly; however, no change was observed in surface water pH and total aluminum. High inter-annual variability in sea salt inputs and increasing (albeit non-significant) dissolved organic carbon may have influenced the response of pH and total aluminum (as ~70% is organic aluminum). Despite their location on the western periphery of Europe, and dominant influence from Atlantic air masses, the repeat survey suggests that the chemistry of small Irish lakes has shown a significant response to reductions in air pollution driven primarily by the implementation of the Gothenburg Protocol under the UNECE Convention on Long-Range Transboundary Air Pollution.
Keywords: Lake chemistry, Sulfate, Emissions, Sea salts, Dissolved organic carbon
Introduction
The impact of acid rain on surface waters has been extensively studied in Europe and North America (Almer et al. 1978; Kähkonen 1996; Skjelkvåle et al. 2005). Since the 1980s, international policies have been implemented to reduce atmospheric emissions of anthropogenic sulfur (S) and nitrogen (N) compounds with the objective to promote chemical and biological recovery of impacted aquatic ecosystems (Bull et al. 2008). In 1999, the effects-based Gothenburg Protocol on long-range transboundary air pollution was signed with the intent to reduce emissions of S and N oxides, and ammonia in Europe (63, 41, and 17%, respectively) by 2010 (UNECE 1999). These emission reductions have stimulated widespread significant changes in the chemistry of acid-sensitive lakes (Stoddard et al. 1999; Skjelkvåle et al. 2005; Kopáček et al. 2004).
Repeat lake surveys in Europe and North America have generally observed a decrease in lake sulfate (SO42−) concentrations consistent with decreases in precipitation concentrations (Skjelkvåle et al. 1998; Pilgrim et al. 2003; Stuchlik et al. 2006). Re-surveys in acid-sensitive regions such as the Adirondack and Catskill Mountains of New York, Tatra Mountains of Slovakia and Poland, and across the Nordic countries, have generally observed chemical recovery from acidification with decreases in SO42−, nitrate (NO3−) and base cations, in conjunction with increases in pH and alkalinity (Skjelkvåle et al. 1998; Kopáček et al. 2006).
Ireland is located on the western periphery of Europe, and predominantly receives clean air masses from the Atlantic. As such, non-marine (nm) SO42− deposition is low (<25 mmolc m−2 year−1 [one mole of charge (molc) is numerically equal to one equivalent (eq)]) compared to other industrial regions of Europe (Aherne and Farrell 2002). Nonetheless, Ireland receives acidifying compounds associated with easterly air masses from Western Europe (Bowman and McGettigan 1994) and, to a lesser extent, pollutants carried in western air masses from North America (Huntrieser et al. 2005). The impact of acidic deposition has been a potential concern since the 1980s (Bailey et al. 1986) owing to the preponderance of surface waters in acid-sensitive regions (Bowman 1991; Flower et al. 1994). During 1997, a survey of predominantly small headwater lakes (n = 200) in remote, acid-sensitive, coastal regions found that nmSO42− (and NO3−) was the dominant source of acidity in 25% of the lakes (Aherne et al. 2002). During the last two decades, decreases in air concentrations of sulfur dioxide (SO2) and nmSO42− in precipitation have been observed in Ireland (Valentia: Bashir et al. 2008).
The objective of this study was to determine differences in the chemistry of small headwater lakes in Ireland between 1997 and 2007, a period which has experienced significant reductions in the deposition of S across Europe owing to the Gothenburg Protocol. To meet the objective, 60 lakes sampled during 1997 (Aherne et al. 2002) were re-surveyed during 2007; chemical observations common to both surveys were compared using the Wilcoxon rank paired test. Trends in atmospheric deposition and meteorology during the study period (1995–2007) were evaluated using the Mann–Kendall test. Emissions of SO2 have decreased by ~55% in European Union member states between 1995 and 2007 (European Environment Agency 2009). As such, it was anticipated that increases in pH and alkalinity would be observed along with decreases in SO42−, base cations and aluminum (Al) concentrations, similar to other regions in Europe and North America (Stoddard et al. 1999; Skjelkvåle et al. 2001).
Materials and Methods
Study Sites
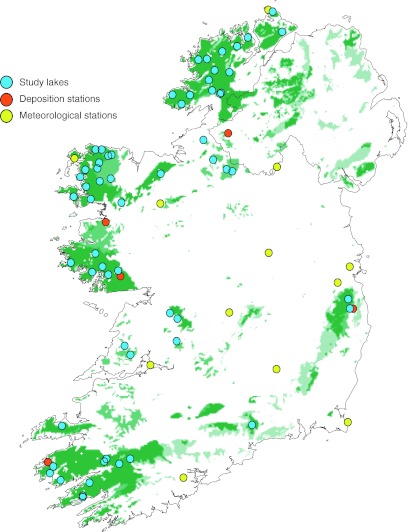
During spring 1997, ~200 small lakes (mean lake area 5.8 ha, 222 m a.s.l.) were sampled in Ireland to determine their hydrochemical characteristics and acid status (Aherne et al. 2002). Lakes were pseudo-randomly selected based on bedrock geology and soil characteristics, with greater weighting towards more remote, higher elevation, acid-sensitive regions. During spring 2007, 60 lakes (mean lake area 4.4 ha, 292 m a.s.l) from the 1997 survey were re-sampled (Fig. 1). The majority of the lakes were located along the coastal margins in relatively undisturbed upland areas; nonetheless, there was a wide range in site characteristics such as lake area, catchment area and elevation (Table 1). The dominant soil type was podzol or peaty podzol, land cover was dominated by moorland and the dominant land use was rough grazing.
