Abstract
Shrubs and trees are expected to expand in the sub-Arctic due to global warming. Our study was conducted in Abisko, sub-arctic Sweden. We recorded the change in coverage of shrub and tree species over a 32– to 34-year period, in three 50 × 50 m plots; in the alpine-tree-line ecotone. The cover of shrubs and trees (<3.5 cm diameter at breast height) were estimated during 2009–2010 and compared with historical documentation from 1976 to 1977. Similarly, all tree stems (≥3.5 cm) were noted and positions determined. There has been a substantial increase of cover of shrubs and trees, particularly dwarf birch (Betula nana), and mountain birch (Betula pubescens ssp. czerepanovii), and an establishment of aspen (Populus tremula). The other species willows (Salix spp.), juniper (Juniperus communis), and rowan (Sorbus aucuparia) revealed inconsistent changes among the plots. Although this study was unable to identify the causes for the change in shrubs and small trees, they are consistent with anticipated changes due to climate change and reduced herbivory.
Keywords: Vegetation change, Tree-line, Shrubs, Sub-Arctic, Herbivory, Human impacts
Introduction
Environmental change is a major issue in the Arctic where global warming is amplified, biodiversity is changing, and impacts from human activities are increasing (ACIA 2005; Anisimov et al. 2007). These factors together affect the structure and function of northern ecosystems, and their services to local people, and the global community (Chapin et al. 2005). Areas of particular concern are the northern altitudinal and latitudinal tree-lines (Callaghan et al. 2002). These ecotones are associated with a rich biodiversity and the provision of important ecosystem services including local resources and regulatory factors of global significance such as climate modification (Callaghan et al. 2002; ACIA 2005). Recently, there has been a developing literature showing observed changes in tree-line forests and the shrubs associated with them (e.g. Kullman 2002; Karlsson et al. 2007; Van Bogaert et al. 2009, 2011; Olofsson et al. 2009; Hallinger et al. 2010).
Interestingly, the mountain birch tree-line was 300–400 m above the present altitude (up to 800 m above sea level) in the Abisko area about 5000–10 000 years ago (Barnekow and Sandgren 2001; Van Bogaert et al. 2011). However, the tree-line became depressed during two closely succeeding periods of climate cooling, often considered as one, the Little Ice age from mid sixteenth century until early twentieth century (Grubb 2008). After the end of the Little Ice age, the tree-line began to increase in altitude (Grubb 2008). In addition, reports show an increased growth and establishment of saplings above the present tree-line (Truong et al. 2006), and a new tree species (aspen) has become naturalized (Van Bogaert et al. 2009).
There is an indication that the shrub layer near the tree-line has expanded, since the 1930s in the Abisko area (Enquist et al. 1933; Sandberg 1963). Data from Canada, Fennoscandia, Alaska and Russia, reveal that there is a Pan-Arctic expansion of shrubs and trees in progress (e.g. Kullman 2002; Tømmervik et al. 2004; ACIA 2005; Tape et al. 2006; Karlsson et al. 2007; Olofsson et al. 2009; Hallinger et al. 2010; Hedenås et al. 2011 [this issue]). The tree-line, in Swedish mountains is predicted to continue to move upward with increasing temperatures in the future (Moen et al. 2004).
The increase of shrub growth maybe related to higher air temperature (Cooper et al. 2004; Walker et al. 2006). A study based on experiments from 11 sites across the Pan-Arctic area revealed that climate warming might cause an increased growth of shrub species (Walker et al. 2006). Indeed, the winter and spring temperature has increased by approximately 2.5°C since the beginning of the twentieth century in the Abisko area (Callaghan et al. 2010). However, exceptions occur: A warming of 3°C had a negative effect on germination and establishment of young seedlings of various sub-arctic tundra species (Shevtsova et al. 2009), while recently observed warming events in winter damage some shrubs (Bokhorst et al. 2009). However, precipitation and especially snow depth have been reported to be even more important factors for shrub expansion (Wahren et al. 2005). The weather record from the Abisko Scientific Research Station shows that the snow depth has increased by approximately 10% per decade, since 1930–1940s to year 2000 (Kohler et al. 2006), although it has declined recently (Callaghan et al. 2010).
Climate is; however, not the only factor that affects tree-line (Hofgaard 1997; Holtmeier and Broll 2005; Karlsson et al. 2007). Herbivory, both vertebrate (i.e. reindeer (Rangifer tarandus), moose (Alces alces), hare (Lepus timidus) and microtine rodents (Clethrionomys spp., Microtus spp. and Lemmuslemmus), and invertebrates (e.g. outburst of autumnal moth (Epirrita autumnata) may limit the expansion and growth of trees and shrubs (e.g. Olofsson et al. 2004; Karlsson et al. 2005, 2007; Olofsson et al. 2009; Van Bogaert et al. 2011). Sami reindeer husbandry spread into the area during the seventeenth century, and around the beginning of twentieth century it expanded to become more of an industry that is now well-established in the northern parts of Sweden (Emanuelsson 1987). Furthermore, there was extensive tree-cutting of mountain birch during the building of the railway in the beginning of twentieth century which probably caused the tree-line to recede (Emanuelsson 1987).
