Abstract
We report on a revisit in 2009 to sites where vegetation was recorded in 1967 and 1970 on Disko Island, West Greenland. Re-sampling of the same clones of the grass Phleum alpinum after 39 years showed complete stability in biometrics but dramatic earlier onset of various phenological stages that were not related to changes in population density. In a fell-field community, there was a net species loss, but in a herb-slope community, species losses balanced those that were gained. The type of species establishing and increasing in frequency and/or cover abundance at the fell-field site, particularly prostrate dwarf shrubs, indicates a possible start of a shift towards a heath, rather than a fell-field community. At the herb-slope site, those species that established or increased markedly in frequency and/or cover abundance indicate a change to drier conditions. This is confirmed both by the decrease in abundance of Alchemilla glomerulans and Epilobium hornemanii, and the drying of a nearby pond. The causes of these changes are unknown, although mean annual temperature has risen since 1984.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-011-0169-x) contains supplementary material, which is available to authorized users.
Keywords: Vegetation change, Arctic, West Greenland, Phenology, Biometrics
Introduction
Impacts of on-going and future climate change on Arctic ecosystems are receiving increasing attention because it is now widely acknowledged that global warming is amplified in the Arctic (ACIA 2005; Anisimov et al. 2007); Arctic ecosystems are particularly vulnerable to climate change (Callaghan et al. 1992, 2005; Chapin et al. 1992); changes in Arctic ecosystems and vegetation zones have major implications for global biodiversity, ecosystem services and climate regulation (Chapin et al. 2005); and impacts of climate change are already measurable (Post et al. 2009; Bhatt et al. 2010; Callaghan and Tweedie 2011 [this issue]). However, not all ecosystems and their components are responding to warming (Daniëls and de Molenaar 2011 [this issue]) as would be expected from many studies and from projections of ecosystem responses derived from modelling (Kaplan et al. 2003; Callaghan et al. 2005; Kaplan and New 2006; Wolf et al. 2008; Euskirchen et al. 2009). Also, some areas that indicate vegetation changes based on remote sensing techniques (e.g. Bhatt et al. 2010) are poorly represented by capacities for field validation, for example, in the Taymyr Peninsula of the Russian Arctic and on Greenland.
Exceptions to the general trend of range extension and increased plant performance are treeline and forest vegetation that have not responded to recent warming (Van Bogaert et al. 2009, 2011), low Arctic vegetation of south-east Greenland (Daniëls and de Molenaar 2011 [this issue]) and high Arctic vegetation on Svalbard (Prach et al. 2010). Furthermore, some observations of ecosystem change, such as recent advances of shrubs into the tundra, assume implicitly or explicitly that the vegetation dynamics are related to climate warming (e.g. Sturm et al. 2001; Tape et al. 2006; Hallinger et al. 2010), but this assumption can sometimes be challenged because many other factors, such as herbivory (e.g. Cairns and Moen 2004; Olofsson et al. 2009; Van Bogaert et al. 2011), extreme winter warming events (Bokhorst et al. 2009), and methodological constraints (Büntgen and Schweingrubber 2010) often confound simple cause–response relationships. An additional challenge to our understanding of responses of ecosystems and their components to climate change are preconceptions that sites, ecosystems and their components are often selected for study because they are expected to be sensitive to change. The literature is probably therefore, currently biased towards studies of treeline and shrub-line ecosystems. Further, an inappropriate ‘currency’ of response variables of plants to climate warming (Forchhammer et al., unpubl. results) is often chosen. For example, biomass and phenology are often measured to indicate responses of vegetation to climate change, but, total biomass, of which over 90% can be below-ground (Shaver and Cutler 1979), could give a significant buffering of inter-annual variations in above ground biomass. Phenology also has a low signal-to-noise ratio. Although phenology has changed significantly in some areas such as high Arctic north-east Greenland (Høye et al. 2007), the consequences for population and ecosystem dynamics are more difficult to record. For example, how important is earlier flowering for a plant species that recruits its populations mainly from clonal growth? Another common currency to indicate ‘greening of the Arctic’ is remotely sensed NDVI (Myneni et al. 1997; Jia et al. 2003; Bhatt et al. 2010), but this parameter records a mixture of leaf area index and plant species composition changes (Campioli et al. 2009) and may also be affected by surface water cover (Goswami et al. 2011).
In this study, we address some of the challenges and ambiguities in the data on plant and vegetation responses to climate change by revisiting sites on Disko Island, West Greenland. The sites were selected for baseline botanical studies in 1967 (Callaghan et al. 1976) and, therefore, independently of the climate change issue. The concept behind the study is that developed within the International Polar Year (IPY) ‘Back to the Future Project’ (Callaghan et al. 2011 [this issue]). Our aims were specifically to
re-establish study plots established and recorded in 1967 and 1970;
repeat measurements to identify changes or stability at the levels of the landscape, plant community, plant population and individual plant;
explore possible causes of change and/or stability;
preserve the sites and old and new data for future generations of researchers.