Fig. 1.
Location of study lakes (n = 60), deposition stations (n = 5), and meteorological stations (n = 13). Also shown is the sensitivity of surface waters to acidification [based on geology and soil, darker shading indicates increasing sensitivity (Aherne et al. 2002)]
Table 1.
Statistical summaries (mean, standard deviation [SD], 5th percentile, median, 95th percentile) of catchment characteristics and lake chemistry (2007) for the study catchments (n = 60; see Fig. 1)
| Variable | Units | Mean | SD | 5%-tile | Median | 95%-tile |
|---|---|---|---|---|---|---|
| Lake size | ha | 4.4 | 8.4 | 0.5 | 1.6 | 13.3 |
| Catchment size | ha | 63.7 | 262.5 | 2.1 | 11.0 | 153.5 |
| Elevation | m | 291.6 | 169.1 | 28.8 | 278.0 | 561.2 |
| pH | 5.1 | 0.6 | 4.3 | 5.1 | 6.2 | |
| Conductivity | μS cm−1 at 25°C | 99.5 | 42.6 | 41.0 | 90.4 | 180.6 |
| Alkalinity (Gran) | μmolc l−1 CaCO3 | −5.6 | 32.3 | −51.7 | −3.6 | 45.9 |
| Ca2+ | μmolc l−1 | 59.5 | 31.5 | 23.1 | 55.1 | 105.4 |
| Mg2+ | μmolc l−1 | 145.5 | 70.4 | 57.0 | 127.3 | 289.1 |
| K+ | μmolc l−1 | 14.2 | 8.1 | 5.2 | 12.2 | 31.6 |
| Na+ | μmolc l−1 | 621.6 | 325.7 | 223.8 | 546.5 | 1323.5 |
| SO42− | μmolc l−1 | 74.1 | 29.0 | 35.9 | 69.3 | 129.9 |
| Cl− | μmolc l−1 | 644.5 | 303.0 | 220.3 | 600.7 | 1227.0 |
| NO3− | μmolc l−1 | 4.6 | 4.0 | 0.7 | 2.8 | 12.5 |
| DOC | mg l−1 | 5.7 | 2.8 | 2.2 | 4.9 | 11.1 |
| AlT | μmol l−1 | 2.5 | 1.9 | 0.5 | 2.1 | 6.9 |
| ANC | μmolc l−1 | 120.0 | 108.1 | 7.4 | 85.2 | 352.5 |
| nmCa2+ | μmolc l−1 | 35.7 | 27.7 | 8.2 | 32.0 | 72.7 |
| nmMg2+ | μmolc l−1 | 17.9 | 17.4 | −0.4 | 13.7 | 59.8 |
| nmK+ | μmolc l−1 | 2.6 | 5.8 | −6.3 | 2.4 | 10.7 |
| nmSO42− | μmolc l−1 | 7.8 | 14.7 | −13.9 | 9.7 | 25.6 |
Nitrate (NO3−) was available for only ~50% of the study lakes, DOC dissolved organic carbon estimated from absorbance at 320 nm (DOC = 49.5 × ABS320 + 1.69), AlT total (labile and non-labile) aluminum, ANC charge balance acid neutralizing capacity, nm non-marine (negative concentrations of non-marine base cations and SO42− may result from short-term variations in deposition). Units: 1 mol of charge (molc) is equal to 1 equivalent (eq)
Sampling and Analysis
Similar field and laboratory procedures were used during both lake surveys where possible. Shore water samples were collected 10–20 cm below the surface in HDPE bottles and kept cool (~4°C) until analysis. Lake samples were analyzed for pH, conductivity, Gran alkalinity, major ions (Ca2+, Mg2+, K+, Na+, Cl− and SO42−) and total aluminum (AlT). Lake pH and conductivity were analyzed using a low conductivity electrode. Gran alkalinity was measured using a PC titration Plus System. Anions (Cl−, NO3−, and SO42−) and cations (Ca2+, Mg2+, K+, and Na+) were analyzed using a Dionex 600 Ion Chromatograph (IC). Dissolved (0.45 μm) organic carbon (DOC) was estimated from absorbance at 320 nm (DOC = 49.5 × ABS320 + 1.69) using a UV–VIS Spectrometer following Gorham (1985), consistent with the 1997 survey (Aherne et al. 2002). Aluminum concentrations were analyzed using an Element2 High Resolution ICP-MS.
Standard QA/QC procedures were followed during sample collection, laboratory analysis and data analysis to ensure accurate, consistent and reliable data. Duplicate water samples (~20%) were sent to an external laboratory (Environment Canada) for independent analysis. Charge balance and conductivity checks were carried out following EMEP (1996) data checking protocols; notably none of the lakes had an invalid ion balance.
Non-marine concentrations of SO42− and base cations were estimated as the difference between total and marine concentrations based on the assumption that all Cl− in lake water originated from sea-spray (i.e., (molc) ratio for SO42−:Cl− is 0.103:1). Negative concentrations of non-marine SO42− and base cations may result from short-term variations in deposition, such as high inputs of sea salts, or variation in deposition ratios with distance from the ocean (Möller 1990), leading to incongruent relations between ions (Aherne and Curtis 2003). Charge balance acid neutralizing capacity (ANC) was calculated as the difference between base cations and acid anions (Reuss and Johnson 1986).
