Abstract
Primary cultures of fetal rat cortical neurons and astrocytes were used to test the hypothesis that astrocyte-mediated control of neuronal glutathione (GSH) is a potent factor in neuroprotection against rotenone and paraquat. In neurons, rotenone (0.025 to 1μM) for 4 and 24 h decreased viability as did paraquat (2 to 100μM). Rotenone (30nM) decreased neuronal viability and GSH by 24% and 30%, while ROS were increased by 56%. Paraquat (30μM) decreased neuronal viability and GSH by 36% and 70%, while ROS were increased by 23%. When neurons were co-cultured with astrocytes, their GSH increased 1.5 fold and 5 fold at 12 and 24 h. Co-culturing with astrocytes blocked neuronal death and damage by rotenone and paraquat. Astrocyte-mediated neuroprotection was dependent on the activity of components of the γ-glutamyl cycle. These studies illustrate the importance of astrocyte-mediated glutathione homeostasis for protection of neurons from rotenone and paraquat and the role of the γ-glutamyl cycle in this neuroprotection
Keywords: glutathione, neurons, astrocytes, γ-glutamyl cycle, rotenone, paraquat
1. Introduction
Pesticides, substances used to repel or destroy insects and herbicides employed for weed control, are chemicals applied to our environment in enormous quantities in rural and urban settings. In the United States, pesticides are applied in quantities of at least 4.5 billion pounds per year, while herbicide application is in excess of 500 million pounds per year (Weiss et al. 2004). While the use of such compounds is arguably necessary to maintain food supplies, a striking reality is the toxicity of these compounds to humans. The scientific literature is dense with studies utilizing common pesticides to generate adult animal models for neurodegenerative diseases (Corasaniti et al. 1998; Sherer et al. 2003a). However, there is an incomplete understanding of the relative toxicities of these compounds to the developing brain and the neuroprotective systems present at various stages of brain development that could mitigate such events.
Rotenone is a pro-oxidant inhibitor of complex I (Sherer et al. 2003a) of the respiratory chain which can generate widespread toxic effects in the central nervous system (Lapointe et al. 2004). This inhibition of complex I by rotenone can recapitulate dopamine (DA) neuronal degeneration and associated neurological disabilities in experimental animals (Betarbet et al. 2000; Dawson and Dawson 2003; Schneider et al. 1987; (Sherer et al. 2003b). Paraquat toxicity is primarily generated by reactive oxygen species (ROS) elicited by redox-cycling with molecular oxygen and NADH-dependent formation of superoxide anions (Kim et al. 2008; Suntres 2002). This herbicide induces oxidative stress in both rat astrocytes and neurons (Schmuck et al. 2002; Mollace et al. 2003). These widely used environmental toxins, with chronic treatment of rodents, induce many key features of Parkinson Disease (PD), motor deficits, loss of dopaminergic neurons, and accumulation of synuclein containing inclusion bodies (Betarbet et al. 2000; Brooks et al. 1999; Manning-Bog et al. 2002; Manning-Bog et al. 2003; McCormack and Di Monte 2003; Sherer et al. 2003; Thiruchelvam et al. 2000; Ferrante et al. 1997, Berarbet et al. 2000).
Astrocytes play a crucial role in the maintenance of brain GSH, which is vital for neuronal survival in baseline or enhanced pro-oxidant settings. A key mechanism underlying this function is the γ-glutamyl cycle. This cycle essentially consists of GSH being extruded from astrocytes where it is hydrolyzed to the Cysgly dipeptide by γ-glutamyl transpeptidase (γ-GT) at the external surface of the astrocyte plasma membrane. This dipeptide is subsequently hydrolyzed by aminopeptidase N (ApN) at the external neuronal plasma membrane, yielding Cys which is transported into the neuronal cytoplasm, driving the synthesis on GSH (Dringen et al. 1999a; Dringen et al. 1999b; Rathinam et al. 2006). We have previously found that this astrocyte-mediated antioxidant system provides protection of neurons from ethanol-mediated oxidative damage, likely a much milder pro-oxidant setting than those generated by either rotenone or paraquat. This neuroprotection occurs due to cerebral cortical astrocytes having a robust GSH synthesizing capacity which is reflected in high GSH content (Cooper 1997; Schulz et al. 2000) and having numerous close contacts with neurons (Wolff 1996). This is an elegant, highly regulated system that is subject to manipulation.
