Abstract
Purpose
A second generation 13 valent pneumococcal conjugate vaccine (PCV13) was licensed and recommended for universal immunization of children through age five years in 2010. Its introduction is intended to address the residual burden of pneumococcal diseases that persists a decade after the introduction of PCV7.
Recent Findings
Immunization with PCV7 has resulted in a substantial decline in pneumococcal diseases caused by vaccine serotypes in both vaccinated and unvaccinated persons in the US. However an increase in disease due to non vaccine serotypes, including empyema; the emergence of multidrug, including ceftriaxone, resistant serotype 19A strains; and the need for broader serotype coverage to address the global disease burden provides a rationale for a second generation conjugate vaccine that includes serotypes 1, 3, 5, 6A, 7F and 19A.
Summary
This article reviews the lessons learned from a decade of experience with PCV7, the increasing problem of disease due to non-vaccine serotypes, and the likelihood of PCV13 to impact the residual disease burden. We contrast the potential differences in prevention of invasive pneumococcal disease (IPD) compared to nonbacteremic pneumonia and acute otitis media. We conclude with the current recommendations for PCV13 providing a rationale for immunization through age 5 years to create both direct and indirect protection in the population.
Keywords: Pneumococcal disease, Conjugate vaccine, Nonvaccine serotypes, AAP recommendations, Catch up regimen
Introduction
A second generation 13 valent pneumococcal conjugate vaccine (PCV13) was licensed and recommended for universal immunization of children through age five years in 2010. Its introduction is intended to address the residual burden of pneumococcal diseases that persists a decade after the introduction of PCV7
Lessons from a Decade of Experience with 7-valent pneumococcal conjugate vaccine (PCV7)
The introduction of a seven-valent pneumococcal conjugate vaccine (PCV7) in 2000 built on the success of the Haemophilus influenzae type B conjugate vaccine in preventing the then major pathogen in childhood bacterial meningitis. Randomized clinical trials of PCV7 had demonstrated the induction of serotype specific antibodies measured by enzyme linked immunosorbent assays (ELISA) and opsonophagocytic activity (OPA) by six months of age as well as evidence of immunologic memory at boosting and protection against vaccine type invasive pneumococcal disease (IPD), primarily bacteremia, in a large clinical trial in Northern California [1]. This large trial also demonstrated significant protection against non-invasive pneumococcal diseases, including pneumonia and acute otitis media [2, 3]. Clinical trials in Finland also demonstrated protection against acute otitis media due to vaccine serotypes, and; importantly, smaller studies reported declines in nasopharyngeal colonization with vaccine serotypes [4, 5].
Post licensure surveillance has demonstrated declines in the incidence of meningitis, bacteremic pneumonia, and bacteremia without a focus across the pediatric age spectrum and rates of hospitalization due to invasive pneumococcal disease and all cause and pneumococcal pneumonia have declined. According to the Centers for Disease Control and Prevention’s Active Bacterial Core surveillance system, from 1998–1999 through 2007, overall and PCV7-serotype specific IPD rates decreased by 45% (from 24.4 to 13.5 cases per 100,000 population) and 94% (from 15.5 to 1.0 cases per 100,000 population), respectively. The largest reductions in IPD incidence has been observed in children aged <5 years, the target population of the vaccination program, with remarkable reductions in all and PCV7-serotype specific IPD rates of 76% and 100%, respectively. [6]
In addition to the substantial reductions in the incidence of IPD among vaccinated children, the introduction of PCV7 in 2000 has also resulted in marked reductions in nasopharyngeal colonization with vaccine serotypes and subsequent reductions in IPD incidence among age groups that were not vaccinated. This indirect population protection benefited both infants that were too young to receive PCV7 [7], and children or adults for whom PCV7 was not recommended [8]. Declines in IPD have been also consistently documented in high risk children including those with HIV [9] and children with Sickle Cell Disease [10].