Study Sites
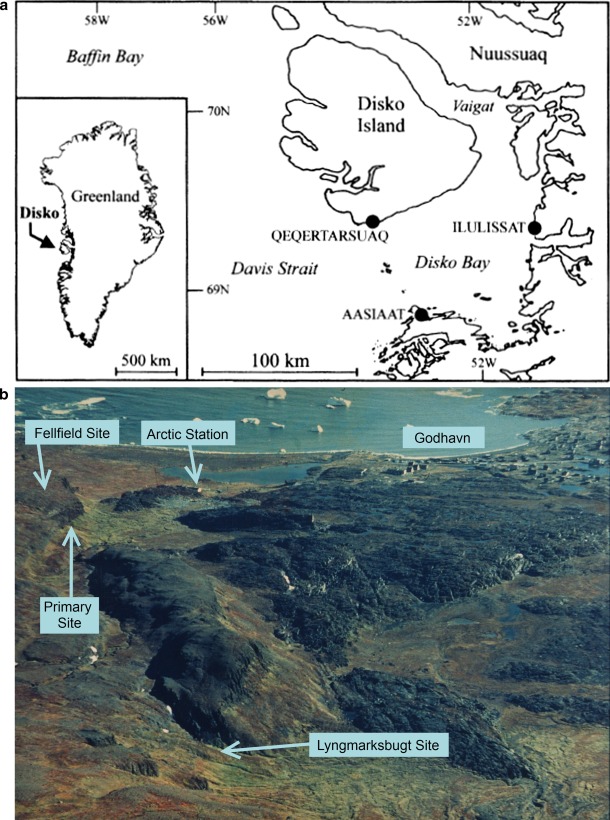
The sites were from the former International Biological Programme (IBP) Tundra Biome Bi-Polar Project (Callaghan et al. 1976; Bliss et al. 1981). They were located close to the Arctic Station (http://arktiskstation.ku.dk/english/), operated by the University of Copenhagen, at Qeqertarsuaq (Godhavn) on the south shore of Disko Island (also called Qeqertarsuaq) at 69°15′N, 53°34′W (Fig. 1a). The sites consisted of an area in Østerlien (Fig. 1b), ranging from a flat area at 75 m above sea level (asl) dominated by the shrub Salix glauca (all nomenclature follows Böcher et al. 1968) to a ridge at 128 m asl dominated by fell-field vegetation. The transition is a slope down which warm springs flow from basalt cliffs. These streams are lined by diverse and productive moss and herb communities. The Primary Site was a south-facing, sub-arctic slope supporting luxuriant closed vegetation of bryophytes, herbs and dense willow shrubs. This site is highly insolated and relatively sheltered from wind. The soil is predominantly humic, flushed and eutrophic; snow cover is thick during winter (up to several metres) and persistent until at least mid-June. An additional site, similar to the stream bank vegetation in Østerlien, was located at the foot of Lyngmarksfjellet (the Lyngmarksbugt Site: LB), and a snow bed site was also located there (Callaghan 1972, 1974). Details of the study sites are given in Table 1. Mean monthly temperatures vary by about 22.5°C and the period above 0°C is about 4.5 months (Hansen et al. 2006).
Fig. 1.
Location of study sites: a general area (illustration from Humlum 1998), b site locations, looking south from the top of Lyngmarkfjellet (photo: T.V. Callaghan 1970). Note that Godhavn = Qeqertarsuaq
Table 1.
Characteristics of the study sites
| Primary site | Fell-field site | Lyngmarksbugten site | |
|---|---|---|---|
| Characteristics | Sub-arctic slope supporting closed vegetation of herbs and Salix scrub, flushed by warm springs issuing from basalt rocks | Exposed gravely plateau. Vegetation sparse and scattered | Sub-arctic slope supporting closed vegetation of herbs and Salix scrub, flushed by warm springs issuing from basalt rocks |
| Location | 69°15.468′N, 53°31.188′W | 69°15.549′N, 53°31.190′W | 69°15.743′N, 53°32.009′W |
| Height above sea level (m) | 75 | 128 | 95 |
| Soil | Humic, flushed | Dry, gravelly | Humic, flushed |
| Vegetation | Low ridges dominated by S.glauca scrub separated by stream valleys lined with mosses and herbs | Fell-field community with low, sparse cover of herbs, prostrate dwarf shrubs, cushion plants and cryptogams | Banks of a stream lined with mosses and herbs rising into a higher area dominated by S.glauca scrub |
| Microclimate | S-facing slope with large accumulations of snow and warmth from perennially unfrozen stream | Wind-exposed ridge/plateau with little snow at times in winter and ice layers on the ground | S-facing slope with large accumulations of snow and warmth from perennially unfrozen stream. In partial shade from neighbouring rocks on some days in summer |
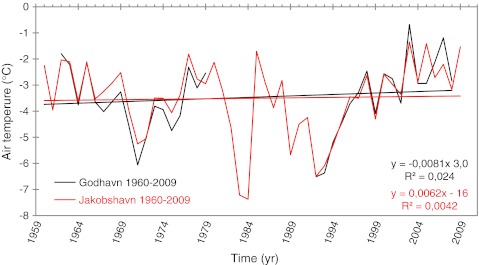
The climate has shown warming since 1984 (Hansen et al. 2006; Fig. 2) when the mean annual minimum is compared with the mean annual maximum. Although there is great inter-annual variability during this warming period, mean annual temperatures have been as high as, or higher than, the highest temperatures recorded since 1924 although over a 130-year period ending in 2004, the high temperatures are not unprecedented (Hansen et al. 2006). Another environmental change is the expansion of amenities for the town including extracting a water supply for the export of spring water from the Lyngmarksfjellet Site.
Fig. 2.
Climate characteristics at Qeqertarssuaq (Godhavn). Mean annual temperatures since 1959 for Godhavn and nearby Illulisat (Jacobshavn): the data for Jacobshavn indicate the temperatures expected at Godhavn between 1980 and 1993 when data at Godhavn are missing. Data from Hansen et al. (2006) and Hansen, pers. comm.
Past Data Sources
A challenge in multi-decadal projects such as this is to identify the original sites accurately and to access original publications, often in the grey literature, and original data. Fortunately, the project had access to an original researcher who worked at the sites in 1967 and 1970. Site re-identification was also facilitated by an 8-mm cine film (now digitised) of the sites and IBP research there in 1967 and 1970, and 35 mm slides (now digitised) taken in 1970. Most of the sites were relocated with great accuracy including individual clones of Phleum alpinum L. Detailed accounts of the first records of the sites were published formally by Callaghan (1974) and Clarke et al. (1971) but other results are included in the grey literature, particularly Callaghan’s 1972 PhD thesis (now digitised) and several IBP Tundra Biome workshop reports (e.g. Lewis and Callaghan 1970). In line with International Panel on Climate Change and International Polar Year recommendations, copies of the photographs and grey literature are held at the Abisko Scientific Research Station. Unfortunately, original data have been lost, and this constrains the statistical analyses of change between past and new measurements. A further constraint is that one site revisit after several decades gives only a snap-shot of changes with no information on inter-annual variations.
Methods
Landscape Scale
Repeat photography (8-mm cine film taken in July and August 1967, and from March to September 1970, and 35-mm slides taken throughout 1970) was employed to compare general landscape features and to aid site identification.