The following studies illustrate the varying toxicity of rotenone and paraquat towards neurons and astrocytes, the critical role of the γ-glutamyl cycle in the maintenance of neuronal GSH homeostasis, and a central role for astrocytes in protection of neurons from rotenone and paraquat.
2. Materials and Methods
2.1. Materials
Minimum Essential Medium (MEM), Dulbecco's Modified Eagle Medium (DMEM), Fetal Bovine Serum (FBS) were from Invitrogen (Carlsbad, CA). Monochlorobimane (MCB), 2,7′-Dichlorofluorescein diacetate (DCF-DA), Horse serum (HS), Bovine serum albumin (BSA), N-acetylcysteine (NAC), rotenone, paraquat, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), acivicin and bestatin were from Sigma (St. Louis, MO). Annexin Cy 3 was from Abcam (Cambridge, MA) and 7-Amino- Actinomycin D (7AAD) from BD Pharmingen (San Diego, CA).
2.2. Cortical Neuron Culture
Primary cortical neuron cultures were prepared from embryonic day 17 rats (Sprague-Dawley) as described (Dutton 1990; Ramachandran et al. 2003). Neurons were suspended in MEM containing 10% horse serum. They were plated in 6 well culture plates (1.2 ×106 cells/well) previously coated with poly-D-lysine. On the following day, neurons were treated with mitotic inhibitors to prevent astrocyte contamination. Cells were fully differentiated by day 5 in-vitro and ready to use for the experiments detailed below.
2.3. Astrocyte Cultures
Cortical Astrocytes were prepared from the cerebral hemispheres of 2-day-old Sprague Dawley rat neonates as described by McCarthy and de Vellis (1980). In brief, brain cortices were removed; tissue was disrupted, treated with 2.5% trypsin and DNase, filtered through 0.25 μm sieve and seeded in 75 cm2 plastic flasks at a density of 6 × 106 cells. The cultures were grown to confluency in DMEM with 10% fetal bovine serum (FBS) and antibiotics. The medium was changed every 3 days and cultures were split on day 7 into the desired plates (100 mm, 60 mm or 6-well). The astrocytes were grown to confluency and were identified as more than 95% pure by staining with glial fibrillary acidic protein. All animal protocols were approved by Institutional Animal Care and Use Committee. Handling and treatment of animals were carried out according to the National Institutes of Health guidelines.
2.4. Co-Culture
Cortical astrocytes prepared from 2-day-old Sprague Dawley rat cortices were suspended in a culture medium consisting of Eagle's minimal essential medium (MEM) with 10% fetal bovine serum and glutamine (2 mM), and plated in 75 cm2 cell culture flasks. At confluency, cells were lightly trypsinized and replated onto cell culture inserts (Fisher-Costar Brand, Fisher Scientific, Pittsburgh, PA, USA) at 2.5 × 105 cells/well and were used 7 days after re-plating, at which time they formed a confluent layer across the surface of the insert. Primary cortical neuron cultures prepared from embryonic day 17 rats were suspended in glial conditioned medium (MEM containing 10% horse serum) and plated into 6-well culture plates (1.2 × 106 cells/ well) previously coated with poly D-lysine. On the following day, neurons were treated with mitotic inhibitors to prevent astrocyte contamination. On the third day in vitro, inserts containing confluent astrocyte cultures were placed in wells containing neurons.
2.5. Toxin Treatments
On day 5 in vitro, neuron cultures were exposed to various concentrations of rotenone (0.025μM-1μM) and paraquat (2μM-100μM) for 4 and 24 h. Confluent astrocyte cultures were treated with 0.1μM- 100μM rotenone and 0.05 - 2mM paraquat. Parallel controls were used for baseline measures within cultures.
2.6. DNA Measures
DNA was assayed using a kit from Trevigen (Trevingen Inc., Gaithersburg, MD, USA). Cells were washed with PBS and re-suspended in serum-free medium. To 150 μL cell suspension, 50 μL diluted Hoechst dye solution were added and incubated at 37°C for 1 h. The fluorescence was recorded at excitation 350 nm and emission 460 nm. DNA content of the sample was calculated from a standard curve which used DNA from salmon testes (Aldrich, Milwaukee, WI, USA).
2.7. Viability Measures
Trypan blue (TB) exclusion
Cells were cultured as stated above and treated in the presence or absence of rotenone or paraquat. Subsequently, they were harvested by light trypsinization and suspended in defined media. A suspension of 0.2 ml was added to 0.5 ml trypan blue (0.4%) and 0.3 ml Hanks' balanced salt solution (HBSS). The number of trypan blue negative cells was counted using a hemocytometer to determine viable cells.