It is important to improve our understanding of vegetation change and the causes of these changes to improve forecasts of future change, which could be used for hypothesis testing (e.g. Johnson et al. 2011 [this issue]), adaptive management, and mitigation. Unfortunately, there are relatively few long-term biologically-focused environmental monitoring programmes in the Arctic (ACIA 2005). Consequently, an international project, Back to the Future (Callaghan and Tweedie 2011 [this issue]), was established during the International Polar Years (2007/2008) for further understanding of the recent decade-time scale vegetation change by rescuing, resampling, and assessing change at sites where historic biotic and abiotic research had been conducted.
The aim of this study is to record changes in the cover and the abundance of trees and shrubs spanning during the period from 1976–1977 and 2009–2010 near Abisko, northern Sweden. This study differs from others that have measured changes in tree and shrubs over decade-time scales in that the change has been assessed based on direct comparison of historic and recent observations (and not as a result of indirect methods such as denrochronology and remote sensing).
Study Area
This study was conducted on an east-facing slope of the mountains Slåttatjåkka/Njulla, 68°21′N 18°49′W in the Abisko Valley, approximately 200 km north of the Arctic Circle, in the county of Norrbotten, Sweden (Figs. 1, 2). The local climate in the Abisko Valley is affected by strong orographic effects. The mean annual precipitation, measured at Abisko Scientific Research Station, is the lowest in Sweden—approximately 310 mm for the period of 1913–2000 (Kohler et al. 2006). Annual mean temperature is nowadays 0.7°C (Kohler et al. 2006).
Fig. 1.
Map of Sweden showing the location of Abisko, the three study plots A2, A3, and A4 (black stars), and the Abisko Scientific Research Station (ANS) where the meteorological station is located. © Lantmäteriet, ärende nr I 2010/0345
Fig. 2.
Photography a was taken in 1977, by N.-Å. Andersson, and photo b was taken in 2009 by H. Hedenås. They are taken from approximately the same location, uphill from plot A2, looking towards plots A3 and A4
Materials and Methods
Four plots, 50 × 50 m, were established in 1976–1977 by Nils-Åke Andersson (unpublished). Three of the plots, A2, A3, and A4 were established in the tree-line ecotone. Another plot, A1, was established in the valley with mixed forest. The plots were established with the aid of measuring tapes and compasses. In each plot, a grid consisting of twenty-five, 10 × 10 m squares, marked with sticks, was lain down over the area. The cover of all shrubs and tree taxa was drawn on a detailed vegetation map. This was done by estimating the outline of each shrub and tree (<3.5 cm DBH; diameter at breast height) with measuring tapes. The number of tree stems ≥3.5 cm DBH was also noted, with the aid of a calliper. They found dwarf birch (Betula nana), juniper (Juniperus communis), mountain birch (Betula pubescens ssp. czerepanovii), rowan (Sorbus aucuparia), and willow species (Salix spp.) (nomenclature according to Karlsson 1997) in 1976–1977.
In September 2009 and August 2010, three of the original plots—A2, A3, and A4 were relocated and resampled (Figs. 1, 2). Loss of original maps made it impossible to exactly locate plot A1, which was therefore omitted from this study. A3 and A4 were located at the tree-line (i.e. sparse occurrences of solitary trees or small tree groups), and A2 was located below A3, at the forest-line (i.e. parts of the plot consist of a denser mountain birch forest). The positions of these three plots were identified almost exactly by comparing the present vegetation structure and location of a few solitary boulders with the original maps that were made in 1976–1977. The most helpful structures were the juniper shrubs as their distribution and cover showed relatively small changes. The field vegetation in plots A2 and A4 was mainly dominated by Empetrum nigrum ssp. hermaphroditum and graminoids with narrow leaves. The field vegetation in plot A3 was dominated by meadow vegetation with both low and tall herbs, while only a minor part of the area was covered with E. nigrum ssp. hermaphroditum and graminoids with narrow leaves (Fig. 2).
The locations and coverage of the different shrub and tree species (<3.5 cm DBH), and number of tree stems (≥3.5 cm DBH) in the three plots were recorded with a Differential Global Positioning System (DGPS, Trimble R7-GNSS receiver with a TSC2 Controller; Trimble 2008). Shrub surveys used the “continuous topo” and fixed real-time kinematic (RTK) functions where the DGPS was configured to record an x, y, and z position every 1 to 2 s as the surveyor slowly moved around each shrub, providing a centimeter-level accuracy for each position. The diameters of trees >3.5 cm were measured with callipers and located using the DGPS “Measure point”, and fixed RTK functions (Trimble 2008). The projected coordinate system used during the measurements was the National Grid of Sweden, SWEREF99 TM.
ArcGIS 9.3.1 was employed to analyze the data. The old hand-made maps of the study sites were digitalized to shape files to enable comparison with the new data. With an uneven terrain, deviation, and different projections, the maps from 2009/2010 in SWEREF99 TM were somewhat tilted compared with the squared maps from 1976/1977. However, the plot corners were at the same locations in 2009/2010 as in 1976/1977. The retrieved coordinates were converted into polygons and the coverage of each species was calculated with the aid of the ArcGIS 9.3.1.