Statistical differences in lake chemistry between the 1997 and 2007 surveys were evaluated using the Wilcoxon rank paired test (compares the medians of non-normally distributed data pairs: O’Brien and Fleming 1978). If p < 0.05, the change in lake chemistry between surveys was assumed to be statistically significant.
Climate and Atmospheric Data
Daily climate data (temperature and precipitation) during the period 1995–2007 was obtained from 13 synoptic meteorological stations (Met Eireann) across Ireland (Fig. 1). Atmospheric deposition data for the period 1995–2007 were obtained from five monitoring stations (Brackloon, Cloosh, Roundwood, Valentia and Lough Navar) remote from local sources of pollution (Fig. 1). All monitoring stations measured precipitation volume, pH, major cations, and anions on a bulk-weekly or wet-only daily (Valentia and Loch Navar) frequency. Monotonic trends in mean annual climate data and atmospheric deposition chemistry were statistically assessed using the non-parametric Mann–Kendall test (Salmi et al. 2002).
Results
Lake Chemistry: 2007 Survey
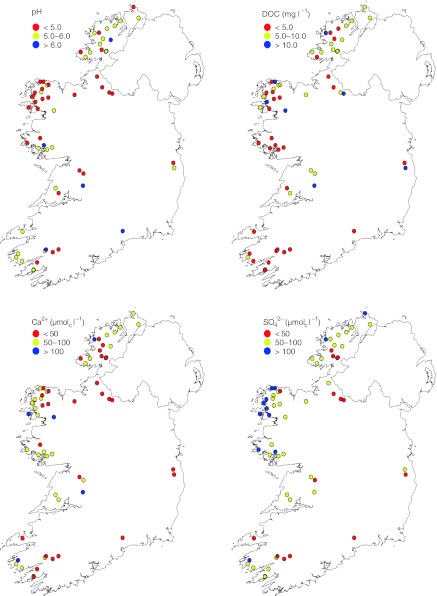
Lake chemistry was dominated by marine ions (Cl− > Na+ > Mg2+), which constituted more than 82% of the total ionic concentration (Table 1), owing to the predominant westerly airflow from the North Atlantic. Chloride concentrations were high, ranging from 196.5 to 1322.0 μmolc l−1 (median of 600.7 μmolc l−1), positively correlated to conductivity (r2 = 0.98) and negatively correlated to elevation (r2 = 0.63). Conductivity values ranged from 34 to 220 μS cm−1; however, 85% of the lakes had values less than 150 μS cm−1. There was a strong negative correlation between elevation and conductivity (r = 0.79 [Pearson product-moment]) owing to lower catchment weathering or reduced marine inputs at higher elevations. In general, surface water pH was low ranging from 4.18 to 6.48 (median: 5.08), and less than or equal to pH 5.5 in 77% of the lakes. The most acidic lakes were in the northwest of the country (Fig. 2). Gran alkalinity was also low, ranging from −58.2 to 135.1 μmolc L−1 (median: −3.6 μmolc l−1), and less than or equal to zero in 57% of the lakes. Dissolved organic carbon (DOC) concentration ranged from 1.8 to 12.7 mg l−1, with a median of 4.9 mg l−1 (88% <10.0 mg l−1). The highest DOC lakes were observed in the northwest consistent with low pH values (Fig. 2). In addition, DOC concentrations were generally higher in the lower elevation lakes (<300 m average: 6.6 mg l−1 [n = 27]) compared with higher (≥300 m average: 4.7 mg l−1 [n = 33]). Total Al was generally low, ranging from 0.4 to 9.5 μmol l−1 (median = 2.1 μmol l−1), and dominated by organic Al (~70%).
Fig. 2.
Geographic distribution of pH, dissolved organic carbon (DOC), calcium (Ca2+), and sulfate (SO42−) for the study lakes in 2007 (n = 60)
Sulfate concentrations in the study lakes ranged from 29.3 to 154.1 μmolc l−1, with a median of 69.3 μmolc l−1. The higher concentrations were observed nearest the west coast (Fig. 2) at the lower elevation lakes (<300 m average of 87.1 μmolc l−1) compared with higher elevation lakes (≥300 m average of 57.6 μmolc l−1). Non-marine sulfate (nmSO42−) ranged from −53.6 to 41.6 μmolc l−1, with a median of 9.7 μmolc l−1 (9 lakes <0 μmolc l−1). The highest concentrations of nmSO42− occurred in the east and near the border with Northern Ireland. This is consistent with previous studies (Bowman and McGettigan 1994) that found higher nmSO42− deposition in the east and north associated with easterly air masses.
Calcium concentration in Irish surface waters had a median of 55.1 μmolc l−1; 92% of the study lakes exhibited concentrations below 100 μmolc l−1, indicating limited catchment sources (e.g., low geochemical weathering). The distribution of low Ca2+ followed the pattern of low pH (and higher DOC), with the lowest concentrations in the northwest and southwest (Fig. 2). Non-marine base cations were dominated by Ca2+ and Mg2+ (Table 1); a small number of lakes (Ca2+ = 1, Mg2+ = 4) had negative concentrations suggesting retention in the catchments. The ANC was low with a median of 85.2 μmolc l−1 and ranged from −2.0 to 461.4 μmolc l−1 (Table 1). Acid neutralizing capacity is used as a water quality criterion for the survival of aquatic organisms (Henriksen et al. 1995); 10% of the study lakes had ANC values less than 20.0 μmolc l−1, which is a widely used limit for protection of fish species (Aherne and Curtis 2003).