The decline in IPD incidence in the US has been accompanied with reductions across the spectrum of pneumococcal diseases including pneumonia and otitis media. In the US, rates of all-cause pneumonia hospitalization in children < 2 years decreased by 33% (95% confidence interval, 28%–37%) from pre-PCV7 (1996–1999) to post PCV7 years (2001–2007). In this age group, pneumococcal pneumonia hospitalization rates decreased 61% (95% confidence interval, 55%–67%) post-PCV7, compared with pre-PCV7 years [11].
Reductions in pneumonia hospitalizations among young infants have been consistently documented in the US and significant reductions have been observed also in other countries where routine vaccination with PCV7 has been implemented with high vaccination coverage attained. For instance, significant reductions in all-cause pneumonia hospitalizations have been reported for Australia, Poland and Canada, following PCV7 introduction. [12, 13, 14]
Similarly, reductions in the incidence of acute otitis media episodes have been reported with concurrent reductions in healthcare utilization and antibiotic use [15, 16, 17]. Decreases of up to 42% in the rate of childhood acute otitis media episodes were documented using a large database of private insurance companies in the US [18]. It is important to note however, that the incidence of acute otitis media started declining even before introduction of PCV7 and thus, disentangling the effects of the vaccination program from ongoing declining trends could be problematic [19]. A recent report from Canada evaluated the effect of secular trends on similar estimates and concluded that from an observed reduction of 25.2% in rates of otitis media visits, only about half this effect could be attributed to vaccination with PCV7 [20].
Two stories in one
Ten years after PCV7 introduction in the US, the declines in pneumococcal diseases can be viewed as two stories in one (Figure 1). Immunization with PCV7 has resulted in the virtual elimination of invasive disease (>98%) and nasopharyngeal carriage (>97%) due to any of the seven vaccine serotypes and significant reductions in the incidence of pneumonia and otitis media. However, we have also observed total replacement of vaccine serotypes with non vaccine serotypes in the nasopharynx and a modest increase in the incidence of IPD due to non vaccine serotypes. [6, 21]
Figure 1.
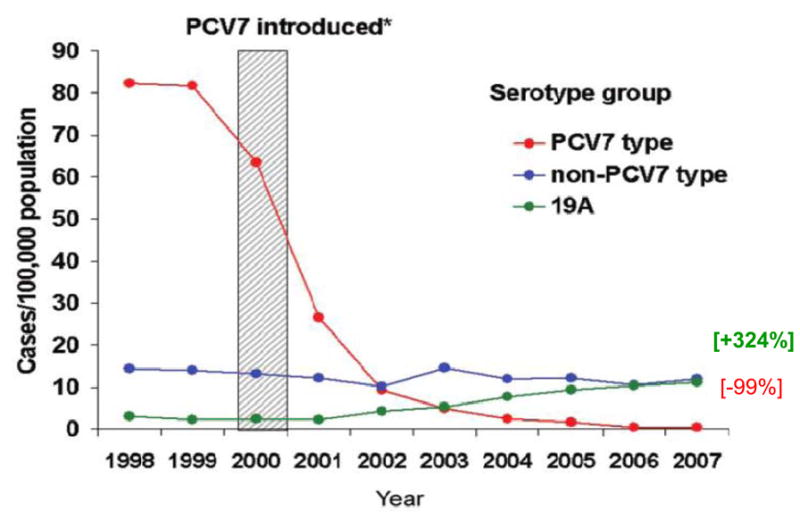
Changes in IPD Incidence by Serotype grouping in Children Less than 5 years by Year: Two Stories in One [6]
[6] Pilishvili T, et al. J Infec Dis 2010;201(1):32–41
Why do we need a second generation pneumococcal conjugate vaccine?