Plant Community Scale
Plant community composition was recorded at the original sites using the original methods as far as possible. At the Primary Site in late July 1967, cover abundance (±5%) was estimated from five 25 × 25 cm quadrats, randomly located within the vegetation dominated by a biologically diverse and productive stream bank vegetation, rather than within the closed S. glauca scrub between streams (Fig. 3). Percentage frequency was obtained from a further 25 randomly located 10 × 10 cm squares. Frequency was the percentage of small quadrats containing a given species, and cover abundance was visually estimated (±5%). On 26 July 2009, a more structured sampling design was applied in which an area of 8.5 m (across slope) × 12.5 m (down slope) was marked out in the same area as sampled in 1967. A grid was established with regular 1.5-m intervals across slope, and 2-5 m intervals down slope. Plant species’ cover abundance was estimated visually in twenty-five 25 × 25 cm squares located in the NW corner of each grid area and frequency was calculated from observations in 10 × 10 cm quadrats placed in the NW corner of each 25 × 25 cm quadrat.
Fig. 3.
Repeat photography of Østerlien, Disko Island. Top summer 1970 (photo T.V. Callaghan); bottom 28th July 2009 (photo T.R. Christensen)
At the Fell-field Site, in 1967, twenty-five 10 × 10 cm quadrats were randomly located over an area of approximately 10 × 10 m. Plant species frequency was calculated. Cover abundance was estimated visually in five randomly located 25 × 25 cm quadrats. On 26 July 2009, a grid of 12 × 12 m was established in an area enclosing that sampled in 1967 (Fig. 4). Sixteen 25 × 25 cm quadrats were located in the NW corner of each 3 m grid cell. In addition, four extra quadrats were located randomly to make a sample size of twenty. Frequency of plant species was estimated in these 25 × 25 cm quadrats and cover abundance was estimated in fifty 10 × 10 cm squares within the larger quadrats. Species formerly present but absent in the sample quadrats in 2009 were sought and, if found, recorded within the overall area, i.e. outside the quadrats. For analytical purposes, these were represented by a nominal 1% frequency value to ensure their presence but rare occurrence.
Fig. 4.
Repeat photography of the Fell-field Site above Østerlien. The semi-transparent frames are from an 8-mm cine film taken in 1967 (T.V. Callaghan) while the background photo was taken on 25 July 2009 by T.R. Christensen. E.J. Jantze arranged the composite picture. In the picture are Dr. Martin Lewis (centre), now deceased, and Elin J. Jantze (extreme right) and Terry Callaghan
Identification of some species was difficult and aggregate groups were therefore established. These include for the Fell-field Site ‘other mosses’ that excluded Polytrichum piliferum and Rhacomitrium lanuginosum, and ‘Carex spp.’ that included C. rupestris and C. capillaris. Because data were not available for individual quadrats in 1970, it is not known if the species that were merged co-occurred in quadrats or were mutually exclusive. To detect change in 2009 therefore, both upper and lower limits of frequency in 1970 were calculated by assuming total mutual exclusion and 100% co-occurrence respectively. At the Primary Site, Philonotis tomentella that had 1% frequency in 1967 was not separated in 2009 from Pohlia wahlenbergii that had large frequencies in both 1967 and 2009.
Plant Population Scale
Population characteristics were compared over time by comparing the number of flowering tillers per m2. In 1970, the number of flowering tillers occurring in 50 random 25 × 25 cm quadrats was counted at the LB Site. On 29 July of 2009, the number of flowering tillers was counted at the Primary Site in eight 25 × 25 cm randomly distributed quadrats where P. alpinum was present, and at the LB Site, it was estimated in 12 quadrats used for the floristic description (not presented here).
Individual Plant Scale
Comparisons of biometrics and phenology were made for P. alpinum between 1970 and 2009 following the same methodology described in Callaghan (1972, 1974). Biometric measurements were made on 25 randomly selected tillers from the LB and Primary Site in 1970 and on 15 tillers from sixteen 25 × 25 cm quadrats at the LB site on 28 and 29 July 2009. Measurements were also made on an additional 24 tillers randomly selected from the eight 25 × 25 cm quadrats used for the floristic descriptions of the Primary Site. Measurements consisted of the maximum length and width of the inflorescence, length of the youngest internode on a flowering tiller, flowering tiller height, length of youngest leaf on the flowering tillers and length of the longest leaf on the vegetative tillers, and number of living leaves per vegetative tiller.
Phenological observations were made on the same clones studied in the 1960s by measuring several variables on 28 July 2009 and estimating from observations the dates in 1970 on which the same values were obtained. In 1970, comparisons were made between three widely separated permanent quadrats at the LB Site spaced along a stream bank. The quadrats had growing seasons of differing lengths and the development of five flowering tillers per sampling site was monitored. Initially tillers at the same developmental stage were selected; this stage was when ¼ of the inflorescence was still enclosed by the youngest leaf sheath. Only ‘Quadrat A’ plants remained in 2009, and these were re-sampled. This sub-site was the first to emerge from the snow in spring and in 1970 the growing season length was 98 days. The variables consisted of a sexual maturity index estimated from a visually determined arbitrary index of the proportion of flowers in an inflorescence that had exerted anthers and styles and a senescence index estimated for the youngest leaves of flowering tillers (Supplementary Table 1). Also, the lengths of the youngest internodes and number of living leaves were measured. For the measurements in 2009, we sampled the same tillers used for biometric measurements.
Results
Landscape Scale
The general landscape had changed relatively little over 40 years: S. glauca still dominated the plateau and hill slopes, fell-field communities still occupied the ridge top and productive, biologically diverse stream bank vegetation still existed in almost identical locations (Fig. 3). However, there was one significant change in 2009 in that a pond on the valley floor was now much reduced in extent and Eriphorum sp. was establishing in areas where the pond had dried. The Fell-field Site had also apparently changed little: it was still dominated by low-growing fell-field vegetation, and approximately the same area of vegetation proportional to bare ground existed (Fig. 4).