MTT assay
Another measure of viability utilized the MTT kit. Cultures were removed from the incubator, washed and MTT was added in an amount equal to 10% of the culture medium volume. Cultures were then returned to the incubator for 2 h. After incubation, the resulting formazan crystals were dissolved by adding an amount of MTT solubilization solution equal to the original media volume. This was mixed until all crystals were dissolved. Absorbance intensity was then measured at 570 nm, with background measured at 690 nm.
2.8. Measurement of ROS using 2, 7′-dichlorofluoroscein diacetate (DCF-DA)
Generation of reactive oxygen species (ROS) was estimated with the fluorescent probe, DCF-DA as previously described by Jung et al. (2001). Cells were harvested and washed with PBS and re-suspended at 1 × 106 cells/ml. DCF-DA was added to a final concentration of 20 μM and incubated for 30 min at 37°C. Following incubation, the cells were washed with PBS and re-suspended in PBS. ROS generation was measured by fluorescence intensity (505 nm Ex, 535 nm Em) of 10,000 cells with a FACS.
2.9. Measurement of Reduced Glutathione
The fluorometric GSH assays were based on those published by Ju et al. (2000), which used a quantization approach described by Fernandez-Checa and Kaplowitz (1990). Confluent monolayers were washed with phosphate buffered saline (PBS), then scraped and incubated with 100 μM monochlorobimane (MCB) for 10 min at 37°C. The cells were lysed with triton X-100 (0.02%) and centrifuged at 13,000 g. Fluorescence of the MCB–GSH complex in the supernatant fluid was measured (360 Ex, 460 Em) on a Perkin Elmer HTS 7000 Bioassay Reader (Perkin Elmer, Wellesley, MA, USA). GSH content was calculated with a standard curve prepared from GSH incubated with MCB and glutathione-S-transferase (0.1U). GSH was expressed as mmoles/mg DNA.
Flow cytometry was also used to measure the MCB fluorescence intensity in large population (10,000 cells). After rotenone and paraquat exposure, cells were labeled with 100μM of MCB for 30 min. cells were scraped, washed once, re-suspended in cold PBS with 1% BSA, and immediately analyzed using FACS.
2.10. Annexin Cy3 and 7 AAD assay
Annexin Cy3 binding was used as a measure of apoptotic cell death. Following treatment, cells (floating and adherent) were harvested and stained with Annexin Cy3. Annexin Cy3 was added to each tube and incubated at room temperature for 5 min in the dark. Cells were washed, resuspended and stained with 5μl of 7 AAD and incubated for 10 min in the dark before analysis. Annexin Cy3 was analyzed by FACS (Ex 543nm and Em 570nm). The 7- AAD fluorescence was detected in the far red range (650nm).
2.11. Statistical Analysis
Results are expressed as mean ± SEM. One-way analysis of variance (ANOVA) followed by Students Newman Kuel's test was used for comparisons between the control and treated groups. A value of P<0.05 was considered statistically significant.
3. Results
3.1. Rotenone or Paraquat Alter the Viability of Fetal Cortical Neurons and Astrocytes Differently
Following treatment of neurons and astrocytes with varying concentrations of rotenone and paraquat, viability of the cells was determined by trypan blue exclusion. Viable cells, excluding the dye were manually counted. Rotenone treatment (0.08μM to 0.5μM) for 4 h (Fig.1a) decreased TB exclusion by 25 – 76% (P<0.05) and by 32 – 95% (P<0.05) when treated for 24 h (0.025μM to 1μM), Similarly, paraquat treatment for 4 h (Fig. 1c, 30μM to 100μM) decreased TB exclusion by 32% and 53% at 24 h (Fig. 1d). Rotenone was clearly more neurotoxic than paraquat and toxicities were dose and time-dependent. Fig. 2a, b, c and d depict the toxic responses in astrocytes. Rotenone treatment (Fig. 2a, b, 0.5μM to 100μM) reduced viability at 4 h by 81% and at 24 h by 44 – 100% (0.1 μM to 100 μM, P<0.05). Astrocytes were highly resistant to paraquat. Paraquat exposure (Fig.2c, d, 50μM to 2mM) had no significant impact on TB exclusion at 4 h and with 24 h exposure, 100 μM was required to elicit a 40% reduction in viability. Higher concentrations of rotenone and paraquat were used for the astrocyte experiments than for neurons due to the greater resistance of this glia to the two toxins.