Trends in Deposition Chemistry and Meteorology Between 1995 and 2007
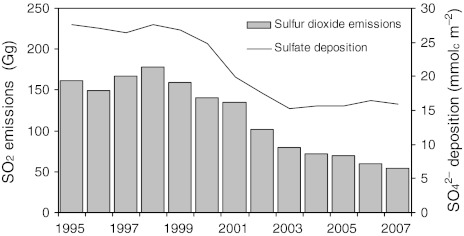
All five monitoring stations (Cloosh, Brackloon, Roundwood, Valentia and Lough Navar) showed temporal changes in precipitation chemistry between 1995 and 2007, with statistically significant (p < 0.05) decreases in nmSO42− deposition, increases in pH, and no change in Cl− deposition. Annual deposition of nmSO42− decreased at Cloosh, Brackloon, Roundwood, Valentia and Lough Navar by 21, 28, 48, 15, and 55%, respectively. A greater reduction in nmSO42− was observed at the more easterly sites (Roundwood and Lough Navar). The deposition of nmSO42− decreased on average from 27.6 mmolc m−2 in 1995 to 15.9 mmolc m−2 in 2007 (~36%), and was strongly correlated to emission reductions (r = 0.95; Fig. 3). In concert, the atmospheric concentration of SO2 at Valentia decreased by ~34% during the same period. Nitrate deposition varied considerably with inter-annual variations showing no significant trend except at Roundwood (east coast: Fig. 1), which had a slight decreasing trend. Observed pH increased significantly (p < 0.05) at all monitoring stations except Lough Navar. There was no trend in Cl− reflecting the strong inter-annual variations associated with weather patterns (e.g., frequency of dominant Atlantic air masses). Depositions of sea salts (Na+ and Cl−) were significantly higher at Cloosh, Brackloon and Lough Navar during 2007 compared with 1997; whereas, sea salt deposition was lower at Roundwood and Valentia.
Fig. 3.
Trends in sulfur dioxide (SO2) emissions for Ireland (EPA 2009) and non-marine sulfate (nmSO42−) deposition (3-year running mean of five deposition stations, see Fig. 1) in Ireland from 1995–2007. Annual emissions and deposition are strongly correlated (r = 0.95 [Pearson product-moment])
Mean annual air temperature (at the 13 synoptic stations; Fig. 1) ranged from 8.5 to 11.7°C, with an overall average of 10.2°C across all stations. There was no trend in mean annual air temperature during the period 1995–2007. Mean annual rainfall varied greatly between years (ranging from 560 to 1923 mm year−1, with an average of 1106 mm year−1); however, no significant trend was observed.
Changes in Lake Chemistry Between 1997 and 2007
Statistically significant differences (p < 0.05) in surface water chemistry were observed between the 1997 and 2007 surveys, with declines in SO42−, nmSO42−, Ca2+ and non-marine base cations (Ca2+ and Mg2+: Table 2). In contrast, statistically significant increases were observed for alkalinity and Cl−. Despite chemical improvements in alkalinity, no change in pH or AlT was observed.
Table 2.
Changes (absolute [Δ] and percent [%]) in mean (and median) lake concentrations between the 1997 and 2007 lake surveys (n = 60). Statistical difference between surveys was assessed with the non-parametric Wilcoxon signed paired test
| Variable | Units | Δ Change | % Change | Wilcoxon§ |
|---|---|---|---|---|
| SO42− | μmolc l−1 | −7.6 (−12.0) | −9.3 (−14.8) | 0.001 |
| nmSO42− | μmolc l−1 | −10.5 (−8.1) | −57.6 (−46.2) | 0.000 |
| pH | −0.03 (0.08) | −0.60 (1.60) | 0.352 | |
| Cl− | μmolc l−1 | 28.6 (30.9) | 4.6 (5.4) | 0.025 |
| Conductivity | μS cm−1 | −1.1 (−0.6) | −1.1 (−0.7) | 0.763 |
| Alkalinity | μmolc l−1 | 5.0 (6.1) | 47.3 (62.5) | 0.003 |
| Ca2+ | μmolc l−1 | −3.7 (0.1) | −5.9 (0.2) | 0.007 |
| Mg2+ | μmolc l−1 | 0.3 (−7.5) | 0.2 (−5.6) | 0.903 |
| nmCa2+ | μmolc l−1 | −4.8 (−0.4) | −11.8 (−1.4) | 0.000 |
| nmMg2+ | μmolc l−1 | −5.3 (−7.8) | −22.9 (−35.9) | 0.004 |
| nmK+ | μmolc l−1 | −0.3 (0.0) | −10.6 (2.2) | 0.594 |
| DOC | mg l−1 | 0.7 (1.6) | 13.7 (47.9) | 0.054 |
| AlT | μmol l−1 | −0.03 (−0.002) | −1.2 (−0.1) | 0.683 |
§Changes in lake chemistry between surveys were assumed to be statistically significant at p < 0.05. Units: 1 mol of charge (molc) is equal to 1 equivalent (eq)
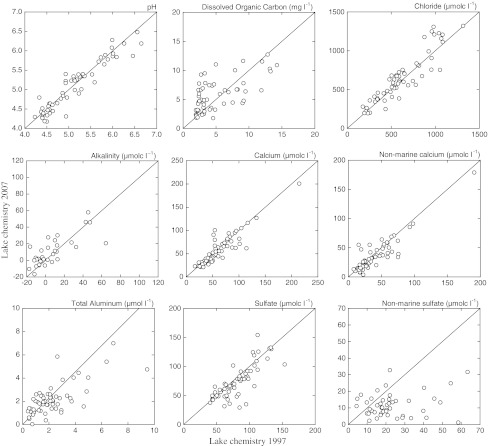
Concentrations of SO42− and nmSO42− decreased on average by 9 and 58%, respectively (Table 2 and Fig. 4). The mean decrease of nmSO42− in surface waters was consistent with the decrease in average deposition (36%; Fig. 3). In concert, concentrations of nmCa2+ (Fig. 4) and nmMg2+ decreased on average by 16%. Despite the decrease in nmSO42− and base cation concentration, no significant difference in conductivity was observed owing to increased inputs of marine ions (Table 2; Fig. 4). Furthermore, no statistically significant difference in DOC was observed between surveys; however, an increase was observed at higher elevation sites. There was no significant change in AlT concentrations (Table 2 and Fig. 4) consistent with DOC. Alkalinity increased significantly by 47%, (from 68 to 57% of lakes with alkalinity less than or equal to zero) while no significant difference in pH was observed, even for the more acidic lakes (i.e., pH < 5.5).