Plant Community Scale
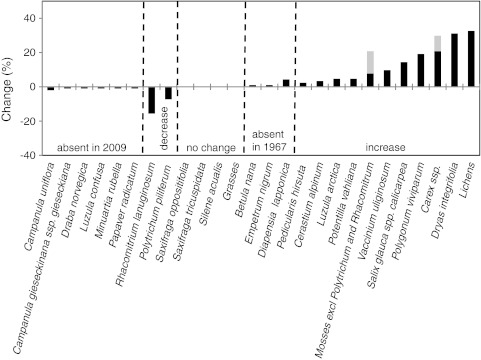
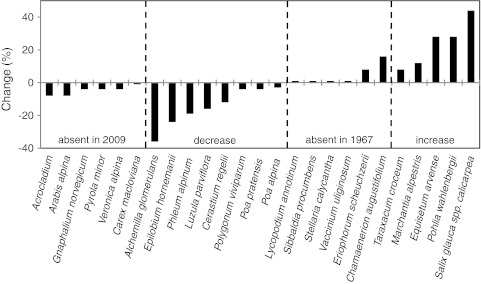
At the Fell-field Site, three species were recorded in 2009 that were not recorded in 1967 (Fig. 5; Supplementary Table 2). However, six species present in 1967 were not recorded in 2009. Eleven species/species aggregates increased their frequencies between 1967 and 2009 whereas only two species (both mosses) decreased in frequency. The greatest increases in frequency (Dryas integrifolia and lichens) were in the order of 30%, and the greatest decrease was about 16%. Estimates of changes in percentage cover of species at the Fell-field Site between 1967 and 2009 (Fig. 6; Supplementary Table 3) showed that there were seven species less in the cover analysis plots than in 1967. However, one of these, Saxifraga tricuspidata, was recorded (but at very low frequency) in the area in 2009 (Fig. 6; Supplementary Table 3). This currently infrequent species at the site had a significant cover (5%) in 1967. Of those species that showed decreases in cover by 2009, only two species (Luzula arctica and the Carex species aggregate) had significant cover values in 1967. Betula nana and Diapensia lapponica had cover values of 7 and 10%, respectively, in 2009, but were not recorded in the cover analysis plots in 1967. The two prostrate dwarf shrubs D. integrifolia and Vaccinium uliginosum increased substantially in cover between 1967 and 2009 (Fig. 6; Supplementary Table 3). About 50% of the ground surface was bare or had mosses and lichens in 2009.
Fig. 5.
Percentage frequency change of species at the Fell-field Site between 1967 and 2009. Note: a the grasses category is probably Poa glauca that was identified in 1967, b for the mosses and Carex spp. aggregates, the grey bars denote the upper limit of change, while the black bars denote the lower limit of change. See Supplementary Table 2 for actual frequency values
Fig. 6.
Changes in percentage cover abundance values for the Disko Island Fell-field site excluding bare ground and rocks between 1967 and 2009
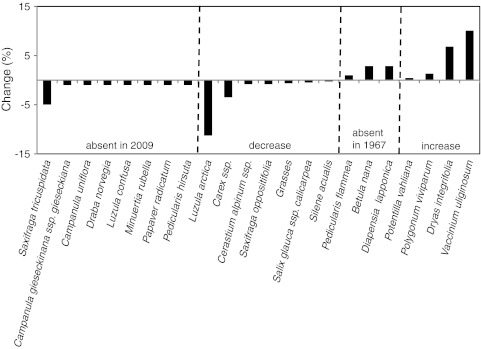
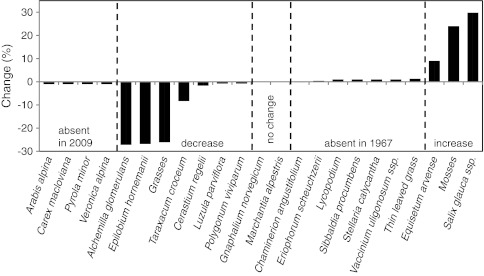
In contrast to the Fell-field Site, no bare ground was present at the Primary Site. Six species present in 1967 were not recorded in 2009 at the Primary Site (Fig. 7; Supplementary Table 4), and there were also six species found in 2009 that were not recorded in 1967. Species that had been present earlier but were not recorded in 2009 had previously been quite frequent (maximum of 8% for the moss Acrocladium sp. and Arabis alpina) (Fig. 7). The six species that appeared since 1967 also had high frequencies (16% for Chamaenerion angustifolium). Almost equal numbers of species showed an increase compared with a decrease in frequency between 1967 and 2009 and many changes in frequency were high (between 15 and 40%). Changes in cover abundance were also high, particularly when compared with the Fell-field Site: five species at the Primary Site had recorded differences of between 20 and 30% (Fig. 8).
Fig. 7.
Change in percentage frequency at the Primary Site between 1967 and 2009. Note that P. tomentella recorded at a very low frequency in 1967 was not distinguished from P. wahlenbergii in 2009. See Supplementary Table 4 for actual frequency values
Fig. 8.
Change in percentage cover at the Primary Site between 1967 and 2009
Plant Population Scale
The number of flowering tillers per m2 at the LB Site was 19.8 in 1970 and 21.3 ± 11.5 (standard error) in 2009. Flowering tiller density at the Primary Site was 104.3 ± 19.7 (standard error) and therefore much higher than at the LB Site in 2009. However, it was not measured at the Primary Site in 1970.
Individual Plant Scale
In 2009, the biometrics of P. alpinum was measured on the same individual clones as in 1970: these were the Quadrat A clones in Callaghan (1972, 1974) that were the only clones to remain undisturbed at the LB Site. Comparisons over the 39 years showed surprisingly little variation in the sizes of inflorescence, youngest internode and youngest leaf and the number of leaves per vegetative tiller (Table 2). Differences ranged between 0.5 (inflorescence width) and 12.4% (length of longest leaf per vegetative tiller). Although variance estimates are not available for 1970, the standard errors associated with the 2009 values suggest no statistically significant differences in most of the biometric comparisons between 1970 and 2009.
Table 2.