Fig.1.
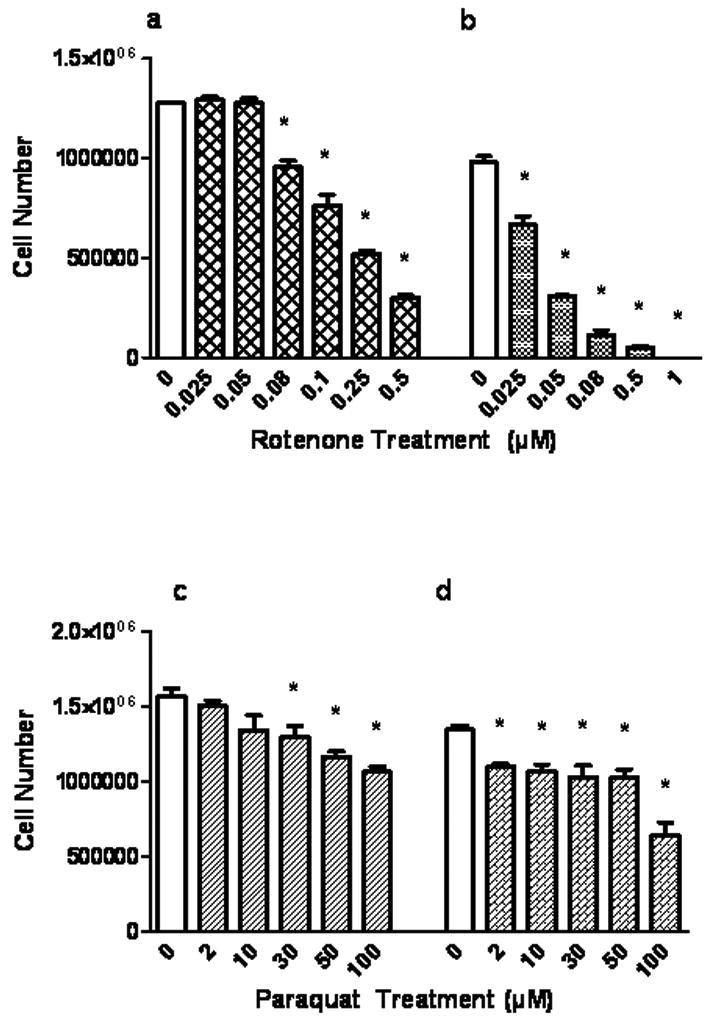
Effect of rotenone and paraquat on primary cortical neuron (PCN) trypan blue exclusion. Cells were treated with rotenone for 4 h (a) and 24 h (b) or with paraquat (c) for 4 h and 24 h (d), with varying concentrations as indicated. Cell number refers to the number of trypan blue negative cells. Values are expressed as the mean±SEM. * denotes P<0.05, treated cells compared to control (0).
Fig.2.
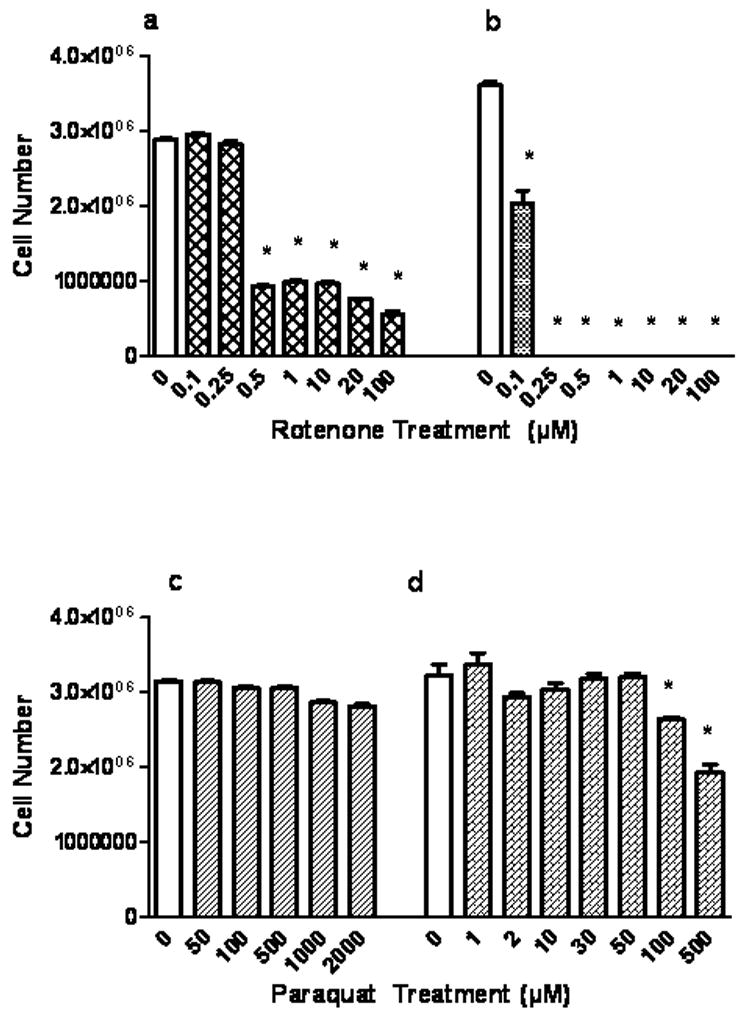
Rotenone and paraquat effects on astrocytic trypan blue exclusion. Cells were treated with rotenone for 4 h (a) and 24 h (b) or with paraquat (c) for 4 h and 24 h (d), with varying concentrations as indicated. Cell number refers to the number of trypan blue negative cells. Values are expressed as the mean±SEM. * denotes P<0.05, treated cells compared to control (0).
3.2. Rotenone or Paraquat Alter GSH Content and ROS Production in Neurons and Astrocytes Differently
Based on an approximate 50% neuron viability decrease by trypan blue exclusion (Fig.1a, b, c and d), the following dosages were chosen for 24 h stressor exposure to assess related toxicity measures on neurons and astrocytes. The 5 day in vitro cultures of neurons were exposed to rotenone 30nM (24 h) or paraquat 30μM (24 h) and confluent astrocytes were exposed to 100nM rotenone for 24 h or 500μM paraquat for 24 h.
Fig. 3a illustrates effects of the two toxins on neuronal viability, as reflected by mitochondrial function (succinate dehydrogenase activity), total GSH, and ROS levels. Fig. 3b depicts the same responses (or lack of) in astrocytes. Rotenone or paraquat treatments for 24 h significantly decreased neuron viability by 76% (P<0.05) and 97% (P<0.05), respectively while