Surveillance studies as early as 2004 in Massachusetts were among the first to identify a significant increase in IPD due to serotype 19A in children aged less than 5 years. [22] This increase was subsequently confirmed by Kaplan and colleagues from their eight Children’s hospital surveillance network and by Pilishvili and colleagues who reported a 4 fold increase in IPD due to serotype 19A, from 2.6 to 11.1 per 100,000 children aged less than 5 years, between baseline (1998–1999) and 2007 at ABC surveillance sites. [23, 6] More recently, our Massachusetts’ data suggest the increase in 19A has plateaued and currently accounts for ~36% of all IPD cases in children less than 18 years of age. [38]
Serotype 19A has also been the most common cause of refractory AOM in Rochester, NY, and acute mastoiditis and chronic sinusitis in hospitalized children at Texas Children’s in Houston. [24, 25, 26] In addition to its frequency, a multidrug resistant strain (MLST 320) has emerged with minimal inhibitory concentrations (MICs) that exceed those achievable with oral antimicrobial agents other than linezolid and levofloxacin. [24, 23] Although the emergence of 19A is likely multifactorial, the minimal anti-19A OPA levels elicited in children immunized with PCV7 supports the need for a second generation PCV that will elicit protective concentrations of functional antibody against this serotype.
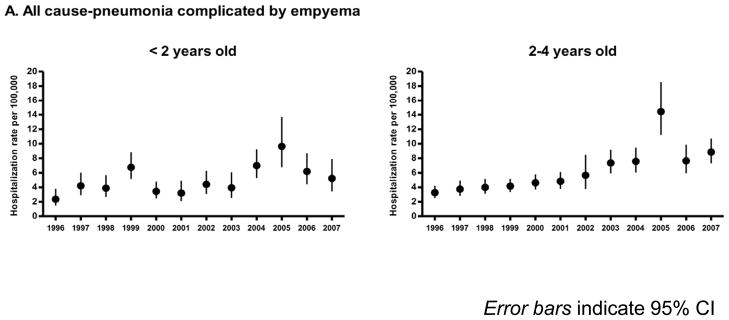
Despite the overall reductions in pneumonia hospitalizations in children less than 2 years of age, pneumonia hospitalizations complicated by empyema have increased 2-fold from 3.5 cases per 100,000 children in 1996–1998 to 7.0 cases per 100,000 children in 2005–2007. Among children aged 2–4 years, pneumonia complicated by empyema increased nearly 3-fold from 3.7 cases per 100,000 children in 1996–1998 to 10.3 cases per 100,000 children in 2005–2007 (Figure 2) [27, 28] In Utah, pediatric pneumococcal empyema also shows an increasing trend that started before PCV7 [29] and continued in more recent years. Between 2001–2007 ninety-eight percent of pneumococcal empyema cases in Utah were due to pneumococcal serotypes not present in PCV7 with serotypes 1, 3, 19A and 7F responsible for 90% of cases (Table 1). Three to 5-fold increases in pediatric empyema have also been reported from Spain [30] and the United Kingdom prior to introduction of the PCV in those countries with most cases due to serotype 1 suggesting that the increase in disease due to this serotype may be unrelated to the introduction of PCV7. Regardless of the cause for this increase, serotypes 1, 3, 7F and 19A are the major causes of pneumococcal empyema in the post PCV7 era. Although empyema remains a relatively uncommon complication of pneumonia, there is a clear global need for effective prevention strategies to prevent this serious disease.
Figure 2.
Annual hospitalization rates for all cause pneumonia complicated by empyema among children aged <5 years, United States, 1996–2007.[*27]
[*27] Modified from Grijalva C, et al Clin Infect Dis2010;50(6):805–13
Table 1.