Fig. 4.
Comparison of lake concentrations for pH, dissolved organic carbon, chloride, alkalinity, calcium, non-marine calcium, total dissolved aluminum, sulfate, and non-marine sulfate sampled during 1997 and 2007 (n = 60)
Discussion
Emission and Deposition Reductions
Ireland is located on the western periphery of Europe and assumed to have generally ‘clean’ rain as it predominantly receives air masses from the Atlantic. Nonetheless, consistent with reductions in SO2 emissions, widespread declines in SO42− deposition have occurred over Ireland. Bashir et al. (2008) reported significant decreases in SO2 air concentration and SO42− in wet precipitation at Valentia monitoring station between 1980 and 2004. Similar declining temporal trends in precipitation chemistry have been reported for other regions in Europe (Fowler et al. 2007). In this study, annual deposition of nmSO42−decreased on average by approximately 36% (~12 mmolc m−2) at five monitoring stations during the period 1995–2007. In addition, pH significantly increased at four of the five monitoring stations. While the deposition of long-range air pollution in Ireland is low, the implementation of the Gothenburg Protocol has led to significant changes in rainfall chemistry.
Changes in Lake Chemistry
The study lakes were predominantly headwater lakes located within rocky catchments characterized by shallow base poor mineral soils underlain by granite, quartzite, schist, and gneiss (Aherne et al. 2002). As such, the lake catchments were considered to be acid sensitive because of their slow geochemical weathering rates and low soil buffering capacity.
There were significant differences in water chemistry between the 1997 and 2007 lake surveys suggesting lakes have responded to reductions in emission of SO2. The most significant observation was the widespread decrease in lake SO42− and nmSO42− concentrations between surveys, which is consistent with other studies in Europe and North America. Skjelkvåle et al. (2005) investigated trends in surface water quality in 23 countries (North America and Europe) and found widespread changes (decreased lake SO42− concentrations) primarily related to reduced emission (and deposition) of S. In this study, the decrease in lake SO42− (average of −0.8 μmolc l−1 year−1) was less compared with regions in North America and Europe (−2.2 μmolc l−1 year−1 [Quebec] to −6.5 μmolc l−1 year−1 [southern Nordic countries] during the period 1990–2001: Skjelkvåle et al. 2005). However, observed differences may be related to the different study periods and different levels of pollution. The ability of catchment soils to retain sulfate (i.e., adsorption or reduction), while Cl− enters surface waters relatively unaltered, may lead to errors in the estimation of non-marine constituents. Negative nmSO42− concentrations were observed in 7 lakes in 1997 compared with 9 in 2007; the slight increase may be due to variation in sea salt inputs between surveys or reduced deposition of nmSO42− rather than changes in catchment retention. Decreased lake SO24 concentration was observed in 73% of the study lakes between the 1997 and 2007 surveys.
Non-marine base cation (Ca2+ and Mg2+) concentration decreased on average by 16% between surveys owing to decreased soil ion exchange under reduced acidic deposition (Reuss and Johnson 1986). The average base cation (Ca2+ and Mg2+) decrease in the study lakes (−3.4 μmolc l−1 year−1) was in agreement with other regions (−2. μmolc l−1 year−1) [east central Europe] to −5.4 μmolc l−1 year−1 [west central Europe] during the period 1990–2001: Skjelkvåle et al. 2005), despite the higher sea salt inputs. Moreover, non-marine base cations decreased in 73% of the study lakes.
Despite significant increases in alkalinity and decreases in lake SO42−, no significant difference in pH and AlT was observed between surveys. In addition to strong acidic deposition, lake pH is largely controlled by organic anions (Sullivan et al. 2005) and sea salt inputs; both DOC (albeit non-significant) and sea salts were higher during the 2007 survey. In concert, the absence of a statistically significant change (decrease) in conductivity was largely related to the constant high input of sea salts. Aluminum concentrations were highly variable (Fig. 4) owing to the dominant influence of DOC and pH. In study lakes with pH >5.5 and DOC <5.0, the average AlT concentration was 1.1 μmol l−1; however, lakes with pH < 5.5 and DOC >5.0 mg l−1, the average AlT concentration was 3.1 μmol L−1. Moreover, the study lakes were dominated by organic Al (~70%), which supports the absence of a significant difference between surveys.