Biometric comparisons of P. alpinum between 1970 and 2009 for the LB site
| Variable | Value 2009 | Value 1970 |
|---|---|---|
| Flowering tillers | ||
| Inflorescence width (mm) | 11.9 ± 0.25 | 12.4 |
| Inflorescence length (mm) | 30.9 ± 1.32 | 30.7 |
| Length of youngest internode (mm) | 167.7 ± 8.3 | 167.3 |
| Flowering tiller height | 311.3 ± 15.4 | 343.8 |
| Number of living leaves per tiller | 2.4 ± 0.18 | 3.1 |
| Length of youngest leaf on flowering tiller (mm) | 35.9 ± 2.98 | 33.3 |
| Vegetative tillers | ||
| Number of leaves per vegetative tiller | 3.2 ± 0.19 | 3.3 |
| Length of longest leaf per vegetative tiller (mm) | 106.9 ± 9.67 | 94.5 |
Values for 1970 interpolated from graphs in Callaghan (1972). Means plus and minus standard errors (2009) are presented
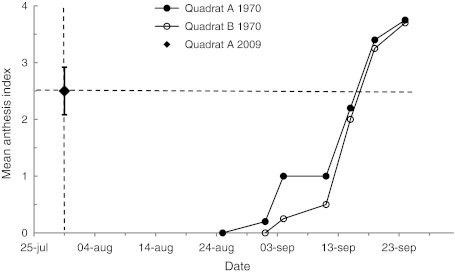
Measurements of phenological variables on one occasion during the 2009 growing season were compared with the seasonal development measured in the 1970 growing season. These comparisons showed that reproductive and vegetative phenology (the latter not shown) had greatly advanced between 1970 and 2009 by up to 48 days (Fig. 9). The anthesis index of 2.5 ± 0.42 recorded on 30 July 2009 was not achieved until 16 September in 1970 whereas in 1970 the value on 30 July was 0 (Callaghan 1972). Similarly, the length of the youngest internode on a flowering tiller was 167.7 ± 9.7 mm on 30 July 2009 whereas it did not achieve this length until 29 August in 1970, and on 30 July 1970 the length was only 97 mm.
Fig. 9.
Example of data for comparisons of phenology. The seasonal phenological development of anthesis index is presented for 1970 (Callaghan 1972) together with the mean value ± standard error observed on 30 July 2009. The difference between the date in 2009 and the date in 1970 when the same value was observed gives the difference in phenological development between the two periods. Estimates for two sub-sites are given for the 1970 data to illustrate variation due to differences in the start of the 1970 growing season (9 days earlier in Quadrat A)
Discussion
Three major issues that emerge from this study are as follows:
Landscape-scale changes in the immediate area studied are happening mainly through the reduction in area of a pond as shown in many areas of arctic North America and Siberia.
The two plant communities were superficially unchanged, but there was net loss of species and colonisation by species that indicated an early transition to a drier habitat at the Primary Site and an early shift to a heath at the Fellfield site.
At the individual plant and population level, phenology of P. alpinum appeared to be irrelevant: although large phenological changes occurred, they had no impact on plant performance in terms of number of tillers m−2 or sexual reproductive capacity.
Landscape Scale Changes
Changes in the local landscape were dominated by the reduction in the size of the small lake. Although the change is based on only two snap-shots separated by 39 years, and inter-annual variation between these snap-shots could be significant, the establishment of an Eriophorum species where the lake had previously been suggests that the change is indeed a long-term phenomenon. Drying of lakes and ponds has been recorded at many locations in the Arctic including high Arctic Canada (Smol and Douglas 2007), Alaska (Hinzman et al. 2005), Siberia (Smith et al. 2005) and in south-east Greenland (Daniëls and de Molenaar 2011 [this issue]). The causes, which vary among locations, include increased evaporation as the duration of winter ice cover decreases and summer temperatures increase, permafrost thaw and drainage and decreased snow cover.
In contrast to the changes in the small lake and colonisation by an Eriophorum species, the major vegetation zones/plant community distribution had remained largely unchanged: S. glauca scrub still dominated the valley floor and lower slopes of the eastern valley wall, and the ridge was still dominated by fell-field vegetation. This contrasts with many areas of the Arctic where shrub expansion has increased substantially [Sturm et al. 2001; Tape et al. 2006; Danby and Hik 2007; Forbes et al. 2009; Hallinger et al. 2010; several papers in this issue; but see Prach et al. 2010; Daniëls and de Molenaar 2011 [this issue]; Jonsdottir et al. 2005 (moss-dominated community) for findings similar to those presented here). However, less is known about the causes of shrub expansion (Büntgen and Schweingrubber 2010] and changes in herbivory could have played a role (Cairns and Moen 2004; Olofsson et al. 2009). On Disko Island, large recent changes in temperature are probably not of sufficient duration to significantly alter vegetation structure yet. Also, herbivory is probably relatively stable because of intensive hunting by the local community.
Plant Community Scale
Superficially, the communities at the two sites appeared to have changed little over a period of 39–42 years: the Primary Site and LB Site were still dominated by a dense moss matrix of P. wahlenbergii that supported herbs and was delineated by S. glauca, and the Fell-field Site still had a cover of only about 50% and was dominated by a mixture of prostrate dwarf shrubs, cushion forbs, graminoids, mosses and lichens. On closer inspection, there had been a net loss in biodiversity. At each of the Fell-field and Primary Sites, six species present in 1967/1970 were not recorded in 2009 whereas six species at the Primary Site and three at the Fell-field Site appeared to have colonised since 1967. The types of species establishing and increasing in frequency and/or cover abundance at the Fell-field Site, such as B. nana, S. glauca, D. integrifolia, Empetrumnigrum and V. uliginosum, as well as the type of species apparently lost, such as Papaver radicatum and Campanula spp. indicate a possible start of a shift towards a heath, rather than a fell-field community (sensu Böcher et al. 1968).
At the Primary Site, those species that established or increased markedly in frequency and/or cover abundance (e.g. S. glauca, Chamaenerion angustifoilium, Lycopodium annotinum, Vaccinium uliginsoum) indicate a change to drier conditions. This is confirmed by the decrease in abundance of Alchemilla glomerulans and Epilobium hornemanii. The causes of these changes are unknown. However, because of the long-lived and clonal nature of almost all the species, the changes over time are not likely to be artefacts of inter-annual variations. The longer term changes could, though, be part of natural succession processes that pre-date recent climate warming, or a response to this warming (Fig. 2) and drying as indicated by the pond shrinkage (Fig. 3) and species change at the Primary Site. Although there is a possibility that there were errors in recording in the past and/or present, this is unlikely to have influenced the results significantly since many of the species that have been gained or lost are easy to see and they were/are now very frequent.