Pneumococcal Serotypes in Pediatric Empyema: 2001–2007 [29]
| Serotype | No. (%) | No. (%) |
|---|---|---|
| Vaccine Serotypes | 1 (2) | |
| 9V............ | ............1 (2) | |
| Nonvaccine Serotypes | 50 (98) | |
| 1.... | 17 (33) | |
| 3 | 14 (27) | |
| 7F | 2 (4) | |
| 17. | 1 (2) | |
| 19A.. | . 13 (26) | |
| 22F | 1 (2) | |
| 38 | . 1 (2) | |
| Nontypeable | 1 (2) |
Reproduced with permission from [29] Byington CL, Hulten KG, Ampofo K, et al. J Clin Microbiol.2010;48(2):520–5
PCV7 was developed primarily to address the serotype distribution of IPD in North American children where coverage was expected to exceed 80% for IPD. However, broader coverage will be needed to address the nearly one million pneumococcal related fatalities annually in developing countries. A recent report on global serotype distribution developed by O’Brien and colleagues at Global Alliance for Vaccines and Immunisation (GAVI), concluded that serotype 14 is the most common serotype in each region of the world among children aged <5 years and serotypes 1 and 5 are among the top 3 ranked serotypes occurring in the GAVI-eligible countries and are among the top 6 ranked serotypes occurring among children aged <5 years in regions with the highest pneumococcal disease burden (Africa, Asia, and Latin American Countries). [31]
The World Health Organization statement on pneumococcal conjugate vaccine noted that the inclusion of additional serotypes beyond the ones included in PCV7 will significantly help to reduce the global burden of pneumococcal disease. The widespread implementation of new pneumococcal conjugate vaccines with expanded serotype coverage will be an important step forward for reducing childhood morbidity and mortality.
Licensure of PCV13
On February 24, 2010, a novel thirteen valent pneumococcal conjugate vaccine (PCV13) was licensed for use among US children aged 6–weeks 71 months. This vaccine succeeds PCV7 and expands its coverage by including capsular polysaccharide antigens of serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F, individually conjugated to a nontoxic diphtheria CRM197 carrier protein (same carrier as in PCV7). [32]
Pre licensure studies demonstrated PCV13 to be at least as immunogenic as PCV7 for common serotypes, and to induce comparable levels of antibodies for serotypes unique to PCV13. [33, 34] PCV13 was evaluated in more than 4,700 infants and 354 older infants and children who had not previously received a pneumococcal vaccine. The criteria for licensure was immunologic responses to the 7 serotypes in PCV7 that were non inferior to those elicited by PCV7 and responses to the 6 additional serotypes that were greater than 0.35 ug/ml as measured by ELISA and OPA titers greater than 1:8 at one month post completion of primary series, and evidence of memory at boosting. All three of these endpoints were achieved with PCV13 for the 6 new serotypes (1, 3, 5, 6A, 7F and 19A). Table 2a and 2b demonstrate the geometric mean antibody concentrations following 3 doses of PCV13 administered at 2, 4 and 6 months of age as well as the OPA titer achieved. [35]
Table 2a.
IgG Sero-responses for the 6 Additional PCV13 Serotypes [35]
| Proportion over 0.35 ug/ml after dose 3 | GMC* After dose 3 | |
|---|---|---|
| Serotype | % | GMC (95% CI) |
| 1 | 95.6 | 2.03 (1.78, 2.32) |
| 3 | 63.5 | 0.49 (0.43, 0.55) |
| 5 | 89.7 | 1.33 (1.18, 1.50) |
| 6A | 96.0 | 2.19 (1.93, 2.48) |
| 7F | 98.4 | 2.57 (2.28, 2.89) |
| 19A | 98.4 | 2.07 (1.87, 2.30) |
Geometric mean concentration 1 month after 3rd dose in Primary series (2, 4 and 6 months of age)
[Data from 35} FDA Briefing Document. Vaccines and Related Biological Products Advisory Committee. November 18, 2009.
AAP recommendations for use of PCV13 are detailed in Table 3. [36] A recommendation for catch up dosing through age 5 even for those who have completed four doses of PCV7 was based on the burden of disease due to serotype 19A in the two to five year age cohort and the goal of hastening the reduction of disease due to the 6 new serotypes, especially 19A, in adults by decreasing carriage in children more rapidly than would be expected to occur without a catch up dose. However, it is unclear if a single dose of PCV13 will elicit sufficient protective antibody as de Wals [37] reported that a single dose of PCV7 in children in their second year of life has relatively low effectiveness against IPD.
Table 2b.