Lake surveys represent a water chemistry ‘snapshot’ at a specific time (or interval), providing spatially extensive assessments of current water quality status. However, surveys may be influenced by short-term or seasonal variations, and are typically carried out with decadal frequency; as such, repeat surveys with long intervals are insufficient to establish long-term trends in water chemistry. Nonetheless, despite the limitations, the extensive differences in water chemistry between surveys in this study are consistent with well-documented changes in surface water chemistry in the United Kingdom; long-term hydrochemical trends in 22 upland acid-sensitive surface waters have shown significant deceases in nmSO42− and base cation concentrations during the period 1988–2007 (United Kingdom Acid Waters Monitoring Network [UKAWMN]: Kernan et al. 2010). However, unlike the UKAWMN, increased pH, DOC and decreased AlT between surveys were not observed in this study.
Despite the dominant westerly airflow and low level of transboundary air pollution, the results of the repeat survey suggest that reductions in SO2 emissions across Europe (and to a lesser extent across North America) have resulted in significant changes in surface water chemistry. However, similar to other lake surveys, the recovery of pH was not observed. Given the short time-scale (one decade), a significant change in pH may be difficult to detect. Moreover, short-term variations in DOC or sea salt episodes may be confounding factors (Skjelkvåle et al. 2003).
Factors Confounding Recovery of Surface Waters
Increases in surface water DOC has been well documented in a number of regions in Europe and North America (Evans et al. 2005; Skjelkvåle et al. 2001; Kopáček et al. 2006; Driscoll et al. 2003; Jeffries et al. 2003). Although the drivers associated with this increase are speculative, suggestions include decreased acidic deposition, increased temperatures owing to global warming, changes in hydrology and changes in land use (Evans et al. 2005). Increased organic acidity in surface waters may offset decreases in mineral acid anions, suppressing the recovery of pH (Sullivan et al. 2005; Evans et al. 2008).
In this study, a statistically significant change in DOC concentration (estimated from absorbance at 320 nm) was not observed between the 1997 and 2007 surveys; however, there was a great deal of variability between surveys (Fig. 4) and a non-significant increase was observed (Table 2). Catchment soils were primarily composed of peaty podzols and blanket peats (high in organic matter), which have a significant influence on surface water DOC concentrations. Nonetheless, DOC was relatively low (53% below 5.0 mg l−1) in the study lakes. Sea salt episodes, typically linked to severe weather conditions, and potentially leading to the transfer of acidity from catchment soils to surface waters have been reported in coastal regions of Norway (Hindar et al. 1994), Scotland, western Ireland, eastern Canada and the United States (Harriman et al. 1995). Moreover, sea salt episodes have also been shown to depress the concentration of DOC in surface waters (Moldan et al. 2011). In this study, there was a significant increase in sea salts between the 1997 and 2007 surveys (Table 2).
Climate patterns in Ireland have experienced changes since the nineteenth century, with increasing temperatures (Butler et al. 2007) and more intense storm events (Sweeney et al. 2003). Climate change may have substantial consequences for the chemistry of Irish surface waters, leading to greater variability in DOC concentrations or sea salt episodes, and potentially confounding the response of lake chemistry to future emission reductions. As suggested by Harriman et al. (1995), caution should be taken when assessing the response of surface waters to declining SO2 emissions in remote coastal regions exposed to sea salt episodes.
Conclusion
Irish lakes situated along the coastal mountain ranges, remote from local pollution sources, are ideal for investigating the impacts of long-range transboundary air pollution. Significant decreases in SO42−, nmSO42− and non-marine base cations were observed between the 1997 and 2007 lake surveys, suggesting that small Irish lakes have responded to reductions in long-range transboundary air pollution. In concert, significant increases in alkalinity were observed; however, there were no significant changes in surface water pH and AlT. It is likely that inter-annual variations in sea salt inputs and DOC concentrations (organic acidity) may have contributed to the delay in recovery of pH. Nonetheless the study supports the far reaching benefits of emission reductions owing to the implementation of the Gothenburg Protocol under the UNECE Convention on Long-Range Transboundary Air Pollution.
Continued improvements in the chemistry of Irish lakes (e.g., decreasing SO42− and increasing pH) are expected into the future under further emission reductions. However, continued monitoring is imperative to assess the combined impacts of changes in atmospheric deposition and climate on the chemical and subsequent biological status of Irish lakes.
Acknowledgements
Financial support for this research was provided by the Irish Environmental Protection Agency under the Climate Change Research Programme (CCRP) 2007–2013 and the Canada Research Chair and NSERC discovery grant programs. We gratefully thank E. P. Farrell and T. Cummins for providing laboratory facilities at University College Dublin, and T. Clair for assistance with lake chemistry quality control. Finally, this work would not have been possible without the extraordinary efforts of the field crew: Jim Johnson, Brent Parsons, Tim Seabert, Koji Tominaga, Colin Whitfield and Antoni Zbieranowski.
Biographies
Andrew W. Burton
is a research scientist at Trent University. His current research focuses on the influence of air pollution on lake chemistry, atmospheric ammonia, and biogeochemical cycling in upland soils.
Julian Aherne
is an Associate Professor in the Department of Environmental and Resource Studies, Trent University. His research concentrates on the impacts of anthropogenic disturbance (air pollution, land use management and climate change) on terrestrial and aquatic ecosystems.
Contributor Information
Andrew W. Burton, Email: andrewburton@trentu.ca
Julian Aherne, Email: jaherne@trentu.ca.
