Abstract
Greening of the Arctic due to climate warming may provide herbivores with richer food supplies, resulting in higher herbivore densities. In turn, this may cause changes in vegetation composition and ecosystem function. In 1982–1984, we studied the ecology of non-breeding moulting geese in Jameson Land, low Arctic East Greenland. By then, geese consumed most of the graminoid production in available moss fens, and it appeared that the geese had filled up the available habitat. In 2008, we revisited the area and found that the number of moulting geese and the temperature sum for June–July had tripled, while the above-ground biomass in a moss fen ungrazed by geese had more than doubled. In a goose-grazed fen, the overall plant composition was unchanged, but the frequency of graminoids had decreased and the area with dead vegetation and open spots had increased. We suggest that climate warming has lead to increased productivity, allowing for higher numbers of moulting geese. However, the reduction of vegetation cover by grazing may have longer term negative consequences for the number of geese the habitat can sustain.
Keywords: Barnacle geese, Carex, Climate change, Grazing impact, Herbivory, Pink-footed geese
Introduction
During the last decades, it has been shown by satellite observation that the Arctic is greening due to climate warming (e.g. Jia et al. 2009; Walker et al. 2009). A variety of tundra manipulative experiments and observations suggest vegetation productivity is increasing with rising temperatures (e.g. Hollister et al. 2005; Walker et al. 2006; Hudson and Henry 2009; Bhatt et al. 2010; Forbes et al. 2010). Even though the ecological responses to climate change may be complex and non-linear (Post et al. 2009), it may be expected that increased vegetation productivity will provide herbivores with richer food supplies, resulting in increased population densities. In turn, increasing densities of herbivores may have a structuring role on the vegetation which may lead to changes in the vegetation composition (van der Wal 2006; Olofsson et al. 2009) and, in extreme cases, degradation of the ecosystem (Jefferies et al. 2006).
Geese are large herbivores in the terrestrial Arctic biome and can exert a substantial grazing pressure, especially in wet graminoid marshes (Massé et al. 2001). In the early 1980s, we studied the ecology of moulting geese in Jameson Land, East Greenland as part of a biological baseline study before oil exploration. It was concluded that Jameson Land was the largest single site for moulting non-breeding pink-footed geese Anser brachyrhynchus and barnacle geese Branta leucopsis in East Greenland and that the area was utilized by geese close to its habitat carrying capacity (Madsen and Mortensen 1987).
Since the early 1980s, the sizes of the two goose populations have increased, as have most Western Palearctic and North American Arctic-breeding goose populations (Madsen et al. 1996; Fox et al. 2010). To investigate the possible effect of increasing numbers of geese at a site that has undergone concurrent warming of goose-grazed habitats, we returned to the study site in Jameson Land 24 years after the original investigation. Hence, we repeated the surveys of geese and analyses of vegetation composition and biomass in goose-grazed and -ungrazed marshes that we originally studied in the early 1980s.
Materials and Methods
Study Area and Goose Populations
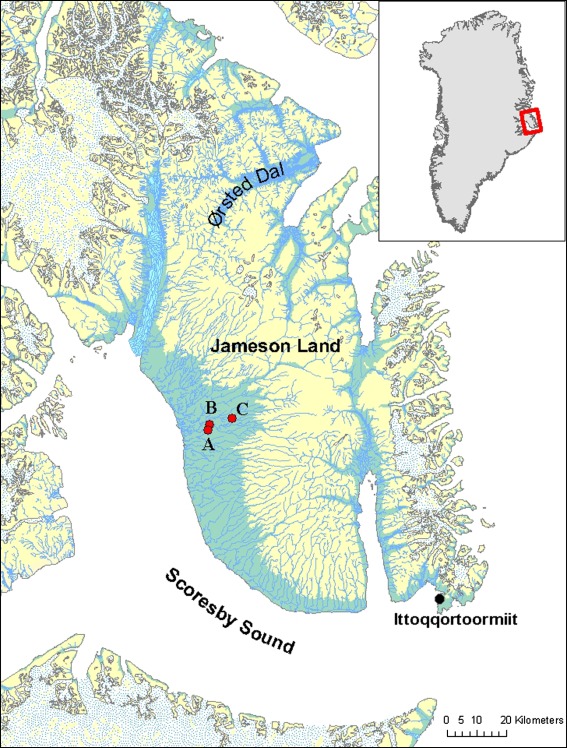
Jameson Land, situated north of the Scoresby Sound Fjord (Fig. 1) on the limit between high and low arctic regions of East Greenland, is lowland tundra that gradually slopes from SW and W towards a northern and eastern plateau (50–1000 m a.s.l.) cut by several valleys, such as Ørsted Dal which connects to the East Greenland Sea. The western part, Heden, is inland tundra intersected by many rivers and dotted with lakes.
Fig. 1.
Jameson Land study area in East Greenland, with the positions of vegetation transects and plots. A Draba Sibirica River transect (sampled 2008), B biomass sampling plot (sampled 1984 and 2008), C lake vegetation transect (sampled 1984 and 2008). Lowland below 200 m a.s.l. is shown by light blue
Fieldwork was carried out in an area around the river Draba Sibirica Elv at Heden (Fig. 1). The dominant vegetation type in the study area on Heden (0–200 m a.s.1.) includes continuous dwarf scrub with 75–100% coverage (with Cassiope tetragona, Vaccinium uliginosum, Empetrum hermaphroditum and Betula nana predominating) and graminoid marshes near rivers, streams and lakes (mosses with protruding Carex stans, C.subspathacea and C.saxatilis and Eriophorum scheuchzeri) (Bay and Holt 1984).