Data Analysis
We used paired t-tests to assess whether there were any differences in cover (<3.5 cm DBH) or the number of tree stems per plot (≥3.5 cm DBH) between 1977–1979 and 2009–2010. This analysis was conducted for all tree and shrub species combined as well as for each species separately, i.e. B. nana, B. pubescens ssp. czerepanovii, J. communis, Populus tremula, Salix spp., and S. aucuparia. Cover values and number of stems were log-transformed before analysis. All t-tests were conducted with the statistical package R 2.11.1.
Results
Cover of Shrubs and Trees <3.5 cm DBH
Our analysis revealed a significant (p = 0.010) increase in the overall cover of shrubs and trees (<3.5 cm DBH) over the last 32–34 years (Table 1; Figs. 3, 4, and 5). One species, B. pubescens showed a significant (p = 0.005) increase in all the plots and a second species B. nana showed a substantial increase in all the plots (p = 0.053). A third species P. tremula, recorded during 2009–2010 occurred in all the plots but was not recorded in any of the earlier surveys. Of the species studied, B. pubescens showed the largest expansion over time with cover increasing by over 500% in all the three plots. The cover of B. pubescens ranged between 47.4 and 521.1 m2 in plots A4 and A2, respectively (Table 1). The cover of B. nana was the highest among the focal species in the 1970s, and is still the highest today, varying between 107.3 m2 in plot A3 and 954.5 m2 in plot A4 (Table 1). P. tremula had a cover during 2009–2010 that varied between 1.6 and 11.0 m2 in plots A4 and A3, respectively (Table 1; Figs. 3, 4, and 5).
Table 1.
Cover of shrub and tree species (<3.5 cm DBH), and the number of stems (≥3.5 cm DBH), during 1976–1977 and 2009–2010, respectively, for plot A2, A3, and A4.
| Species | A2 | A3 | A4 | Paired t-test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1976–1977 | 2010 | % Change | 1976–1977 | 2009 | % Change | 1976–1977 | 2009 | % Change | t | df | p | |
| Cover | ||||||||||||
| B. nana (m2) | 421.4 | 653.1 | 55.0 | 38.6 | 107.3 | 178.0 | 501.5 | 954.5 | 90.3 | 4.18 | 2 | 0.05 |
| B. pubescens ssp. czerepanowii (m2) | 75.7 | 521.1 | 588.4 | 39.6 | 241.1 | 508.8 | 6.3 | 47.4 | 652.4 | 46.10 | 2 | <0.01 |
| J. communis (m2) | 41.5 | 28.9 | −30.4 | 356.5 | 387.2 | 8.6 | 164.7 | 170.0 | 3.2 | −0.58 | 2 | 0.62 |
| P. tremula (m2) | 0 | 2.8 | – | 0 | 11.0 | – | 0 | 1.6 | – | 3.46 | 2 | 0.07 |
| Salix spp. (m2) | 153.3 | 104.4 | −31.9 | 213.3 | 253.0 | 18.6 | 33.3 | 100.6 | 202.1 | 0.68 | 2 | 0.57 |
| S. aucuparia (m2) | 13.0 | 8.6 | −33.8 | 4.4 | 14.9 | 238.6 | 0.1 | 0.1 | 0 | 0.54 | 2 | 0.64 |
| Total cover of shrubs and trees (m2) | 704.8 | 1319.0 | 87.1 | 652.4 | 1014.5 | 55.5 | 705.9 | 1274.2 | 80.5 | 7.75 | 2 | 0.01 |
| No. of stems | ||||||||||||
| B. pubescens ssp. czerepanowii (≥3.5 cm) | 193 | 186 | 3.6 | 33 | 81 | 145.5 | 1 | 3 | 200.0 | 1.80 | 2 | 0.21 |
| Salix spp. (≥3.5 cm) | 2 | 5 | 150.0 | 5 | 0 | – | 0 | 0 | – | −0.50 | 2 | 0.67 |
| S. aucuparia (≥3.5 cm) | 3 | 0 | −100.0 | 3 | 0 | – | 0 | 0 | – | −2.00 | 2 | 0.18 |
| Total number of stems (≥3.5 cm) | 198 | 191 | −3.5 | 41 | 81 | 97.6 | 1 | 3 | 200.0 | 1.81 | 2 | 0.21 |
Paired t-test results from the comparison of cover of shrubs and trees <3.5 cm and number of trees ≥3.5 cm between the years. Bold figures denote significant changes
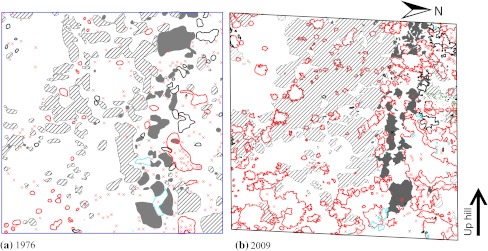
Fig. 3.