References
- Aherne J, Curtis CJ. Critical loads of acidity for Irish lakes. Aquatic Sciences. 2003;65:21–35. doi: 10.1007/s000270300002. [DOI] [Google Scholar]
- Aherne J, Farrell EP. Deposition of sulfur, nitrogen and acidity in precipitation over Ireland: Chemistry, spatial distribution and long-term trends. Atmospheric Environment. 2002;36:1379–1389. doi: 10.1016/S1352-2310(01)00507-6. [DOI] [Google Scholar]
- Aherne J, Kelly-Quinn M, Farrell EP. A survey of lakes in the Republic of Ireland: Hydrochemical characteristics and acid sensitivity. Ambio. 2002;31(6):452–459. doi: 10.1579/0044-7447-31.6.452. [DOI] [PubMed] [Google Scholar]
- Almer B, Dickson W, Ekström C, Hornström E. Sulphur pollution and the aquatic ecosystem. In: Nriagu JO, editor. Sulfur in the environment. New York: Wiley; 1978. pp. 271–311. [Google Scholar]
- Bailey MD, Bowman JJ, O’Connell C, Flanagan PJ. Air quality in Ireland. Dublin: An Foras Forbartha; 1986. [Google Scholar]
- Bashir W, McGovern F, Ryan M, Burke L. Chemical trends in background air quality and the ionic composition of precipitation for the period 1980–2004 from samples collected at Valentia Observatory, CoKerry, Ireland. Journal of Environmental Monitoring. 2008;10:730–738. doi: 10.1039/b803010c. [DOI] [PubMed] [Google Scholar]
- Bowman JJ. Acid sensitive waters in Ireland: The impact of a major new sulfur emission on sensitive surface waters in an unacidified region. Dublin: Environmental Research Unit; 1991. [Google Scholar]
- Bowman JJ, McGettigan M. Atmospheric deposition in acid sensitive areas of Ireland: the influence of wind direction and a new coal burning electricity generation station on precipitation quality. Water, Air, and Soil pollution. 1994;75:159–175. doi: 10.1007/BF01100407. [DOI] [Google Scholar]
- Bull K, Johannson M, Kryzanowski M. Impacts of the convention on long-range transboundary air pollution on air quality in Europe. Journal of Toxicology and Environmental Health: Part A. 2008;71(1):51–55. doi: 10.1080/15287390701557883. [DOI] [PubMed] [Google Scholar]
- Butler CJ, García-Suárez A, Pallé E. Trends in cycles in long Irish Meteorological Series. Biology and Environment: Proceedings of the Royal Irish Academy. 2007;107B(3):157–165. doi: 10.3318/BIOE.2007.107.3.157. [DOI] [Google Scholar]
- Driscoll CT, Driscoll KM, Roy KM, Mitchels MJ. Chemical response of lakes in the Adirondack region of New York to declines in acidic deposition. Environmental Science and Technology. 2003;37:2036–2042. doi: 10.1021/es020924h. [DOI] [PubMed] [Google Scholar]
- EMEP. 1996. Manual for sampling and chemical analysis. http://www.tarantula.nilu.no/projects/ccc/qa.
- European Environmental Agency. 2009. European community emission inventory report 1990–2007 under the UNECE Convention on Long-range Transboundary Air Pollution (LRTAP). EEA Technical Report No 8/2009, Copenhagen. doi:10.2800/12414.
- Evans CD, Monteith DT, Cooper DM. Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environmental Pollution. 2005;137:55–71. doi: 10.1016/j.envpol.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Evans CD, Monteith DT, Reynolds B, Clark JM. Buffering of recovery from acidification by organic acids. Science of the Total Environment. 2008;404:316–325. doi: 10.1016/j.scitotenv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Flower RJ, Rippey B, Rose NL, Appleby P, Battarbee RW. Paleolimnological evidence for the acidification and contamination of lakes by atmospheric pollution in western Ireland. Journal of Ecology. 1994;82(3):581–596. doi: 10.2307/2261266. [DOI] [Google Scholar]
- Fowler D, Smith R, Muller J, Cape JN, Sutton M, Erisman JW, Fagerli H. Long term trends in sulfur and nitrogen deposition in Europe and the cause of non-linearities. Water, Air, and Soil Pollution: Focus. 2007;7:41–47. doi: 10.1007/s11267-006-9102-x. [DOI] [Google Scholar]
- Gorham E. The chemistry of bog waters. In: Stumm W, editor. Chemical processes in lakes. New York: Wiley; 1985. pp. 330–363. [Google Scholar]
- Harriman R, Anderson H, Miller JD. The role of sea-salts in enhancing and mitigating surface water acidity. Water, Air, and Soil pollution. 1995;85:553–558. doi: 10.1007/BF00476887. [DOI] [Google Scholar]
- Henriksen A, Posch M, Hultberg H, Lien L. Critical loads of acidity for surface waters—can the ANClimit be considered variable? Water, Air, and Soil pollution. 1995;85(4):2419–2424. doi: 10.1007/BF01186196. [DOI] [Google Scholar]
- Hindar A, Henriksen A, Torseth K, Semb A.Acid water and fish death Nature 1994372327–328. 10.1038/372327b07969489 [DOI] [Google Scholar]
- Huntrieser H, Heland J, Schlager H, Forster C, Stohl A, Aufmhoff H, Arnold F, Scheel HE, et al. Intercontinental air pollution transport from North America to Europe: Experimental evidence from airborne measurements and surface observations. Journal of Geophysical Research Atmospheres. 2005;110:1–22. [Google Scholar]