OPA Geometric mean concentrations for the 6 Additional PCV13 Serotypes [35]
| PCV 13 (N=91–94; post dose 3) | PCV13 (N=89–94; post dose 4) | |
|---|---|---|
| Serotype | GMT*(95% CI) | GMT* (95% CI) |
| 1 | 52 (39, 69) | 164 (114, 237) |
| 3 | 121 (92, 158) | 380 (300, 482) |
| 5 | 91 (67, 123) | 300 (229, 393) |
| 6A | 980 (783, 1226) | 2242 (1707, 2945) |
| 7F | 9494 (7339, 12281) | 11629 (9054, 14938) |
| 19A | 152 (105, 220) | 1024 (774, 1355) |
N = range in the number of subjects with a determinate antibody titer to the given serotype.
GMT geometric mean titer
Data from {35} FDA Briefing Document. Vaccines and Related Biological Products Advisory Committee. November 18, 2009.
Another potential difference between PCV13 and PCV7 might also be the impact of infant immunization on disease due to serotypes 1, 3 and 5 as well as the development of herd immunity for these serotypes. Immunogenicity for serotypes 1, 3 and 5 was in general lower than for other serotypes (Table 2) and whether protective concentrations of OPA activity have been achieved will require post licensure surveillance studies. In addition, these serotypes are not commonly carried by children, sometimes cause disease in clusters or outbreaks, and may have different sources or patterns of transmission in the community. Whether infant immunization with PCV13 will confer serotype specific indirect protection to adults is unclear.
Potential Impact of PCV 13?
IPD surveillance conducted by the CDC’s Active Bacterial Core (ABC) Surveillance system has reported consistent rates of IPD (22–25 cases per 100,000 children aged <5 years) since 2002. Despite this relative stability in all IPD (caused by any serotype) after introduction of PCV7, the burden of IPD caused by serotypes not covered by PCV7 has gradually increased over time. By 2007, ABCs identified 427 IPD cases with known serotype (overall total 493) in children aged <5 years. Among IPD with known serotype, 274 (64%) were caused by serotypes contained in PCV13 and 95% of these cases were caused by serotypes 3, 7F and 19A, not included in PCV7. The proportions of IPD cases caused the 13 vaccine serotypes were similar among black (61%), white (67%) and other races (62%). [32] During 2007, an estimated 4,600 IPD cases occurred among US children aged <5 years. Approximately, 2,900 (63%) of these IPD cases were caused by PCV13 serotypes and thus, are potentially preventable with PCV13. [32]
Each of the 6 new serotypes selected for PCV13 represents specific targets for prevention that are either important causes of ‘epidemic’ IPD on a global basis [1, 5] or have emerged as frequent causes of invasive disease in at least some of the countries where PCV7 has been introduced (19A, 7F, 3) or represents a cross reactive serotype that has persisted as a potential cause of IPD (6A) or expanded its role in colonization in children but may not as yet have been demonstrated to be a frequent cause of IPD (6C).
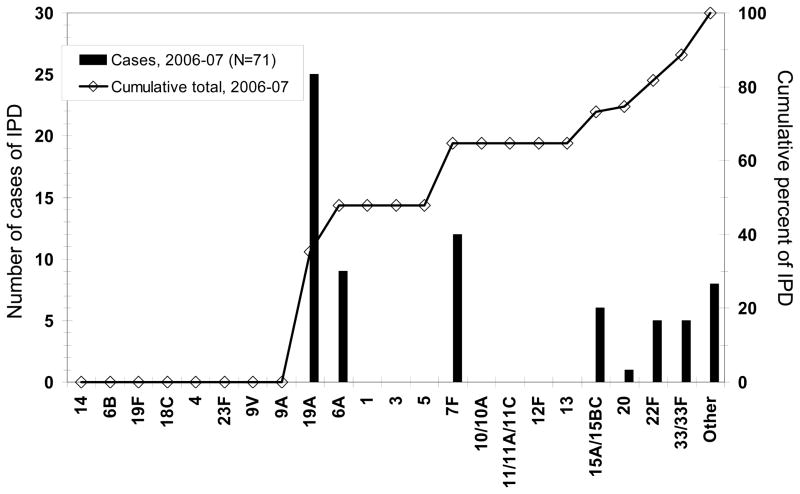
Our studies in Massachusetts demonstrated that ~ 65% of IPD in children in 2009 was due to serotypes 19A, 7F or 3 (Figure 3). [38] Data from the ABC surveillance system reports a similar burden of disease due to these serotypes. Thus, if IPD due to PCV13 serotypes is virtually eliminated as occurred with PCV7, we would achieve a nearly 90% reduction in IPD from baseline (1998/1999) in children.