Cover of trees and shrubs <3.5 cm DBH, and position of tree stems ≥3.5 cm DBH, in plot A2 a 1976–1977 and b 2009. Lines and filled areas denote coverage and crosses indicate tree stems. Both plots are 50 × 50 m. Red crosses denote B. pubescens ssp. czerepanovii stems ≥3.5 cm DBH, black crosses denote Salix spp. ≥3.5 cm DBH, blue crosses denote S. aucuparia ≥3.5 cm DBH, hatched pattern denotes B. nana <3.5 cm DBH, red outline denote B. pubescens ssp. czerepanovii <3.5 cm DBH, black outline denotes J. communis <3.5. cm, blue outline denotes S. aucuparia <3.5 cm DBH, gray filling denotes Salix spp <3.5 cm DBH, and green filling denotes P. tremula <3.5 cm DBH
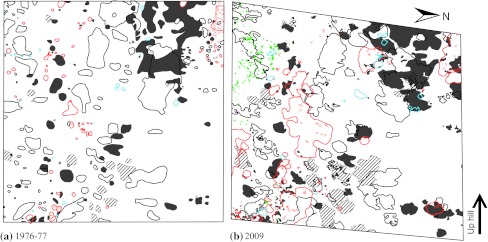
Fig. 4.
Cover of trees and shrubs <3.5 cm DBH, and position of tree stems ≥3.5 cm DBH, in plot A3 a 1976–1977 and b 2009. Both plots are 50 × 50 m. For key to the symbols see legend to Fig. 3
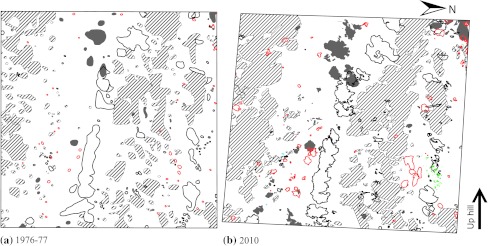
Fig. 5.
Cover of trees and shrubs <3.5 cm DBH, and position of tree stems ≥3.5 cm DBH, in plot A4 a 1976–1977 and b 2010. Both plots are 50 × 50 m. For key to the symbols see legend to Fig. 3
All three other focal species, J. communis, Salix spp., and S. aucuparia showed an inconsistent pattern among the plots. J. communis increased slightly in two of the plots; from 356.5 to 387.2 m2 in A3 and from 164.7 to 170 m2 in A4; which corresponds to an increase of 8.6 and 3.2%, respectively. However, this species decreased in plot A2 from 38.9 to 28.9 m2, which corresponds to a 25.7% decrease (Table 1; Figs. 3, 4, and 5). The cover of Salix spp. decreased by 27.6%, from 144.2 to 104.4 m2 in A2, while it increased by 18.6% (213.3–253.0 m2) in plot A3 and 202.1% in plot A4 (33.3–100.6 m2, Table 1; Figs. 3, 4, and 5). S. aucuparia showed a decrease in the coverage in plot A2 from 12.2 to 8.6 m2 which corresponds to a 29.5% change, and an increase in coverage in plot A3 from 4.4 to 14.9 m2 from 1976–1977 to 2009, which corresponds to a 238.6% change (Table 1). There was no change of coverage of S. aucuparia in plot A4 (Table 1).
Number of Stems ≥3.5 cm DBH
There were no overall significant changes in the number of tree stems ≥3.5 cm DBH between surveys (Table 1). The total number of tree stems decreased 3.5% in plot A2, and increased by 97.6% in plot A3, and 200.0% in plot A4 (Table 1; Figs. 3, 4, and 5). The number of B. pubescens stems in plot A3 showed a 145.5%, increase (i.e. from 33 stems in the 1970s to 81 stems in the year 2009) and a 200.0% increase in plot A4 (i.e. from one stem in 1970s to three stems in the year 2009, Table 1). The number of B. pubescens stems decreased in plot A2 from 198 to 191 stems (a 3.5% decrease). The number of Salix spp. stems increased from two to five in plot A2, and decreased from five to zero in plot A3 (Table 1). The number of S. aucuparia stems decreased from three stems, in the 1970s, to zero stems during 2009/2010 (Table 1). No P. tremula stems were found.
Discussion
Our study revealed that the total shrub cover and the number of tree stems increased considerably since the mid 1970s. Our general results are in agreement with other studies that have observed an expansion of deciduous shrub and tree species throughout the Arctic (Sturm et al. 2001; Tape et al. 2006; Olofsson et al. 2009; Hallinger et al. 2010). However, there was no consistent transition over time among species. B. pubescens, B. nana, and P. tremula increased considerably in all the plots. A particularly important observation was that P. tremula, not observed in the 1970s, had become established in all the plots and has also been noted nearby by Van Bogaert et al. (2011). The trends of the other species revealed a more complex pattern. In general, they increased or were stable in the two plots (A3 and A4) located at the tree-line while they decreased in the nearby plot (A2) located at the forest-line below. The coverage of the gymnosperm J. communis remained almost unchanged in the two plots at the tree-line (A3 and A4), while it decreased in the plot located at the forest-line (A2). The coverage of Salix spp., however, increased in the two plots located at the tree-line (A3 and A4), while it decreased in the plot located at the forest-line (A2). The contrasting response of J. communis agreed with findings from a study based on dendrochronology from the same area (Hallinger et al. 2010), and with observations from the Central European Alps (Zeidler et al. 2009). The increase of Salix spp. in the tree-line plots also agrees with other studies that have reported a considerable expansion of willows in arctic and sub-arctic areas (e.g. Sturm et al. 2001; Tape et al. 2006; Forbes et al. 2009). However, the decrease of willows at the forest-line does not follow this pattern.