- Jeffries DS, Clair TA, Couture S, Dillon P, Dupont J, Keller B, McNicol D, Turner M, et al. Assessing the recovery of lakes in southeastern Canada from the effects of acidic deposition. Ambio. 2003;32(3):176–182. doi: 10.1579/0044-7447-32.3.176. [DOI] [PubMed] [Google Scholar]
- Kähkonen AM. Soil geochemistry in relation to water chemistry and sensitivity to acid deposition in Finnish Lapland. Water, Air, and Soil pollution. 1996;87:311–327. doi: 10.1007/BF00696844. [DOI] [Google Scholar]
- Kernan M, Batterbee RW, Curtis CJ, Monteith DT, Shilland EM. Recovery of lakes and streams in the UK from the effects of acid rain: UK acid waters monitoring network 20 year interpretive report. London: University College London; 2010. p. 465. [Google Scholar]
- Kopáček J, Hardekopf D, Majer V, Psenakova P, Stuchlik P, Vesely J. Response of alpine lakes and soils to changes in acid deposition: the MAGIC model applied to the Tatra Mountain region, Slovakia-Poland. Journal of Limnology. 2004;63:143–156. [Google Scholar]
- Kopáček J, Stuchlik E, Hardekopf D. Chemical composition of the Tatra Mountain lakes: Recovery from acidification. Biologia Bratislava. 2006;61:21–33. doi: 10.2478/s11756-006-0117-6. [DOI] [Google Scholar]
- Moldan, F., J. Hruška, C. Evans, and M. Hauhs. 2011. Experimental simulation of the effects of extreme climatic events on major ions, acidity and dissolved organic carbon leaching from a forested catchment, Gårdsjön, Sweden. Biogeochemistry. doi:10.1007/s10533-010-9567-6
- Möller D. The Na/Cl ratio in rainwater and the seasalt chloride cycle. Tellus. 1990;42B:254–262. [Google Scholar]
- O’Brien PC, Fleming TR. A paired Prentice–Wilcoxon test for censored paired data. Biometrics. 1987;43:169–180. doi: 10.2307/2531957. [DOI] [Google Scholar]
- Pilgrim W, Clair TA, Choate J, Hughes R. Changes in acid precipitation related water chemistry of lakes from southwestern New Brunswick, Canada, 1986–2001. Environmental Monitoring and Assessment. 2003;88:39–52. doi: 10.1023/A:1025592202153. [DOI] [PubMed] [Google Scholar]
- Reuss JO, Johnson DW. Acid deposition and the acidification of soils and waters. New York: Springer-Verlag; 1986. [Google Scholar]
- Salmi, T., A. Määttä, P. Anttila, T. Ruoho-Airola, and T. Amnell. 2002. Detecting trends of annual values of atmospheric pollutants by the Mann-Kendall test and Sen’s slope estimates—the Excel template application MAKESENS. Publications on Air Quality No. 31. Helsinki: Finnish Meteorological Institute.
- Skjelkvåle BL, Wright R, Henriksen A. Norwegian lakes show widespread recovery from acidification; Results from national surveys of lakewater chemistry 1986–1997. Hydrology and Earth System Sciences. 1998;2(4):555–562. doi: 10.5194/hess-2-555-1998. [DOI] [Google Scholar]
- Skjelkvåle BL, Mannio J, Wilander A, Anderson T. Recovery from acidification of lakes in Finland, Norway, and Sweden 1990–1999. Hydrology and Earth System Science. 2001;5(3):327–337. doi: 10.5194/hess-5-327-2001. [DOI] [Google Scholar]
- Skjelkvåle BL, Evans CD, Larsson T, Hindar A, Raddum GG. Recovery from acidification in European surface waters: A view to the future. Ambio. 2003;32(3):170–175. doi: 10.1579/0044-7447-32.3.170. [DOI] [PubMed] [Google Scholar]
- Skjelkvåle BL, Stoddard J, Jeffries DS, Torseth K, Hogasen T, Bowman JJ, Mannio J, Monteith DT, et al. Regional scale evidence for improvements in surface water chemistry 1991–2001. Environmental Pollution. 2005;137:165–176. doi: 10.1016/j.envpol.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Jeffries DS, Lükewille A, Clair TA, Dillon PJ, Driscoll CT, Forsius M, Johannesson M, et al. Regional trends in aquatic recovery from acidification in North America and Europe. Nature. 1999;401:575–578. doi: 10.1038/44114. [DOI] [Google Scholar]
- Stuchlik E, Kopáček J, Fott J, Horicka Z. Chemical composition of the Tatra Mountain lakes: Response to acidification. Biologia Bratislava. 2006;61:11–20. doi: 10.2478/s11756-006-0116-7. [DOI] [Google Scholar]
- Sullivan TJ, Saunders MC, Tonnesson KA, Nash BL, Miller BJ. Application of a regionalized knowledge-based model for classifying the impacts of nitrogen, sulfur, and organic acids on lakewater chemistry. Knowledge-Based Systems. 2005;18:65–68. doi: 10.1016/j.knosys.2004.04.007. [DOI] [Google Scholar]
- Sweeney, J., T. Brereton, C. Byrne, R. Charlton, C. Emblow, C. Fealy, N. Holden, M. Jones, et al. 2003. Climate change: Scenarios and impacts. Final report. Environmental RTDI Programme 2000–2006. Environmental Protection Agency, Ireland.
- UNECE. 1999. The 1999 protocol to abate acidification, eutrophication and ground-level ozone. Document ECE/EB.AIR/67. New York, Geneva: United Nations Economic Commission for Europe.