Population Scale
Phleum alpinum still retained the same habitat preferences as it had at least 42 years earlier, i.e. in stream banks dominated by P. wahlenbergii in the moss layer and with herbs and grasses characteristic of wet areas (Böcher et al. 1968; Callaghan 1972, 1974). A visual impression was that it was more widespread in 2009. Although there are no data to compare this with in 1970, plant ecologists had failed to find the species between 1970 and 2009 (S. Jonasson and U. Molau, pers. comm.) suggesting that it was not widespread then. When the same patches were located at the LB Site, tiller density had remained almost identical over the 39 years. However, tiller density was almost ten times higher at the Primary Site in 2009 compared with the LB Site. Although there are no data for 1967, a main reason for the establishment of the study site for P. alpinum at the LB Site was because the grass was relatively rare at the Primary Site and sensitive to damage during recording. It is therefore very likely that P. alpinum has spread significantly at the Primary Site over the past decades.
Individual Plant Scale
It is unusual to revisit the same clones of a grass after 39 years. The biometric characteristics were surprisingly stable. For example, inflorescence width and length varied by only −4% (−0.5 mm) and 1% (0.2 mm), respectively, and this can indicate that we had indeed sampled the same population—even clones—as earlier. In contrast, however, phenological characters had changed dramatically: anthesis (% of flowers/inflorescence in anthesis) was 48 days earlier than in 1970 and this has occurred within a short growing season (only ca. 98 days in 1970: Callaghan 1974). Similar dramatic advances in phenology have been recorded at Zackenberg in Northeast Greenland (Høye et al. 2007), but over a much shorter period (10 years). Other studies exist showing either no overall trend over a 11-year period (Molau et al. 2005) or smaller advances in phenology as a result of experimental increases in air temperature within the ITEX (International Tundra Experiment) project (Henry and Molau 1997; Arft et al. 1999). However, no other studies in the Arctic have apparently shown that large advances in phenology are insignificant in terms of performance of the individual plant and local population density. This is surprising as a marked spread of the grass was inferred at the Primary Site and maximum density and growth indicated by studies at other sites had not been achieved (Callaghan 1974). Furthermore, Callaghan (1972, 1974) showed that the completion of the flowering cycle with production of viable seed was strongly related to growing season length and that following apparently long growing seasons before 1970, P. alpinum had established in uncharacteristic habitats including even a snow bed site with a very short growing season of 42 days in 1970. However, examination of this and other neighbouring sites failed to find any P. alpinum, despite the earlier penology and increased chances of seed set seen in 2009. Furthermore, inspection of 10 inflorescences produced in 2008 failed to find any viable seed.
The changes demonstrated in phenology again represent two snap-shots widely separated in time and any inter-annual variability is unknown. However, the lack of association between phenological advancement, increased plant performance and population density argue for extreme caution of the frequent use of reproductive phenological variables as ‘indicators’ of plant responses to climate change, although genetic analysis would be needed to confirm this.
The rather small changes in plant community structure recorded here compared with many dramatic changes published earlier for the Arctic (e.g. Sturm et al. 2001; Tape et al. 2006) establish some balance. On one hand, the relatively small changes could reflect relatively small long-term changes in climate on Disko Island (Hansen et al. 2006), whereas it might simply reflect that some recent publications that record dramatic changes are perhaps biased by selection of sites since climate warming became an issue. However, although the community changes were relatively small, the overall loss of biodiversity and tendency towards later successional stages might indicate the start of more important future changes that will reinforce modern trends of major loss of biodiversity (Butchart et al. 2010) and projections of plant community change in the Arctic (Kaplan et al. 2003; Sitch et al. 2003; Callaghan et al. 2005; Kaplan and New 2006; Wolf et al. 2008; Euskirchen et al. 2009).
Electronic supplementary material
Acknowledgments
This study formed part of the IPY project ‘Back to the Future’ (http://www.btf.utep.edu/). and the authors are grateful to our colleagues Professor Pat Webber and Dr. Craig Tweedie for collaboration in the establishment of this project. Dr. Thomas Bjoernebo Berg kindly helped with arrangements for the field visit and we would like to thank the Arctic Station for welcoming us and providing facilities. Of course the project would have been impossible without the formative studies of the others who established the IBP Tundra Biome Bi-Polar Botanical Project—Dr. S.W. Greene, Mrs. D.M. Greene and Dr. M.C. Lewis (all now deceased), and Dr. G.C.S. Clarke and B. Philipps. The project was financed by a grant from the Swedish Research Council (Vetenskapsrådet) 327-2007-833 as part of the International project ‘Retrospective and prospective vegetation change in the polar regions: Back to the Future’ Project (BTF; IPY Project number ID No 512).
Biographies
Terry V. Callaghan
is a Distinguished Research Professor at the Royal Swedish Academy of Sciences and Professor of Arctic Ecology at the Universities of Sheffield, UK and Lund, Sweden. He specializes in arctic ecology, and climate and UV-B radiation impacts on arctic ecosystems.
Torben R. Christensen
is Professor at the Department of Earth and Ecosystem Sciences, Lund University, Sweden and at the Greenland Climate Research Centre, Nuuk, Greenland. He specializes in how vegetation and ecosystem processes respond and provide feedback effects on climate under changing environmental conditions.
Elin J. Jantze
is a PhD student at the Department of Physical Geography and Quaternary Geology of Stockholm University, Sweden. She specialises on the role of the cryosphere, hydrological and ecosystem shifts for arctic hydro-climatic interactions and carbon fluxes.
Contributor Information
Terry V. Callaghan, Email: terry_callaghan@btinternet.com
Torben R. Christensen, Email: torben.christensen@nateko.lu.se
Elin J. Jantze, Email: elin.jantze@natgeo.su.se
References
- Arctic climate impact assessment. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Anisimov, O.A., D.G. Vaughan, T.V. Callaghan, C. Furgal, H. Marchant, T.D. Prowse, H. Vilhjálmsson, and J.E. Walsh. 2007. Polar regions (Arctic and Antarctic). In Climate Change 2007: Impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, ed. M.L. Parry, O.F. Canziani, J.P. Palutikof, C.E. Hanson, and P.J. van der Linden, 655–685. Cambridge: Cambridge University Press.
- Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, Dale M, Diemer M, Gugerli F, et al. Responses of tundra plants to experimental warming: Meta-analysis of the international tundra experiment. Ecological Monographs. 1999;69:491–511. [Google Scholar]
- Bhatt US, Walker DA, Raynolds MK, Comiso JC, Epstein HE, Jia G, Gens R, Pinzon JE, et al. Circumpolar Arctic tundra vegetation change is linked to sea ice decline. Earth Interactions. 2010;14:1–20. doi: 10.1175/2010EI315.1. [DOI] [Google Scholar]
- Bliss LC, Heal OW, Moore JJ. Tundra ecosystems: A comparative analysis. Cambridge, UK: Cambridge University Press; 1981. [Google Scholar]
- Böcher TW, Holmen K, Jacobsen K. The flora of Greenland. Copenhagen: P. Haase & Son; 1968. [Google Scholar]
- Bokhorst S, Bjerke JW, Tømmervik H, Callaghan TV, Phoenix GK. Winter warming events damage sub-Arctic vegetation: Consistent evidence from an experimental manipulation and a natural event. Journal of Ecology. 2009;97:1408–1415. doi: 10.1111/j.1365-2745.2009.01554.x. [DOI] [Google Scholar]
- Büntgen U, Schweingrubber FH. Environmental change without climate change? New Phytologist. 2010;188:646–651. doi: 10.1111/j.1469-8137.2010.03342.x. [DOI] [PubMed] [Google Scholar]
- Butchart SHM, Walpole M, Collen B, Strien A, Scharlemann JPW, Rosamunde I, Almond EA, Baillie JEM, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- Cairns DM, Moen J. Herbivory influences tree lines. Journal of Ecology. 2004;92:1019–1024. doi: 10.1111/j.1365-2745.2004.00945.x. [DOI] [Google Scholar]
- Callaghan, T.V. 1972. Ecophysiological and taxonomic studies on bipolar Phleum alpinum L. PhD thesis, University of Birmingham.
- Callaghan TV. Intraspecific variation in Phleum alpinum L. with special reference to polar populations. Arctic Alpine Research. 1974;6:361–401. doi: 10.2307/1550331. [DOI] [Google Scholar]
- Callaghan, T.V., and C.E. Tweedie (eds.) 2011. Multi-decadal changes in Tundra environments and ecosystems—The International Polar Year Back to the Future Project. Ambio Special Issue 40(6). [DOI] [PMC free article] [PubMed]
- Callaghan, T.V., C.E. Tweedie, and P.J. Webber. 2011. Multi-decadal changes in Tundra environments and ecosystems: The International Polar Year Back to the Future Project (IPY-BTF). Ambio. doi:10.1007/s13280-011-0162-4. [DOI] [PMC free article] [PubMed]
- Callaghan TV, Björn LO, Chernov Y, Chapin FS, Christensen TR, Huntley B, Ims R, Jonasson S, et al. Tundra and polar desert ecosystems. In: Symon C, Arris L, Heal B, et al., editors. ACIA Arctic climate impacts assessment. Cambridge: Cambridge University Press; 2005. pp. 243–352. [Google Scholar]
- Callaghan TV, Smith RIL, Walton DWH. The IBP bipolar botanical project. Philosophical Transactions of the Royal Society London Biological Sciences. 1976;274:15–319. [Google Scholar]
- Callaghan TV, Sonesson M, Somme L. Responses of terrestrial plants and invertebrates to environmental change at high latitudes. Philosophical Transactions of the Royal Society London Biological Sciences. 1992;338:279–288. doi: 10.1098/rstb.1992.0148. [DOI] [Google Scholar]
- Campioli M, Street LE, Michelse A, Shaver GR, Maere T, Samson R, Lemeur R. Determination of Leaf Area Index, total foliar nitrogen, and Normalised Difference Vegetation Index for arctic ecosystems dominated by Cassiope tetragona L. Arctic, Antarctic, and Alpine Research. 2009;41(4):426–433. doi: 10.1657/1938-4246-41.4.426. [DOI] [Google Scholar]
- Chapin FS, III, Berman M, Callaghan TV, Convey P, Crepin ASE, Danell K, Ducklow H, Forbes B. Polar ecosystems. In: Hassan R, Scholes R, Ash N, editors. Ecosystems, human well-being: Current state, trends. Washington: Island Press; 2005. pp. 719–743. [Google Scholar]
- Chapin FS, III, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J. Arctic ecosystems in a changing climate: An ecophysiological perspective. San Diego: Academic Press; 1992. [Google Scholar]
- Clarke, G.C.S., S.W. Greene, and D.M. Greene. 1971. Productivity of bryophytes in polar regions. Annals of Botany 35: 99–108.
- Danby RK, Hik DS. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. Journal of Ecology. 2007;95:352–363. doi: 10.1111/j.1365-2745.2006.01200.x. [DOI] [Google Scholar]
- Daniëls, F.J.A., and J.G. de Molenaar. 2011. Flora and vegetation of Tasiilaq, Formerly Angmagssalik, Southeast Greenland—a comparison of data from between around 1900 and 2007. Ambio. doi:10.1007/s13280-011-0171-3. [DOI] [PMC free article] [PubMed]
- Euskirchen ES, McGuire AD, Chapin FSIII, Yi S, Thompson CC. Changes in vegetation in northern Alaska under scenarios of climate change 2003–2100: Implications for climate feedbacks. Ecological Applications. 2009;19:1022–1043. doi: 10.1890/08-0806.1. [DOI] [PubMed] [Google Scholar]
- Forbes B, Fauria M, Zetterberg P. Russian Arctic warming and greening are closely tracked by tundra shrub willows. Global Change Biology. 2009;15(7):1–13. [Google Scholar]
- Goswami, S., J.A. Gamon, and C.E. Tweedie. 2011. Surface hydrology of an arctic ecosystem: Multiscale analysis of a flooding and draining experiment using spectral reflectance. Journal of Geophysical Research 116: G00I07. doi:10.1029/2010JG001346.