Today, climate change is very much in focus, particularly in the Arctic (ACIA 2005; Anisimov et al. 2007), and changes in the climatic parameters over a 30-year period are often inferred to explain the observed pattern. Indeed, increased temperatures and increased snow depth in the area over the last century (Kohler et al. 2006) could have contributed to the increase in deciduous shrub and tree cover (cf. Weih and Karlsson. 1997; Sturm et al. 2005; Kohler et al. 2006; Tape et al. 2006; Torp 2010). Further, increased length of growing season has been suggested as one of the major factors causing tree and shrub expansion (Torp 2010). Kohler et al. (2006) found no indication of any net change in the start of the growing season over the last century. However, it is likely that the growing season had started earlier, at our plots, in the past decade (Callaghan et al. 2010; Andrews et al. 2011 [this issue]).
Furthermore, the temporary lower temperatures during the “Little Ice Age”, i.e. two periods of colder climate from middle of the sixteenth century until the beginning of the twentieth century, caused species to retreat downslope (Grubb 2008). Thus, upward movement of species observed today could be interpreted as an ongoing natural re-establishment of plants at higher altitudes due to a natural increase in the temperature since the “Little Ice Age” (Kammer et al. 2007). Indeed, already in the 1930s, an upward movement of the tree-line was observed adjacent to the study area (Enquist et al. 1933). Thus, if tree-line responded to this “natural” increase in temperature, then it would be expected to also respond to the generally accepted (IPCC 2007) recent increases in temperatures such as those observed at Abisko over the past 100 years (Callaghan et al. 2010). Furthermore, if the trees and shrubs have responded to natural or anthropogenic warming, then future changes should be observed in response to future global warming.
A change in the temperatures was discussed already in 1963 as one of the main factors behind the observed shrub expansion (Sandberg 1963). The other factor discussed was changes in reindeer grazing (Sandberg 1963). Indeed a release in reindeer grazing pressure may facilitate growth and expansion of shrubs and trees in the tree-line ecotone (e.g. Olofsson et al. 2009). The reindeer grazing pressure has probably decreased locally, on the slope of Slåttatjåkka and Njulla, due to a change in reindeer migration patterns that occurred following the construction of a chair-lift in 1965, and increased disturbance from tourists, although the reindeer numbers generally has increased over the past century in the Abisko area (Van Bogaert et al. 2011). Similarly, a release in microtine rodent pressure may facilitate growth and expansion of the dwarf-shrub B. nana (Olofsson et al. 2004, 2009). Voles (Clethrionomys spp., Microtus spp.) and possibly lemmings (L.lemmus), have decreased in the mountain range over the last 20–30 years (Yoccoz and Ims 1999; Ims and Fuglei 2005).
Historic and extant anthropogenic activities associated with Sami settlements, reindeer husbandry, railway construction, mining, tourism, hunting, and fishing have affected the landscape in the Abisko area (Emanuelsson 1987; Bäck and Jonasson 1998). Emanuelsson (1987) concluded that the cutting of trees for construction and firewood have led to an unnaturally low tree-line and tree cover in the Abisko area. Karlsson et al. (2007) noticed in an area south of Abisko that the deforestation was followed by an increased cover of shrub species in particular B. nana when a nearby Sami settlement was abandoned. This might partly explain the observed tree and shrub expansion.
The establishment of P. tremula might have been facilitated by an increase in air temperature in the area over the last 30 years (Van Bogaert et al. 2009), and low abundances of herbivores in the study area. For instance, moose (A. alces) generally avoids such altitudes (Lundmark 2008), but might otherwise severely affect the growth and establishment of P. tremula (Zakrisson et al. 2007; Van Bogaert et al. 2009). In addition, low abundances of microtine rodents and reindeer might also contribute to the establishment of P. tremula.
The main reasons for the lack of growth and expansion of the J. communis population might be competition and shading from trees in combination with a scarcity of suitable sites for colonization and insufficient disturbance (Zeidler et al. 2009). Further, Tape et al. (2006) found that the greatest expansion of shrub species occurred in areas with the highest amount of disturbance, as there was a relationship between the amount of disturbance and the nutrient availability.
We have documented rapid and substantial increases in the abundance of prominent tree and shrub species near tree-line and forest-line in sub-Arctic Sweden and have recorded an invasion by a thermophilic tree species not present in the plots 34 years ago. Land-use, grazing pressure, temperature, snow depth, and length of growing season are all important variables that have changed over the last decades which one by one or together may influence the observed changes in shrub and tree cover. In addition, past changes in reindeer grazing (Van Bogaert et al. 2011), temperature, and land-use may influence present changes in shrub and tree cover.