The summer climate is usually continental with much sun and low precipitation. This was prevalent in 1982 and 1984, while in 1983, the summer was rainy and cold with little sun. The summer of 2008 was sunny and with little rain (see ‘Results’ section for more detail).
Two species of geese, the pink-footed goose and barnacle goose, occur as summering and breeding species in Jameson Land. The pink-footed geese belong to the population which breeds in Iceland and East Greenland and winters in Scotland and England. Between the early 1980s and ca. 2008, the population size increased from ca. 90 000 (Ogilvie 1983a) to 350 000 individuals (Fox et al. 2010). The barnacle geese are part of the East Greenland breeding population that winters in Scotland and Ireland. This population has grown from ca. 25 000 (Ogilvie 1983b) to 70 500 individuals (Fox et al. 2010). Aerial surveys and ground counts (Madsen et al. 1984) revealed that in the early 1980s Jameson Land was primarily a moulting area for non-breeding geese undertaking a moult migration to the area. Immatures and failed breeders of pink-footed geese arrive from Iceland at the end of June; at the same time, barnacle geese arrive from the East Greenland breeding range. During a 3–4 week period (pink-footed geese ca. 7 July–1 August, barnacle geese ca. 5–28 July), the geese moult their remiges and are flightless. The moulting flocks are extremely wary and concentrate along rivers and coastlines, and around lakes, which serve as refuges from predators, mainly arctic foxes Vulpex lagopus. They feed on leaves and shoots, rather than rhizomes, in graminoid marshes in a narrow zone along the shores, and pink-footed geese move further inland (up to ca. 150 m) than barnacle geese (up to ca. 75 m). Having regained the power of flight following the moult, the geese immediately abandon the moulting grounds (Madsen and Mortensen 1987).
In July 1982–1984, the moulting populations consisted of 5000–5500 pink-footed geese and 5000–6100 barnacle geese. By comparison, in 2008, numbers had increased to ca. 19 000 and 16 600 individuals, respectively (Glahder et al. 2010). In the early 1980s, the numbers of breeding pairs of each species were a few hundred (Madsen et al. 1984), whereas in 2008, the numbers had increased, in case of pink-footed geese approximately tenfold (Glahder et al. 2010).
Climate Data
We used the North American Regional Reanalysis (NARR-A) dataset from the National Centers for Environmental Prediction (NCEP; http://www.emc.ncep.noaa.gov/mmb/rreanl/) for the climate trend analysis. The NARR data is the best available regional scale, long-term climate data for North America and Greenland. It is superior to global reanalysis datasets and climate models due to the precipitation assimilation, direct assimilation of radiances, land-surface model updates, etc. Also, direct observations from climate stations, radio sondes, ship, buoys and satellites are used in the reanalysis. The data are based on the Eta Model and has a resolution of 32 km. We downloaded data (daily with averages of 3 h intervals) for temperature, short wave incoming radiation, cloud cover, relative humidity, snow depth and total precipitation for the period 1980–2009.
To validate the quality of the data in East Greenland, we compared yearly mean air temperatures from the climate station at Illoqqortoormiut (Cappelen 2010) with 10 m surface temperatures from the closest corresponding NARR pixel. The temperatures were very close (ANOVA, n = 28, R2 = 0.71, p < 0.0001) even though the comparison was made between a spot measurement and a 1024 km2 large pixel.
Goose Counts
On the basis of aerial and ground surveys of geese performed during mid-July 1983 and 2008, respectively (Madsen et al. 1984; Glahder et al. 2010), we identified ten areas for which we were sure that counts covered discrete flocks of geese which were unlikely to move between sites. On the basis of a vegetation map (Bay and Holt 1984) and knowledge about how far inland moulting geese are foraging, defined as 100 m from the periphery of the shore of the refuge coast, lake or river (Madsen and Mortensen 1987), we estimated the available marsh fen for each site. A linear relationship between the marsh size and numbers of geese will be an indication whether geese fill up the area according to resource availability, i.e. habitat matching.