Figure 3.
Invasive Pneumococcal Disease: Cases in Massachusetts Children Less than 18 Years Age By Serotype [38]
[38] Hsu KK, Shea KM, Stevenson AE, and Pelton. Pediatr Infect Dis J 2010;29(4):289–93
Are there serotypes of concern not in pcv13?
Studies of the ‘invasive capacity’ of pneumococcal serotypes describe differences that appear to explain, at least in part, the success of PCV7 in the US. Following introduction of PCV7, nasopharyngeal carriage of vaccine serotypes has been replaced with non vaccine serotypes, primarily 19A, 6C, 15B/C, 35B, 11A and 23A, each of which has a relatively low propensity to produce IPD other than serotype 19A. This could explain why IPD disease rates remains consistently lower than prior to introduction of PCV7 in the setting of complete replacement in the nasopharynx and the absence of type specific protection against these serotypes.
ABC surveillance reported that increases in the incidence of IPD due to many non-vaccine serotypes have been observed since 2000, but the increases have been modest in the general US population. In part, non vaccine serotypes most likely to result in invasive disease such as 22F and 33F, have not demonstrated increased prevalence in carriage. However, the potential for expansion of more invasive serotype persists and further surveillance will be necessary following introduction of PCV13 to determine if a new ‘19A’ will emerge.
The scenario for prevention of AOM and nonbacteremic pneumonia is less clear as the differences in ‘invasive capacity’ among serotypes with regard to their ability to ascend the Eustachian tube or overcome host defenses of the respiratory track appear to be small. Pichichero reported that serotypes/groups 19A, 6A and 6C, 23B, 15, and 11 were the most common causes of pneumococcal otitis media in the post PCV7 era. [39]
Conclusion
The new PCV13 is likely to reduce the incidence of IPD, pneumonia and otitis media beyond what PCV7 achieved. Surveillance of these conditions and their complications is warranted to assess the effectiveness of the new vaccination program and to monitor the development and effects of serotype replacement.
Table 3.
|
Key Points.
Non-vaccine serotypes are the leading cause of residual invasive and mucosal pneumococcal disease in children currently.
Serotype 19A is currently the major cause of invasive pneumococcal disease, mastoiditis, and refractory AOM in infants and children and may be multidrug resistant.
Empyema is increasing in US children; serotypes 1, 3, 7F and 19A are the major causes of pneumococcal empyema in the post PCV7 era.
A new 13 valent vaccine has been licensed by the FDA that includes six additional polysaccharides (1, 3, 5, 6A, 7F, 19A) conjugated to CRM197 in addition to those in PCV7.
AAP recommendations for the new 13 valent PCV include universal immunization of children less than 2 years of age and catch up for Children through age 5 years who have completed the recommended 4 dose regimen of PCV7
Acknowledgments
Dr. Grijalva received lecture fees and research support from Pfizer. Dr. Grijalva is supported by a CDC career development award (K01 CI000163)
Stephen I. Pelton has investigator initiated research grants from Novartis Vaccine and Diagnostics, Pfizer, Inc, Intercell and GSK bio and has received honorarium for participation in advisory board meeting on conjugate vaccines or as symposium moderators from Novartis Vaccine and Diagnostics, GSK bio and Pfizer, Inc.
Contributor Information
Carlos G. Grijalva, Email: carlos.grijalva@vanderbilt.edu, Assistant Professor of Preventive Medicine, Vanderbilt University Medical Center, Department of Preventive Medicine, 1500 21st. Avenue S, The Village at Vanderbilt - Suite N. 2650, Nashville, TN 37212.
Stephen I. Pelton, Email: spelton@bu.edu, Professor of Pediatrics and Epidemiology, Boston University Schools of Medicine and Public Health Chief, Section of Pediatric Infectious Diseases, Boston Medical Center, 670 Albany Street, 6th Floor, Boston, MA 02118, 617-414-5194.