Acknowledgments
The authors would like to acknowledge Nils-Åke Andersson for providing them with unpublished data and photographs from 1976–1977, Craig Tweedie, and an anonymous reviewer for valuable comments. The authors also sincerely thank the staff on Abisko Arctic Scientific Research Station for their logistic support. The project was financed by a grant from the Swedish Research Council (Vetenskapsrådet) 327-2007-833.
Biographies
Sara Rundqvist
has a master’s degree in biology from the Department of Ecology and Environmental Science (EMG), Umeå University. Sara is currently working as a project assistant at Umeå University in EMG.
Henrik Hedenås
has a Ph.D. in ecology from the Department of Ecology and Environmental Science (EMG), Umeå University. He has worked at different positions at EMG, and is currently a researcher at Abisko Scientific Research Station and temporary forest habitat advisor at Species Information Center, Swedish University of Agricultural Science.
Anneli Sandström
has a bachelor degree in biology from the Department of Ecology and Environmental Science (EMG) at Umeå University. She is continuing for a master degree in ecology and conservation at Biology Education Center (IBG), Uppsala University.
Urban Emanuelsson
has a Ph.D. from University of Lund and is appointed professor by the Swedish Government in January 2007. He has worked as Director of the Swedish Biodiversity Center 1995–2008 at The Swedish University of Agriculture and Uppsala University, where he today is senior adviser. He is also guest professor at Högskolan Kristianstad, Member of the Board, for Ajtte (Swedish same museum), Member of the Board, WWF Sweden, Member of the Royal Academy of Forestry and Agriculture.
Håkan Eriksson
has a degree in civil engineering from KTH Royal Institute of Technology in Stockholm. In addition studies in physical geography at Umeå University and now teaches geographic information systems at the Department of Ecology and Environmental Science (EMG) at Umeå University.
Christer Jonasson
has a Ph.D. in physical geography from Uppsala University and is associate professor at Stockholm University. He is station manager for the Abisko Scientific Research Station.
Terry V. Callaghan
has a Ph.D. from the University of Birmingham, UK, honorary Ph.D.s from the Universities of Lund, Sweden, and Oulu, Finland, and a D.Sc. from the University of Manchester, UK. He is a Royal Swedish Academy of Sciences’ distinguished research professor and member and also professor of arctic ecology at the University of Sheffield, UK.
Contributor Information
Sara Rundqvist, Email: sara.rundqvist@gmail.com.
Henrik Hedenås, Email: henrik.hedenas@slu.se, Email: henrik.hedenas@gmail.com.
Anneli Sandström, Email: ansa1992@student.uu.se.
Urban Emanuelsson, Email: urban.emanuelsson@slu.se.
Håkan Eriksson, Email: hakan.eriksson@emg.umu.se.
Christer Jonasson, Email: christer.jonasson@ans.polar.se.
Terry V. Callaghan, Email: terry_callaghan@btinternet.com
References
- Arctic Climate Impact Assessment. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Andrews, C., J. Dick, C. Jonasson, and T.V. Callaghan. 2011. Assessment of biological and environmental phenology at a landscape level from 30 years of fixed date repeat photography in Northern Sweden. Ambio. doi:10.1007/s13280-011-0167-z. [DOI] [PMC free article] [PubMed]
- Anisimov, O.A., D.G. Vaughan, T.V. Callaghan, C. Furgal, H. Marchant, T.D. Prowse, H. Vilhjálmsson, and J.E. Walsh. 2007. Polar regions (Arctic and Antarctic). In Climate Change 2007: Impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, ed. M.L. Parry, O.F. Canziani, J.P. Palutikof, C.E. Hanson, and P.J. van der Linden, 655–685. Cambridge: Cambridge University Press.
- Bäck L, Jonasson C. The Kiruna-Narvik road and its impact on the environment and the recreational land-use. Ambio. 1998;27:345–350. [Google Scholar]
- Barnekow L, Sandgren P. Palaeoclimate and tree-line changes during the Holocene based on pollen and plant microfossil records from six lakes at different altitudes in northern Sweden. Review of Palaeobotany and Palynology. 2001;117:109–118. doi: 10.1016/S0034-6667(01)00080-X. [DOI] [Google Scholar]
- Bokhorst S, Bjerke JW, Tömmervik H, Callaghan TV, Phoenix GK. Winter warming events damage sub-Arctic vegetation: Consistent evidence from an experimental manipulation and a natural event. Journal of Ecology. 2009;97:1408–1415. doi: 10.1111/j.1365-2745.2009.01554.x. [DOI] [Google Scholar]
- Callaghan, T.V., F. Bergholm, T.R. Christensen, C. Jonasson, U. Kokfelt, and M. Johansson 2010. A new climate era in the sub-Arctic: Accelerating climate changes and multiple impacts. Geophysical Research Letters 37. doi:10.1029/2009GL042064.