Vegetation Composition and Biomass
Vegetation Composition
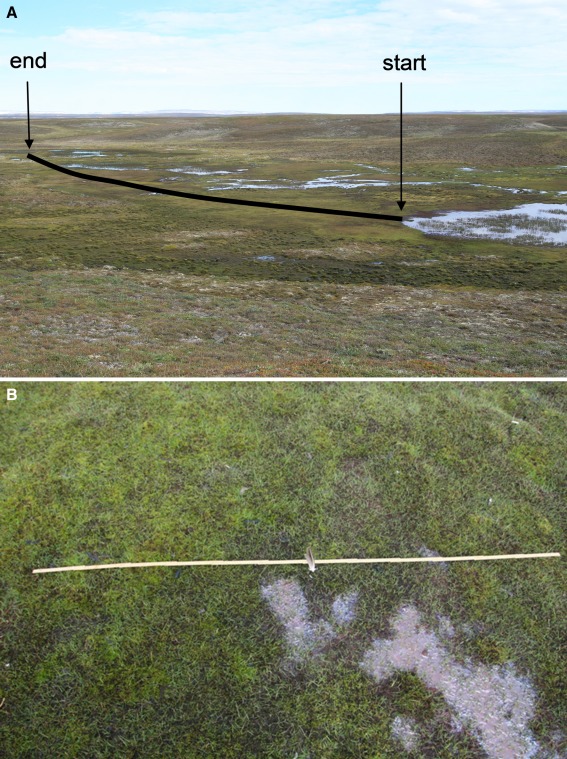
On 30 July 1984, plant species frequency was examined using a point-intercept method in a C. subspathacea—moss dominated sward grazed by moulting pink-footed geese. A 165 m-long transect was laid out in the marsh perpendicular to the lake edge where geese were seeking refuge (Fig. 2a). At 15-m intervals, a secondary transect was laid at right angles to the main transect. Depending on the width of the marsh, the presence or absence of species was recorded in up to 180 contact points. In 2008, we repeated the analysis following the same methodology. Since we did not have exact positions of the 1984 primary and secondary transect lines, we visually judged the position of the former main transect line from pictures. Owing to the configuration of the marsh, we believe we have positioned the 2008-primary transect line within few metres of that used in 1984. We took GPS positions of the centre of each secondary transect line. Owing to the presence of breeding waterbirds on the nearby lake, we wanted to avoid disturbance and did the point-intercept analysis based on closeup vertical digital pictures of the secondary transect lines. For each secondary transect, we took a picture in up to three plots at 2 m-intervals on each side of the main transect, placing a ruler in the picture. Subsequently, the plant species presence was sampled in 32 contact points within the picture frame (which was 50 × 30 cm), overlaying the picture with a grid. Furthermore, for graminoids grazed by the geese, we recorded whether the leaf had been grazed by geese or not. For each centre point of the secondary transects, we also took an oblique picture of the vegetation community, with a 2-m ruler as the frame (Fig. 2b). The picture thus represents an area of approximately 4 m2. From each picture, we counted the number of flowering E. scheuchzeri.
Fig. 2.
Photos showing a the 165 m long transect line in Jameson Land, East Greenland where point-intersect analysis was performed in 1984 and 2008 and b the goose-grazed marsh, with signs of removal of moss and creation of open spots without vegetation. Leaves of C. subspathacea are protruding from the moss carpet. The ruler is 2 m long (photos: Cornelia Jaspers)
On 31 July 2008, we made an additional transect in a C. subspathacea dominated marsh along the Draba Sibirica River, using the same method and sampling protocol as described above. This marsh was primarily grazed by moulting barnacle geese but some pink-footed geese used the area as evidenced by the primary feathers found shed on the river banks. Here, we not only made the point-intercept recordings directly in the field but also took digital photos to compare the direct field sampling with the photo-based sampling. Based on data from three secondary transects (in total 576 contact points), we found that the two methods yielded highly comparable results (paired Wilcoxon signed rank test, p > 0.05).
Plant Biomass and Productivity of Vegetation
To estimate potential food supplies available to moulting geese, the net above-ground primary production (NAPP) of marsh vegetation was studied in 1983 and 1984 (Madsen and Mortensen 1987). In 1984, an exclosure experiment was set up in a C. stans–Poa pratensis dominated marsh along a tributary to a small river. The marsh sloped upwards towards a snow bed supplying the marsh with melt water. The ground was sandy and with a low coverage of moss. We simulated goose grazing by clipping at weekly intervals during the period 21 June and 30 July (see Madsen and Mortensen 1987). On 27 July 2008, we revisited the marsh. We were not able to set up an exclosure, but we took six biomass samples at random locations in the previous exclosure plot. The area showed no signs of having been grazed by geese; hence, we regarded this as being comparable to the exclosure plots sampled in 1984. In six plots of 16 cm × 16 cm (0.0256 m2) each, we clipped the above-ground vegetation to the ground and sorted the vegetation into species; only leaves which were alive were collected. If leaf tips were dead, but the lower part was still alive, the whole leaf was sampled. Samples were stored in paper bags and wind dried in the field. In the laboratory, samples were dried at 50°C for 48 h, stored in an exicator for 24 h and weighed.
Statistical Analyses
To evaluate the climate data trends, a generalized additive model (GAM) fit with a smoothing spline (using the software R vers. 2.11.1 with the mgcv package) was used (Wood 2008). We used Akaikes Information Criterion (AIC) (Burnham and Anderson 2002) to choose the most parsimonious models within a select degrees of freedom range (df: 2, 3, 4, 5). A difference in AIC value of two or greater between the four models was employed to indicate significant difference. We only tested up to five degrees of freedom to avoid models being affected too much by the inter-annual variance.
The relationship between the goose numbers and available marsh size was analysed using linear regression analyses, where the regression was constrained to run through the origin (intercept equals 0). For statistical comparison of the 1984 and 2008 point-intersect data, we used the non-parametric version of the paired t-test, viz. a paired Wilcoxon signed rank test. For analysis of trends along transects, we used a Mann–Kendall test for mononic trend because the frequency data was not normally distributed, even after transformation.