References
- 1.Black S, Shinefield H, Fireman B. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22(1):10–6. doi: 10.1097/00006454-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–9. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 5.Kayhty H, Auranen K, Nohynek H, et al. Nasopharyngeal colonization: a target for pneumococcal vaccination. Expert Rev Vaccines. 2006;5(5):651–67. doi: 10.1586/14760584.5.5.651. [DOI] [PubMed] [Google Scholar]
- **6.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648593. Thorough analysis of the data from ABC surveillance on the reduction in invasive pneumococcal disease in children and the current picture of residual invasive disease. [DOI] [PubMed] [Google Scholar]
- 7.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295(14):1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 8.Direct and Indirect Effects of Routine Vaccination of Children with 7-Valent Pneumococcal Conjugate Vaccine on Incidence of Invasive Pneumococcal Disease --- United States, 1998---2003. MMWR. 2005;54(36):893–897. [PubMed] [Google Scholar]
- 9.Cohen AL, Harrison LH, Farley MM, et al. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS. 2010;24(14):2253–2262. doi: 10.1097/QAD.0b013e32833d46fd. [DOI] [PubMed] [Google Scholar]
- 10.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–33. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 11.Grijalva CG, Nuorti JP, Arbogast PG, et al. Decline in pneumonia admissions after routine childhood immunization with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369(9568):1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 12.Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J. 2010;29(7):607–12. doi: 10.1097/inf.0b013e3181d7d09c. [DOI] [PubMed] [Google Scholar]
- *13.Patrzalek M, Albrecht P, Sobczynski M. Significant decline in pneumonia admission rate after the introduction of routine 2+1 dose schedule heptavalent pneumococcal conjugate vaccine (PCV7) in children under 5 years of age in Kielce, Poland. Eur J Clin Microbiol Infect Dis. 2010;29(7):787–92. doi: 10.1007/s10096-010-0928-9. Confirms decline in pneumonia admissions as observed in US studies. [DOI] [PubMed] [Google Scholar]
- 14.de Wals P, Robin E, Fortin E, et al. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J. 2008;27(11):963–8. doi: 10.1097/INF.0b013e31817cf76f. [DOI] [PubMed] [Google Scholar]
- 15.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119(4):707–15. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 16.Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118(3):865–73. doi: 10.1542/peds.2006-0492. [DOI] [PubMed] [Google Scholar]
- 17.Sox CM, Finkelstein JA, Yin R, et al. Trends in otitis media treatment failure and relapse. Pediatrics. 2008;121(4):674–9. doi: 10.1542/peds.2007-1565. [DOI] [PubMed] [Google Scholar]
- 18.Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics. 2008;121(2):253–60. doi: 10.1542/peds.2007-0619. [DOI] [PubMed] [Google Scholar]
- 19.Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10(3):195–203. doi: 10.1016/S1473-3099(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 20.de Wals P, Carbon M, Elodie S, et al. Reduced Physician Claims for Otitis Media After Implementation of Pneumococcal Conjugate Vaccine Program in the Province of Quebec, Canada. Pediatr Infect Dis J. 2009;28(9):e271–275. doi: 10.1097/INF.0b013e3181bad212. [DOI] [PubMed] [Google Scholar]
- *21.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124(1):e1–11. doi: 10.1542/peds.2008-3099. Nine year experience in the evolution of pneumococcal nasopharyngeal carriage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emergence of antimicrobial-resistant serotype 19A Streptococcus Pneumoniae --Massachusetts, 2001–2006. MMWR. 2007;56(41):1077–80. [PubMed] [Google Scholar]
- **23.Reinert R, Jacobs MR, Kaplan SL. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine. 2010;28(26):4249–59. doi: 10.1016/j.vaccine.2010.04.020. Review of the emergence of 19A and specifically the multidrug resistance profile of this pathogen in pediatric infections. [DOI] [PubMed] [Google Scholar]