- Hallinger M, Manthey M, Wilmking M. Establishing a missing link: Warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist. 2010;186:890–899. doi: 10.1111/j.1469-8137.2010.03223.x. [DOI] [PubMed] [Google Scholar]
- Hansen BU, Elberling B, Humlum O, Nielsen N. Meteorological trends (1991–2004) at Arctic Station, Central West Greenland (69°15′N) in a 130 year perspective. Danish Journal of Geography. 2006;106(1):45–55. [Google Scholar]
- Henry GHR, Molau U. Tundra plants and climate change: The International Tundra Experiment (ITEX) Global Change Biology. 1997;3(S1):1–9. doi: 10.1111/j.1365-2486.1997.gcb132.x. [DOI] [Google Scholar]
- Hinzman, L.D., N.D. Bettez, W.R. Bolton, F.S. Chapin, N.B. Dyurgerov, C.L. Fastie, B. Griffith, R.D. Hollister, et al. 2005. Evidence and implications of recent climate change in northern Alaska and other arctic regions. Climatic Change 72: 251–298.
- Høye TT, Post E, Meltofte H, Schmidt NM, Forchhammer MC. Rapid advancement of spring in the High-Arctic. Current Biology. 2007;17:R449–R451. doi: 10.1016/j.cub.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Humlum O. Active layer thermal regime 1991–1996 at Qeqertarsuaq, Disko Island, Central West Greenland. Arctic and Alpine Research. 1998;30(3):295–305. doi: 10.2307/1551977. [DOI] [Google Scholar]
- Jia GJ, Epstein HE, Walker DA. Greening of arctic Alaska, 1981–2001. Geophysical Research Letters. 2003;30:2067. doi: 10.1029/2003GL018268. [DOI] [Google Scholar]
- Jonsdottir IS, Magnusson B, Gudmundsson J, Elmarsdottir A, Hjartason H. Variable sensitivity of plant communities in Iceland to experimental warming. Global Change Biology. 2005;11(4):553–563. doi: 10.1111/j.1365-2486.2005.00928.x. [DOI] [Google Scholar]
- Kaplan JO, Bigelow NH, Prentice IC, Harrison SP, Bartlein PJ, Christensen TR, Cramer W, Matveyeva NV, et al. Climate change and Arctic ecosystems: 2. Modeling, paleodata-model comparisons, and future projections. Journal of Geophysical Research. 2003;108(D19):8171. doi: 10.1029/2002JD002559. [DOI] [Google Scholar]
- Kaplan JO, New M. Arctic climate change with a 2°C global warming: Timing, climate patterns and vegetation change. Climatic Change. 2006;79:213–241. doi: 10.1007/s10584-006-9113-7. [DOI] [Google Scholar]
- Lewis MC, Callaghan TV. Bipolar botanical project: Primary productivity studies on Disko Island, West Greenland. In: Heal OW, editor. Tundra biome working meeting on analysis of ecosystems, Kevo, Finland. London: Tundra Biome Steering Committee; 1970. pp. 34–50. [Google Scholar]
- Molau U, Nordenhall U, Eriksen B. Onset of flowering and climate variability in an alpine landscape: A 10-year study from Swedish Lapland. American Journal of Botany. 2005;92(3):422–431. doi: 10.3732/ajb.92.3.422. [DOI] [PubMed] [Google Scholar]
- Myneni RB, Keeling CD, Tucker CJ, Asra G, Nemani RR. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. 1997;386:698–702. doi: 10.1038/386698a0. [DOI] [Google Scholar]
- Olofsson J, Oksanen L, Callaghan TV, Hulme PE, Oksanen T, Suominen O. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biology. 2009;15:2681–2693. doi: 10.1111/j.1365-2486.2009.01935.x. [DOI] [Google Scholar]
- Post E, Forchhammer MC, Bret-Harte S, Callaghan TV, Christensen TR, Elberling B, Fox AD, Gilg O, et al. Ecological dynamics across the Arctic associated with recent climate change. Science. 2009;325:1355–1358. doi: 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- Prach K, Kosnar J, Klimesova J, Hais M. High Arctic vegetation after 70 years: A repeated analysis from Svalbard. Polar Biology. 2010;33:635–639. doi: 10.1007/s00300-009-0739-6. [DOI] [Google Scholar]
- Shaver GR, Cutler JC. The vertical distribution of live vascular phytomass in cottongrass tussock tundra. Arctic and Alpine Research. 1979;11:335–342. doi: 10.2307/1550421. [DOI] [Google Scholar]
- Sitch S, Smith B, Prentice I, Arneth A, Bondeau A, Cramer W, Kaplan J, Levis S, et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Global Change Biology. 2003;9(2):161–185. doi: 10.1046/j.1365-2486.2003.00569.x. [DOI] [Google Scholar]
- Smith LC, Sheng Y, MacDonald GM, Hinzman LD. Disappearing Arctic lakes. Science. 2005;308:1429. doi: 10.1126/science.1108142. [DOI] [PubMed] [Google Scholar]
- Smol JP, Douglas MSV. Crossing the final ecological threshold in high Arctic ponds. Proceedings of the National Academy of Sciences. 2007;104(30):12395–12397. doi: 10.1073/pnas.0702777104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm M, Racine C, Tape K. Climate change—increasing shrub abundance in the Arctic. Nature. 2001;411:546–547. doi: 10.1038/35079180. [DOI] [PubMed] [Google Scholar]
- Tape K, Sturm M, Racine C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology. 2006;12:686–702. doi: 10.1111/j.1365-2486.2006.01128.x. [DOI] [Google Scholar]
- Bogaert R, Haneca K, Hoogesteger J, Jonasson C, Dapper M, Callaghan TV. A century of tree line changes in sub-Arctic Sweden show local and regional variability and only a minor role of 20th century climate warming. Journal of Biogeography. 2011;38:907–921. doi: 10.1111/j.1365-2699.2010.02453.x. [DOI] [Google Scholar]
- Bogaert R, Jonasson C, Dapper M, Callaghan TV. Competitive interaction between aspen and birch moderated by invertebrate and vertebrate herbivores and climate warming. Plant Ecology and Diversity. 2009;2:221–232. doi: 10.1080/17550870903487456. [DOI] [Google Scholar]
- Wolf AK, Larsson K, Callaghan TV. Future vegetation changes in the Barents Region. Climatic Change. 2008;87:51–73. doi: 10.1007/s10584-007-9342-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.