Results
Climate
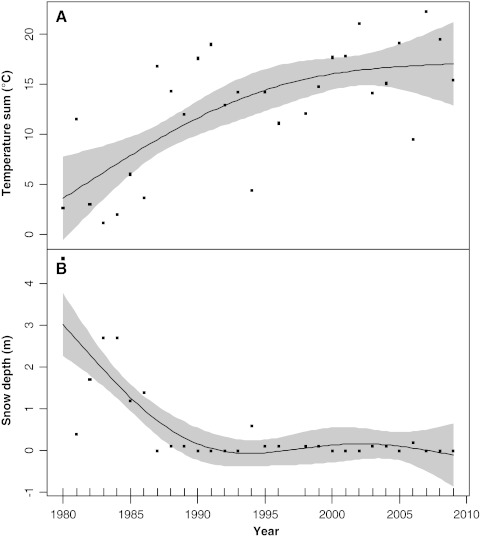
We found changes in mean and maximum temperatures, temperature sums, snow depth and relative humidity between 1980 and 2009. There were no significant trends in cloud cover and hence solar radiation. Table 1 summarizes the selected GAM models. The annual average temperature increased almost linearly through the entire period. However, most of this increase was due to a rise in summer temperatures, especially June and July although the increase declined somewhat in the last 5–10 years. Nevertheless, the June–July sum of temperatures had almost tripled during the 30-year period (Fig. 3). Snow depth had decreased through the study period and melted earlier, extending the growing season. The average snow depth in June in the beginning of the 1980s was several metres, whereas the snow had now almost disappeared in June since the early 1990s. Relative humidity during summer showed a similar decrease during the study period, most likely due to less melting snow in these months and therefore a drier atmosphere.
Table 1.
Summary of GAM smoothing spline fit results for the climate variables with significant changes between 1980 and 2009 for a 32 km × 32 km grid cell in Jameson Land, East Greenland
| Variable | Period | n | df | AIC | Dev. expl. (%) | R2 | p |
|---|---|---|---|---|---|---|---|
| Temp. (sum > 0) | June/July | 29 | 2 | 175.1 | 49.3 | 0.454 | <0.001 |
| Temp. (sum > 0) | June/July/August | 29 | 2 | 179.3 | 57.1 | 0.540 | <0.0001 |
| Temp. (mean) | Year | 28 | 2 | 68.6 | 86.1 | 0.850 | <0.0001 |
| Temp. (mean) | July | 28 | 2 | 129.8 | 55.6 | 0.520 | <0.0001 |
| Temp. (max) | July | 28 | 2 | 144.8 | 44.5 | 0.400 | <0.001 |
| Snow depth | June | 29 | 3 | 64.8 | 66.4 | 0.620 | <0.0001 |
| Snow depth | July | 29 | 4 | 33.3 | 51.7 | 0.440 | <0.005 |
| Relative humidity | July | 29 | 2 | 210.3 | 43.8 | 0.400 | <0.001 |
Columns include: climatic variable (variable), time of year included in the model (period), number of sampling times included (n), degrees of freedom for the model (df), Akaikes Information Criterion for the most parsimonious model (AIC), deviance explained by the model (Dev.expl.), R2 and level of significance for the model
Fig. 3.
Example of GAM smoothing spline fits for climate variables for a 32 × 32 km grid cell in Jameson Land, East Greenland in the period 1980–2009: a Temperature sum (°C) × 100 for June and July, and b snow cover in June. Shaded areas are smoothing fit confidence intervals
Goose Numbers
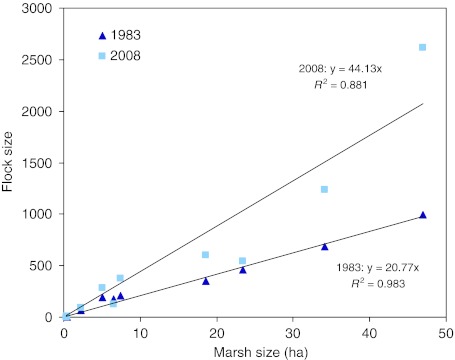
The relationships between the moulting goose flock size and marsh size in 1983 and 2008 are shown in Fig. 4. In both years, there was a significant linear relationship suggesting habitat matching. In 2008, the slope of the regression was estimated at 44.1 (95% confidence limit 34.5–53.8), which was significantly higher than the slope of 20.8 (95% confidence limit 19.3–22.2) estimated for 1983, indicated by the non-overlapping confidence limits. For the ten sites included in the analysis as a whole, the density of geese was 1.9 times higher in 2008 compared to 1983.
Fig. 4.
Relationship between size of available graminoid marsh and numbers of moulting geese in ten discrete sites which were surveyed in both 1983 and 2008 in Jameson Land, East Greenland. Marsh size was defined as a 100 m zone inland from refuge (shoreline, lake edge, river), and marsh habitat was extracted from a vegetation map prepared by Bay and Holt (1984)
Marsh Vegetation Composition
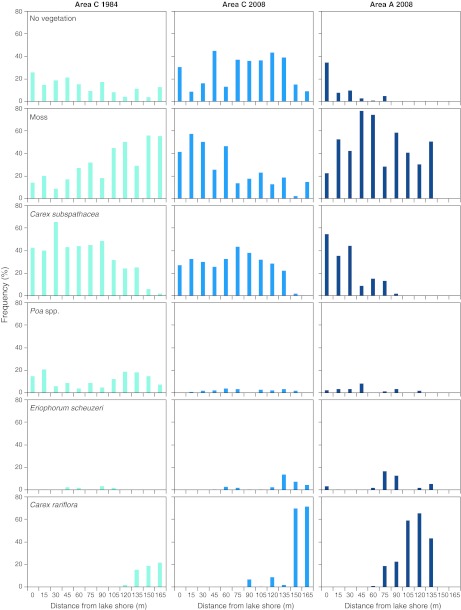
Comparison of the point-intersect sampling from the goose-grazed marsh in the Draba Sibirica area, 1984 and 2008, suggests the overall composition was similar (Fig. 5). Less frequent species (less than 1% in overall frequency) are not shown, but included Salix herbacea, Saxifraga foliosa, Bistorta viviparum, Juncus spp., C. stans and Equisetum spp. At a distance of up to approximately 120 m from the lake shore, C. subspathacea, dominated and protruded from the moss layer. Further away from the lake, Carex rariflora became dominant. However, between years, there were significant differences. First, the frequency of dead biomass (no vegetation) increased from 1984 to 2008 (Table 2); in 1984, the frequency decreased with distance from the lake shore, whereas there was no trend in 2008. In patches of the grazed marsh, we observed open spots and signs of removal of mosses in 2008 (Fig. 2b). Second, in 1984, the frequency of moss increased with distance from lake shore, whereas the opposite was the case in 2008. Third, the frequency of C. subspathacea and Poa spp. were higher in 1984 compared to 2008. Fourth, E. scheuchzeri increased in frequency with distance away from the lake shore in 2008, but showed no trend in 1984 (Fig. 5).