- 24.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298(15):1772–8. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- *25.McNeil JC, Hulten KG, Mason EO, Kaplan SL. Serotype 19A is the most common Streptococcus pneumoniae isolate in children with chronic sinusitis. Pediatr Infect Dis J. 2009;28(9):766–8. doi: 10.1097/INF.0b013e3181a24557. Role of serotypes 19A in chronic sinus infection in children. [DOI] [PubMed] [Google Scholar]
- 26.Ongkasuwan J, Valdez TA, Hulten KG, Mason EO, Jr, Kaplan SL. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics. 2008;122(1):34–9. doi: 10.1542/peds.2007-2703. [DOI] [PubMed] [Google Scholar]
- *27.Grijalva CG, Nuorti JP, Zhu Y, Griffin MR. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin Infect Dis. 2010;50(6):805–13. doi: 10.1086/650573. Highlights increase in empyema in US in post PCV7 era. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li ST, Tancredi DJ. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics. 2010;125(1):26–33. doi: 10.1542/peds.2009-0184. [DOI] [PubMed] [Google Scholar]
- **29.Byington CL, Hulten KG, Ampofo K, et al. Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah. J Clin Microbiol. 2010;48(2):520–5. doi: 10.1128/JCM.01200-09. Identifies importance of nonvaccine pneumococcal serotypes as etiology of empyema in post PCV7 era role of nonvacc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez-Bou S, Garcia-Garcia JJ, Esteva C, et al. Pediatric parapneumonic pleural effusion: epidemiology, clinical characteristics, and microbiological diagnosis. Pediatr Pulmonol. 2009;44(12):1192–200. doi: 10.1002/ppul.21114. [DOI] [PubMed] [Google Scholar]
- **31.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project. PLOS MEDICINE. 2010;7(10):e1000348. doi: 10.1371/journal.pmed.1000348. Provides insight in global burden of pneumococcal disease and serotype distribution by region. Establishes need for broader serotype coverage to reduce global mortality and morbidity from invasive pneumococcal disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine -United States, 2007. MMWR. 2010;59(9):253–7. (March 12, 2010) [PubMed] [Google Scholar]
- *33.Esposito S, Tansey S, Thompson A, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol. 2010;17(6):1017–26. doi: 10.1128/CVI.00062-10. Supports safety and immunogenicity of PCV13 which wascriteria for licensure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Kieninger DM, Kueper K, Steul K, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28(25):4192–203. doi: 10.1016/j.vaccine.2010.04.008. Supports safety and immunogenicity of PCV13 which was criteria for licensure. [DOI] [PubMed] [Google Scholar]
- 35.Bryant-Genevier M, Khoie T, Lee L, Vaillancourt J. FDA Briefing Document. Vaccines and Related Biological Products Advisory Committee. Pneumococcal 13-valent Conjugate Vaccine (Diphtheria CRM197 Protein) 2009 Nov 18; accessed at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM190734.pdf.
- **36.AAP Committee on Infectious Diseases. Pediatrics. 2010;126(1):186–90. Details of AAP recommendations for use of PCV13 and PPV23. [Google Scholar]
- 37.Deceuninck G, de Wals P, Boulianne N, et al. Effectiveness of Pneumococcal Conjugate Vaccine Using a 2+1 Infant Schedule in Quebec. Canada Pediatr Infect Dis J. 2010;29(6):546–549. doi: 10.1097/INF.0b013e3181cffa2a. [DOI] [PubMed] [Google Scholar]
- *38.Hsu KK, Shea KM, Stevenson AE, Pelton SI. Changing serotypes causing childhood invasive pneumococcal disease: Massachusetts, 2001–2007. Pediatr Infect Dis J. 2010;29(4):289–93. doi: 10.1097/INF.0b013e3181c15471. Details pneumococcal serotype distribution of residual invasive pneumococcal disease in children. [DOI] [PubMed] [Google Scholar]
- **39.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. Reports on serotypes of Streptococcus pneumoniae now recovered from middle ear in children with acute OM and recurrent OM. [DOI] [PMC free article] [PubMed] [Google Scholar]