- Callaghan, T.V., and C.E. Tweedie (eds.) 2011. Multi-decadal changes in Tundra environments and ecosystems—The International Polar Year Back to the Future Project. Ambio Special Issue 40(6). [DOI] [PMC free article] [PubMed]
- Callaghan TV, Werkman BR, Crawford RMM. The tundra-taiga interface and its dynamics: Concepts and applications. Ambio Special Issue. 2002;12:6–14. [PubMed] [Google Scholar]
- Chapin FS, III, Berman M, Callaghan TV, Convey P, Crepin A-S, Danell K, Ducklow H, Forbes B, et al. Polar systems. In: Hassan R, Scholes R, Ash N, et al., editors. Ecosystems and human well-being: Current state and trends. Washington: Island Press; 2005. pp. 717–743. [Google Scholar]
- Cooper EJ, Alsos IG, Hagen D, Smith FM, Coulson SJ, Hodkinson ID. Plant recruitment in the high arctic: Seed bank and emergence on Svalbard. Journal of Vegetation Science. 2004;15:115–124. doi: 10.1111/j.1654-1103.2004.tb02244.x. [DOI] [Google Scholar]
- Emanuelsson U. Human influence on vegetation in the Torneträsk area during the last three centuries. Ecological Bulletins. 1987;30:95–111. [Google Scholar]
- Enquist, F. 1933. Trädgränsundersökningar. Svenska Skogsvårdsföreningens Tidskrift 31: 145–191 (in Swedish, German summary).
- Forbes BC, Fauria MM, Zetterberg P. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Global Change Biology. 2009;15:1–13. doi: 10.1111/j.1365-2486.2008.01704.x. [DOI] [Google Scholar]
- Grubb H. Torneträsk tree-ring width and density AD 500–2004: A test of climatic sensitivity and a new 1500-year reconstruction of north Fennoscandian summers. Climate Dynamics. 2008;31:843–857. doi: 10.1007/s00382-007-0358-2. [DOI] [Google Scholar]
- Hallinger M, Manthey M, Wilmking M. Establishing a missing link: Warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist. 2010;186:890–899. doi: 10.1111/j.1469-8137.2010.03223.x. [DOI] [PubMed] [Google Scholar]
- Hedenås, H., H. Olsson, C. Jonasson, J. Bergstedt, U. Dahlberg, and T.V. Callaghan. 2011. Changes in tree growth, biomass and vegetation over a thirteen-year period in the Swedish sub-Arctic. Ambio. doi:10.1007/s13280-011-0173-1 [DOI] [PMC free article] [PubMed]
- Hofgaard A. Inter-relationships between tree line position, species diversity, land use and climate change in the central Scandes Mountains of Norway. Global Ecology and Biogeography Letters. 1997;6:419–429. doi: 10.2307/2997351. [DOI] [Google Scholar]
- Holtmeier FK, Broll G. Sensitivity and response of Northern Hemisphere elevational and polar tree lines to environmental change at landscape and local scales. Global Ecology and Biogeography. 2005;14:395–410. doi: 10.1111/j.1466-822X.2005.00168.x. [DOI] [Google Scholar]
- Ims RA, Fuglei E. Trophic interaction cycles in tundra ecosystems and the impact of climate change. BioScience. 2005;554:311–322. doi: 10.1641/0006-3568(2005)055[0311:TICITE]2.0.CO;2. [DOI] [Google Scholar]
- IPCC. 2007. Climate Change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment. Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
- Johnson, D.R., D. Ebert-May, P.J. Webber, and C.E. Tweedie. 2011. Forecasting Alpine vegetation change using repeat sampling and a novel modeling approach. Ambio. doi:10.1007/s13280-011-0175-z. [DOI] [PMC free article] [PubMed]
- Kammer PM, Schöb C, Choler P. Increasing species richness on mountain summits: Upward migration due to anthropogenic climate change or re-colonization? Journal of Vegetation Science. 2007;18:301–306. doi: 10.1111/j.1654-1103.2007.tb02541.x. [DOI] [Google Scholar]
- Karlsson, T. 1997. The vascular plants of Sweden—a checklist. Svensk Botanisk Tidsskrift 91: 241–560 (in Swedish, English summary).
- Karlsson H, Hörnberg G, Hannon G, Nordström E-M. Long-term vegetation changes in the northern Scandinavian forest limit: A human impact-climate synergy? The Holocene. 2007;17:37–49. doi: 10.1177/0959683607073277. [DOI] [Google Scholar]
- Karlsson PS, Weih M, Borg C. Mountain birch growth in relation to climate and herbivores. In: Wielgolaski FE, editor. Plant ecology, herbivory, and human impact in Nordic mountain birch forests. Berlin: Springer-Verlag; 2005. pp. 71–86. [Google Scholar]
- Kohler J, Brandt O, Johansson M, Callaghan T. A long-term Arctic snow depth record from Abisko, northern Sweden 1913–2004. Polar Research. 2006;25:91–113. doi: 10.1111/j.1751-8369.2006.tb00026.x. [DOI] [Google Scholar]
- Kullman L. Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. Journal of Ecology. 2002;90:68–77. doi: 10.1046/j.0022-0477.2001.00630.x. [DOI] [Google Scholar]
- Lundmark, C. 2008. Morphological and behavioral adaptations of moose to climate, snow, and forage. PhD thesis, Swedish University of Agricultural Sciences, Umeå, Sweden.