Fig. 5.
Frequency distribution of plants and non vegetated points (including dead biomass) in relation to the lake shore in a goose-grazed sedge and moss-dominated marsh north of the Draba Sibirica area (Area C) studied in 1984 and revisited in 2008, and in a marsh along the Draba Sibirica River (Area A) studied in 2008 in Jameson Land, East Greenland. The Area C transect was 165 m long and the Area A transect 135 m
Table 2.
Statistical comparison of vegetation coverage and composition along a transect in a goose-grazed marsh in the Draba Sibirica area (Area C), 1984 vs. 2008
| Difference 1984–2008 V |
Trend 1984 τ |
Trend 2008 τ |
|
|---|---|---|---|
| No/dead vegetation | 11* | −0.576* | 0.06n.s. |
| Moss | 42n.s. | 0.758** | −0.606** |
| C. subspathacea | 72** | −0.485* | −0.382n.s. |
| Poa spp. | 78** | 0.091n.s. | 0.095n.s. |
| Eriophorum scheuzeri | 16n.s. | −0.224n.s. | 0.688** |
| C. rariflora | 4n.s. | 0.759** | 0.776** |
Trends in coverage are from the lake shore to 165 m inland. Difference in coverage between 1984 and 2008 was tested using a paired Wilcoxon signed rank test (V-value) (non-parametric equivalent of a paired t-test). Trends are expressed by a Mann–Kendall test for a mononic trend (τ-value)
* p < 0.05 (two-sided); ** p < 0.01 (two-sided); n.s. not significant
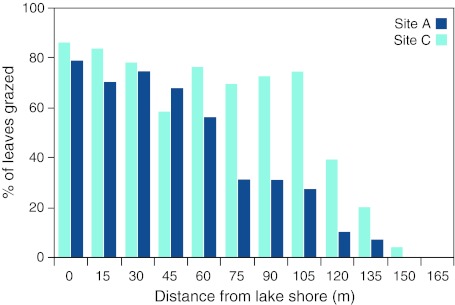
The geese grazed the vegetation intensively in 2008. Hence, within 100 m from the lake shore, approximately 80% of all leaves of Carex, Poa and Eriophorum were grazed. Beyond that distance, grazing pressure decreased, and at a distance of 165 m from the lake, none of the leaves were grazed (Fig. 6). Furthermore, up to 130 m from the lake shore, no E. scheuchzeri was recorded flowering in the photographed plots; beyond that distance, flowering plants occurred frequently (with 4–9 flowers per 4 m2).
Fig. 6.
The proportion of graminoid leaves grazed by moulting geese in relation to the distance to the lake shore at Site A and C, respectively, in 2008, Jameson Land, East Greenland. The Area C transect was 165 m long and the Area A transect 135 m
In the marsh adjacent to the Draba Sibirica River, the overall vegetation composition and spatial trends in 2008 were similar to the lake marsh (Fig. 5). However, frequency of moss was higher and C. subspathacea was replaced by C. rariflora at a distance of 75 m from the river. Poa spp. and E. scheuchzeri were infrequent; Eriophorum started to flower at a distance of 90 m from the river (with 1–13 flowers per 4 m2 picture frame). The grazing pressure, expressed by the proportion of leaves grazed, was high close to the river, but decreased at a distance of 60 m from the river (Fig. 6).
Biomass and Productivity
The biomass clipping in six marsh plots in late July 2008 yielded a standing crop of on average 98.2 g dw m−2 (SD = 36.0), comprised of 88.8% C. stans and 11.2% Poa spp. This compares to a cumulative above-ground biomass of on average 42 g dw m−2, measured in the same plot in late July 1984 (Fig. 9b in Madsen and Mortensen 1987). In 1984, C. stans made up 87% of the biomass and Poa spp. 13%. Because the 1984 standing crop was based on repeated vegetation clipping of the plots, it represented an estimate of net primary productivity for June–July, while the 2008 biomass was based on live plant material measured once in late July. Hence, the 2008 biomass was a conservative estimate of productivity.
Discussion
The field studies in 1982–1984 concluded that habitat capacity of Jameson Land for moulting geese was close to being reached. This was based on three lines of evidence. First, all suitable sites, i.e. coastlines as well as rivers and lakes with a combination of a certain minimum width providing refuge against mammalian predators and surrounding marsh feeding habitat, were occupied. Habitat matching, i.e. a linear correlation between the size of available habitat and the number of geese, suggested that the geese had filled up the area. Second, in the C. subspathacea dominated marsh, i.e. the most common moulting foraging habitat, the geese removed a significant part of the NAPP. Third, by the end of the moulting period, the vegetation was grazed below 0.5 cm height, and the geese abandoned the sites immediately after having regained flight (Madsen and Mortensen 1987).
Between 1982–1984 and 2008, we observed that the number of moulting geese in Jameson Land tripled and both pink-footed geese and barnacle geese increased in numbers. The habitat matching in both 1983 and 2008 suggests that sites were filled up according to available food resources, however, with an almost doubling in average goose density in 2008 compared to 1983. The grazing gradients found in the goose-grazed marshes in 2008 were comparable to what we found in the early 1980s, which shows that the moulting geese had not expanded their marsh use as a possible response to increasing densities, but that the increase in density was real.
The climate in East Greenland has been warming during the last 30 years, not only in the Scoresby Sound region but also further north. In high-Arctic Zackenberg, for example, the average July air temperature has increased by 0.28°C per year during 1996–2005 (Forchhammer et al. 2008), although with a more recent high variability (M. Forchhammer, unpubl. data). The warming and extension of the growing season due to earlier snow melt may have had a positive influence on the productivity of the graminoid vegetation, possibly explaining the more than doubling in biomass of the C. stans–Poa plots from 1984 to 2008. Even though the 2008 biomass sampling was a snapshot at the end of the goose moulting period, this one measure exceeded the productivity estimate for 1984 by a factor of 2.3, i.e. in terms of productivity the increase was even higher. Similarly, on Bylot Island, northeast Canada, graminoid above-ground production in wetlands has increased by 84% between 1990 and 2007, most likely as a consequence of climate warming (Cadieux et al. 2008). Therefore, we suggest that through warming and extended growth season, productivity has increased, allowing for higher numbers of moulting geese.
In addition to the improvement of the habitat capacity for moulting geese, the earlier snow melt has improved the potential for breeding geese, in particular pink-footed geese, which nest on the open tundra. In the early 1980s, we only observed scattered nests and family groups and mainly in Ørsted Dal. However, in 2008, flocks of families were highly abundant all over Jameson Land (Glahder et al. 2010). On Svalbard, it is known that early snow melt has a dramatic positive effect on the density of nesting geese and their fecundity (Madsen et al. 2007). In the longer term, the advancement of spring in East Greenland may contribute substantially to the continued growth of the populations, such as has been predicted to be the case in Svalbard (Jensen et al. 2008). In Jameson Land, the goose families feed in marshes away from the moulting grounds of the non-breeders; hence, the growth in density of breeding geese will not necessarily affect the densities of non-breeding moulting geese.
With our study, it is difficult to evaluate the inter-annual temperature and snow variability and their effects on the system. Because the marsh plant species are long-lived clonal, the species composition does not respond readily to inter-annual variations in temperatures and snow cover but productivity does. This was evident in the early 1980s, when the NAPP estimate in C. subspathacea dominated marshes in Jameson Land varied between less than 1 g dw m−2 in the cold summer of 1983 and 13 g dw m−2 in the warm and sunny 1984 (Madsen and Mortensen 1987). Since both 1984 and 2008 can be regarded as relatively highly productive years for their time periods, we believe that the increase in biomass is a reflection of the long-term trend in climate conditions. How the moulting geese respond to inter-annual variation is unknown; in the early 1980s, there was little inter-annual variation in numbers of moulting geese between years (Madsen et al. 1984), but we have no information on how geese cope in terms of daily food intake rates and grazing pressures. The longer term build-up of numbers is probably a reflection of the interaction between the general gradual improvement of the moulting habitat and the overall growth in the sizes of the two goose populations and their demand for moulting habitats. Little is known about the population processes and possible ‘bottlenecks’ in their annual cycles and hence, whether the moulting grounds constitute a limiting factor. If so, however, climate warming appears to be a factor which will be a contributor to further population growth.
The repeated vegetation transect in the Draba Sibirica area showed a significant increase in dead vegetation, open spots and decrease in cover of C. subspathacea and Poa. This may suggest that moulting geese have a long-term effect on the vegetation composition and cover in marshes subject to intensive grazing. Furthermore, the succession in composition of plants as well as flowering of E. scheuchzeri where goose-grazing ceased suggest that the grazing structures plant communities. In the marsh grazed by pink-footed geese, the geese entered around 120 m into the marsh, whereas in the marsh primarily grazed by barnacle geese, the geese only used the zone 60–80 m from the river shore. These distances corresponded to where we found the succession of Carex, and only beyond these distances we found flowering of Eriophorum. More generally, we observed that in marshes grazed by moulting geese, C. subspathacea dominated, whereas in marshes without such intensive grazing, C. rariflora or C. stans dominated, and there was highly abundant flowering in E. scheuchzeri (J. Madsen and C. Jaspers, unpubl.). The structuring role of goose grazing has also been demonstrated in studies employing long-term exclosures in grazed marshes (Person et al. 2003; Gauthier et al. 2004). Owing to its small and low leaves protruding from the moss carpet, C. subspathacea is possibly the most grazing tolerant species amongst the Carex species which may explain its dominance in the grazed part of the marsh.
The suggested grazing effects are, however, still rather subtle and not comparable to what has been reported from La Perouse Bay in the Hudson Bay, Canada, for example, where foraging lesser snow geese Anser c. caerulescens have caused a massive and large-scale degradation of salt marshes (e.g. Jefferies et al. 2006). The effect of below-ground foraging (so-called grubbing) by pink-footed geese on Svalbard has also caused dramatic changes in plant species composition and the establishment of open depressions void of vegetation (Gornall et al. 2009; Speed et al. 2009). A likely explanation for the more subtle effects observed in Jameson Land is that the moulting geese exclusively graze the above-ground vegetation instead of below-ground material whereby moss and roots are pulled out of the vegetation carpet, and a larger component of the plant community other than the forage is disturbed in the process.