- Moen J, Aune K, Edenius L, Angerbjörn A. Potential effects of climate change on tree-line position in the Swedish mountains. Ecology and Society. 2004;9:16. [Google Scholar]
- Olofsson J, Hulme PE, Oksanen L, Suominen O. Importance of large and small mammalian herbivores for the plant community structure in the forest tundra ecotone. Oikos. 2004;106:324–334. doi: 10.1111/j.0030-1299.2004.13224.x. [DOI] [Google Scholar]
- Olofsson J, Oksanen L, Callaghan T, Hulme EP, Oksanen T, Suominen O. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biology. 2009;15:2681–2693. doi: 10.1111/j.1365-2486.2009.01935.x. [DOI] [Google Scholar]
- Sandberg, G. 1963. Växtvärlden i Abisko nationalpark. In Natur i Lappland. II., ed. K. Curry-Lindahl, 885–909. Uppsala: Bokförlaget Svensk Natur (in Swedish).
- Shevtsova A, Jessen Graae B, Jochum T, Milbau A, Kockelbergh F, Beyens L, Nijs I. Critical periods for impact of climate warming on early seedling establishment in sub-arctic tundra. Global Change Biology. 2009;15:2662–2680. doi: 10.1111/j.1365-2486.2009.01947.x. [DOI] [Google Scholar]
- Sturm M, Racine C, Tape K. Climate change-increasing shrub abundance in the Arctic. Nature. 2001;411:546–547. doi: 10.1038/35079180. [DOI] [PubMed] [Google Scholar]
- Sturm M, Schimel J, Michaelson G, Welker JM, Oberbauer SF, Liston GE, Fahnestock J, Romanovsky VE. Winter biological processes could help convert arctic tundra to shrubland. BioScience. 2005;55:17–26. doi: 10.1641/0006-3568(2005)055[0017:WBPCHC]2.0.CO;2. [DOI] [Google Scholar]
- Tape K, Sturm M, Racine C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology. 2006;12:686–702. doi: 10.1111/j.1365-2486.2006.01128.x. [DOI] [Google Scholar]
- Tømmervik H, Johansen B, Tombre I, Thannheiser D, Hogda K, Gaare E. Vegetation changes in the Nordic mountain birch forest: The influence of grazing and climate change. Arctic, Antarctic, and Alpine Research. 2004;36:323–332. doi: 10.1657/1523-0430(2004)036[0323:VCITNM]2.0.CO;2. [DOI] [Google Scholar]
- Torp, M. 2010. The effects of snow on plants and their interactions with herbivores. PhD thesis, Umeå University, Umeå, Sweden.
- Trimble. 2008. Trimble survey controller software help, version 12.22. Revision A. April 2008.
- Truong C, Palme AE, Felber F. Recent invasion of the mountain birch Betula pubescens ssp. tortuosa above the tree line due to climate change: Genetic and ecological study in northern Sweden. Journal of Evolutionary Biology. 2006;20:369–380. doi: 10.1111/j.1420-9101.2006.01190.x. [DOI] [PubMed] [Google Scholar]
- Van Bogaert, R., K. Haneca, J. Hoogesteger, C. Jonasson, M. De Dapper, and T.V. Callaghan. 2011. A century of tree line changes in sub-Arctic Sweden show local and regional variability and only a minor role of 20th century climate warming. Journal of Biogeography. doi:10.1111/j.1365-2699.2010.02453.x.
- Bogaert R, Jonasson C, Dapper M, Callaghan TV. Competitive interaction between aspen and birch moderated by invertebrate and vertebrate herbivores and climate warming. Plant Ecology & Diversity. 2009;2:221–232. doi: 10.1080/17550870903487456. [DOI] [Google Scholar]
- Wahren CHA, Walker MD, Bret-Harte MS. Vegetation responses in Alaskan arctic tundra 8 years of a summer warming and winter snow manipulation experiment. Global Change Biology. 2005;11:537–552. doi: 10.1111/j.1365-2486.2005.00927.x. [DOI] [Google Scholar]
- Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, et al. Plant community response to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih M, Karlsson PS. Growth and nitrogen utilization in seedlings of mountain birch (Betula pubescens ssp. tortosa) as related to plant nitrogen status and temperature: A two-year study. Écosience. 1997;4:365–373. [Google Scholar]
- Yoccoz NG, Ims RA. Demography of small mammals in cold regions: The importance of environmental variability. Ecological Bulletins. 1999;47:137–144. [Google Scholar]
- Zakrisson C, Ericsson G, Edenius L. Effects of browsing on recruitment and mortality of European aspen (Populus tremula L.) Scandinavian Journal of Forest Research. 2007;22:324–332. doi: 10.1080/02827580701442186. [DOI] [Google Scholar]
- Zeidler M, Banas M, Zenata M. Ecological conditions and the distribution of alpine juniper in the Hruby Jesenik Mts. Biologia. 2009;64:687–693. doi: 10.2478/s11756-009-0068-9. [DOI] [Google Scholar]