The earlier snow melt and advancement of spring will probably lead to an earlier and extended plant growing season. Unless geese are able to adjust their timing of moult migration to Jameson Land, this may result in a mismatch between peak availability of high quality forage and moulting by geese, which are reliant on a narrow foraging zone; however, by repeated grazing of the growing foraging plants, geese may be able to compensate, maintaining high food quality during the moulting period.
Conclusions
This study suggests that between the early 1980s and 2008, climate warming has increased productivity of graminoid vegetation in tundra marshes in East Greenland, thereby allowing for an increase in numbers of moulting geese. However, reduced vegetation cover due to goose grazing may have longer-term negative consequences for the quality of the habitat and its capacity to support geese. It is recommended that more detailed studies of the phenology and productivity of the forage plants are carried out and that the long-term effects of increased goose grazing pressure in Jameson Land are studied experimentally.
Acknowledgments
This study was part of the IPY project ‘Back to the Future’. The authors are grateful to Terry Callaghan and Craig Tweedie for taking them onboard. The re-visit in 2008 was part of a study financed by the Bureau of Minerals and Petroleum, Greenland Government. The authors would like to thank the National Centers for Environmental Prediction (NCEP) for making the North American Regional Reanalysis (NARR) data freely available (http://www.emc.ncep.noaa.gov/mmb/rreanl/). The authors thank the editors and anonymous reviewers for their constructive comments on the manuscript. This is a contribution by the Greenland Climate Research Centre (GCRC), Greenland Institute of Natural Resources, Nuuk, Greenland.
Biographies
Jesper Madsen
D.Sc., is professor in Arctic conservation ecology at Aarhus University; he has studied the ecology and population dynamics of Arctic geese for more than 30 years and initiated the goose studies in Jameson Land in 1982–1984.
Cornelia Jaspers
M.Sc., is a doctoral candidate in biology at DTU Aqua, Technical University of Denmark. She has been participating in terrestrial field work in temperate and arctic areas since 2007.
Mikkel Tamstorf
Ph.D., senior researcher at Aarhus University, is a physical geographer. He has worked with climate change in the Arctic since 1995, with expertise on snow–vegetation interactions.
Christian Ebbe Mortensen
M.Sc., is biologist and worked with Arctic goose grazing ecology in Jameson Land, East Greenland for several years in the 1980s.
Frank Rigét
M.Sc., is senior researcher at Aarhus University working with environmental science and is particularly involved with contaminants and their bioaccumulation in the Arctic.
Contributor Information
Jesper Madsen, Email: jm@dmu.dk.
Cornelia Jaspers, Email: coja@aqua.dtu.dk.
Mikkel Tamstorf, Email: mpt@dmu.dk.
Christian Ebbe Mortensen, Email: ebbe@tiscali.dk.
Frank Rigét, Email: ffr@dmu.dk.
References
- Bay, C., and S. Holt. 1984. Botanical studies in Jameson Land (Botaniske undersøgelser i Jameson Land), 1983. Greenland Fisheries and Environment Research Institute, Report, Copenhagen, Denmark, 89 pp (in Danish).
- Bhatt US, Walker DA, Raynolds MK, Comiso JC, Epstein HE, Jia GS, Gens R, Pinzon JE, et al. Circumpolar Arctic tundra vegetation change is linked to sea ice decline. Earth Interactions. 2010;14:1–20. doi: 10.1175/2010EI315.1. [DOI] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. 2. New York: Springer; 2002. [Google Scholar]
- Cadieux, M.C., G. Gauthier, C.A. Gagnon, J. Bêty, and D. Berteaux. 2008. Monitoring the environmental and ecological impacts of climate change on Bylot Island, Sirmilik National Park. Université Laval, Report, Quebec, Canada, 112 pp.
- Cappelen, J. 2010. DMI monthly climate data collection 1768–2009, Denmark, the Faroe Islands and Greenland. Danish Meteorological Institute, Technical Report 10-05 DMI, Copenhagen, Denmark, 52 pp.
- Forbes BC, Fauria MM, Zetterberg P. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Global Change Biology. 2010;16:1542–1554. doi: 10.1111/j.1365-2486.2009.02047.x. [DOI] [Google Scholar]
- Forchhammer MC, Christensen TR, Hansen BU, Tamstorf MP, Schmidt NM, Hoye TT, Nabe-Nielsen J, Rasch M, et al. Zackenberg in a circumpolar context. Advances in Ecological Research. 2008;40:499–544. doi: 10.1016/S0065-2504(07)00021-9. [DOI] [Google Scholar]
- Fox AD, Ebbinge BS, Mitchell C, Heinicke T, Aarvak T, Colhoun K, Clausen P, Dereliev S, et al. Current estimates of goose population sizes in Western Europe, a gap analysis and an assessment of trends. Ornis Svecica. 2010;20:115–127. [Google Scholar]
- Gauthier G, Bety J, Giroux JF, Rochefort L. Trophic interactions in a high arctic snow goose colony. Integrative and Comparative Biology. 2004;44:119–129. doi: 10.1093/icb/44.2.119. [DOI] [PubMed] [Google Scholar]
- Glahder, C., D. Boertmann, J. Madsen, M. Tamstorf, P. Johansen, J. Hansen, A. Walsh, C. Jaspers, et al. 2010. Biological baseline study in the Ramsar site “Heden” and the entire Jameson Land, East Greenland. National Environmental Research Institute, Aarhus University, Technical Report 769, Roskilde, Denmark, 86 pp.
- Gornall JL, Woodin SJ, Jonsdottir IS, Wal R. Herbivore impacts to the moss layer determine tundra ecosystem response to grazing and warming. Oecologia. 2009;161:747–758. doi: 10.1007/s00442-009-1427-5. [DOI] [PubMed] [Google Scholar]
- Hollister RD, Webber PJ, Tweedie CE. The response of Alaskan arctic tundra to experimental warming: Differences between short- and long-term responses. Global Change Biology. 2005;11:525–536. doi: 10.1111/j.1365-2486.2005.00926.x. [DOI] [Google Scholar]
- Hudson JMG, Henry GHR. Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology. 2009;90:2657–2663. doi: 10.1890/09-0102.1. [DOI] [PubMed] [Google Scholar]
- Jefferies RL, Jano AP, Abraham KF. A biotic agent promotes large-scale catastrophic change in the coastal marshes of Hudson Bay. Journal of Ecology. 2006;94:234–242. doi: 10.1111/j.1365-2745.2005.01086.x. [DOI] [Google Scholar]
- Jensen RA, Madsen J, O’Connell M, Wisz MS, Tømmervik H, Mehlum F. Prediction of the distribution of Arctic-nesting pink-footed geese under a warmer climate scenario. Global Change Biology. 2008;14:1–10. [Google Scholar]
- Jia GSJ, Epstein HE, Walker DA. Vegetation greening in the Canadian arctic related to decadal warming. Journal of Environmental Monitoring. 2009;11:2231–2238. doi: 10.1039/b911677j. [DOI] [PubMed] [Google Scholar]
- Madsen J, Boertmann D, Mortensen CE. The significance of Jameson Land, East Greenland, as a moulting and breeding area for geese: Results of censuses 1982. Dansk Ornitologisk Forenings Tidsskrift. 1984;78:121–131. [Google Scholar]
- Madsen J, Mortensen CE. Habitat exploitation and interspecific competition of moulting geese in East-Greenland. Ibis. 1987;129:25–44. doi: 10.1111/j.1474-919X.1987.tb03157.x. [DOI] [Google Scholar]
- Madsen J, Reed A, Andreev A. Status and trends of geese (Anser spp., Branta spp.) in the world: A review, updating and evaluation. Gibier Faune Sauvage. 1996;13:337–353. [Google Scholar]
- Madsen J, Tamstorf M, Klaassen M, Eide N, Glahder C, Riget F, Nyegaard H, Cottaar F. Effects of snow cover on the timing and success of reproduction in high-Arctic pink-footed geese Anser brachyrhynchus. Polar Biology. 2007;30:1363–1372. doi: 10.1007/s00300-007-0296-9. [DOI] [Google Scholar]
- Massé H, Rochefort L, Gauthier G. Carrying capacity of wetland habitats used by breeding greater snow geese. Journal of Wildlife Management. 2001;65:271–281. doi: 10.2307/3802906. [DOI] [Google Scholar]
- Ogilvie MA. Number of geese in Britain and Ireland, 1982–1983. Wildfowl. 1983;34:172. [Google Scholar]
- Ogilvie MA. The numbers of Greenland barnacle geese in Britain and Ireland. Wildfowl. 1983;34:77–88. [Google Scholar]
- Olofsson J, Oksanen L, Callaghan T, Hulme PE, Oksanen T, Suominen O. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biology. 2009;15:2681–2693. doi: 10.1111/j.1365-2486.2009.01935.x. [DOI] [Google Scholar]
- Person BT, Herzog MP, Ruess RW, Sedinger JS, Anthony RM, Babcock CA. Feedback dynamics of grazing lawns: Coupling vegetation change with animal growth. Oecologia. 2003;135:583–592. doi: 10.1007/s00442-003-1197-4. [DOI] [PubMed] [Google Scholar]
- Post E, Forchhammer MC, Bret-Harte MS, Callaghan TV, Christensen TR, Elberling B, Fox AD, Gilg O, et al. Ecological dynamics across the Arctic associated with recent climate change. Science. 2009;325:1355–1358. doi: 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- Speed JDM, Woodin SJ, Tømmervik H, Tamstorf MP, Wal R. Predicting habitat utilization and extent of ecosystem disturbance by an increasing herbivore population. Ecosystems. 2009;12:349–359. doi: 10.1007/s10021-009-9227-7. [DOI] [Google Scholar]
- Wal R. Do herbivores cause habitat degradation or vegetation state transition? Evidence from the tundra. Oikos. 2006;114:177–186. doi: 10.1111/j.2006.0030-1299.14264.x. [DOI] [Google Scholar]
- Walker DA, Leibman MO, Epstein HE, Forbes BC, Bhatt US, Raynolds MK, Comiso JC, Gubarkov AA, et al. Spatial and temporal patterns of greenness on the Yamal Peninsula, Russia: Interactions of ecological and social factors affecting the Arctic normalized difference vegetation index. Environmental Research Letters. 2009;4:1–16. doi: 10.1088/1748-9326/4/4/045004. [DOI] [Google Scholar]
- Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, et al. Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. Fast stable direct fitting and smoothness selection for generalized additive models. Journal of the Royal Statistical Society Series B: Statistical Methodology. 2008;70:495–518. doi: 10.1111/j.1467-9868.2007.00646.x. [DOI] [Google Scholar]