concomitantly reducing GSH by 30% and 70% (P<0.05), respectively and increasing ROS by 41% (P<0.05) and 89% (P<0.05), respectively. Astrocytes (fig. 3b) exposed to rotenone or paraquat (albeit at considerably higher concentrations) expressed decreased viability by 70 and 80% (P<0.05), respectively while GSH content was decreased by 14% (P<0.05), only with paraquat exposure. ROS was assessed in terms of DCF fluorescence and was increased by 18 and 22% (P<0.05) when treated with rotenone or paraquat. These two figures clearly illustrate the more robust GSH homeostasis machinery in the astrocyte than in the neuron.
Fig.3.
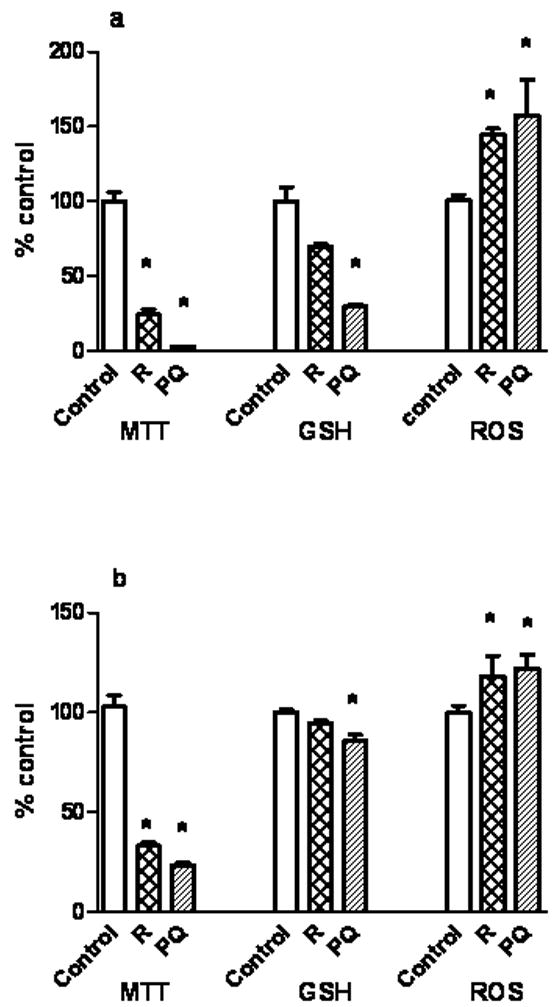
Rotenone and paraquat treatment for 24 h alters cell viability (MTT assay), GSH content, and reactive oxygen species (ROS) in neurons and astrocytes. The 5 day in vitro cultures of neurons were exposed to rotenone 30nM and paraquat 30μM (fig. 3a.). Confluent astrocytes were exposed to 100nM rotenone and 500μM paraquat (fig. 3b). MTT was assayed as a measure of cell viability based on mitochondrial succinate dehydrogenase activity. GSH was measured using MCB fluorescence. ROS was assayed using DCF fluorescence by FACS. Values are expressed as the mean±SEM. N=3. * denotes P<0.05, treated cells compared to control
3.3. Astrocytes Augment Neuron GSH Content and Protect Neurons From Rotenone and Paraquat
Typically, cerebral cortical astrocytes contain higher concentrations of GSH than neurons (Dringen et al. 2000). This likely reflects a more robust GSH homeostasis machinery and possibly an increased Nrf2/ARE pathway activation (Johnson et al. 2002) the former being supported by the experiments illustrated in Fig. 3. The enhanced basal GSH levels and efflux from astrocytes (Rathinam et al. 2006) are key components of the γ-glutamyl cycle and can protect neurons from oxidative insult in a co-cultured environment (Dringen et al. 2000; Dringen et al. 1999b; Rathinam et al. 2006; Shih et al. 2003). Therefore, neurons were co-cultured with astrocytes to assess their protective capacity towards the well documented toxicity of rotenone and paraquat. The experiments in Fig. 4 demonstrate the ability of co-cultured astrocytes to elevate baseline neuronal GSH to a degree equivalent to N-acetylcysteine (NAC) pre-treatment. Neurons in co-culture with astrocytes had a significant increase in GSH by 1.5 fold at 12 h and by 5 fold (p <0.05) at 24 h which is comparable to that occurring when neurons are treated with NAC treatment.
Fig.4.
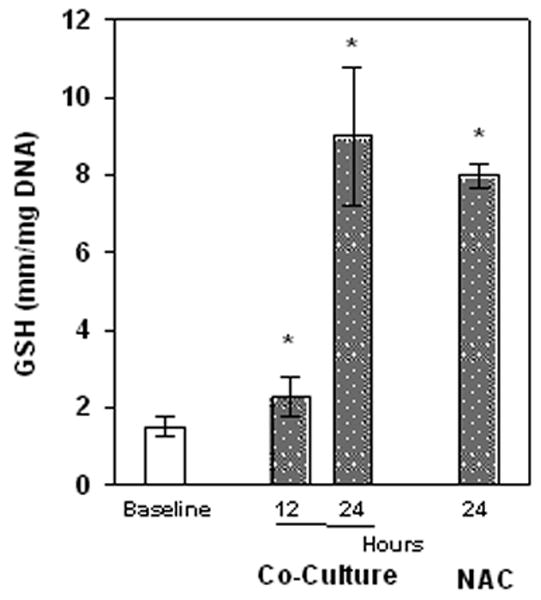
GSH levels in neurons with NAC pretreatment or co-cultured with cortical astrocytes. Neurons alone or co-cultured with astrocytes for 12 or 24 h or incubated with 5mM NAC (24 h). Values are expressed as the mean±SEM. n=3.* denotes P<0.05, treated cells compared to Baseline values.
Fig. 5 illustrates a striking degree of neuroprotection from rotenone (5a) and paraquat (5b) when neuronal GSH content is enhanced by pretreatment with NAC, using trypan blue exclusion as the viability measure. These should be compared to Fig. 1a and 1c. Neurons co-cultured with astrocytes were also strikingly protected from rotenone (Fig. 5c) or paraquat (Fig. 5d) toxicity. Survival was clearly enhanced for the rotenone exposed neurons in co-culture, as reflected by the decrease in neuronal death to 21% (P<0.05, fig. 5c) from 76% (Fig.1 a). Enhanced neuronal GSH provided almost complete protection against paraquat (Fig. 5d compared to Fig. 1c).
Fig.5.
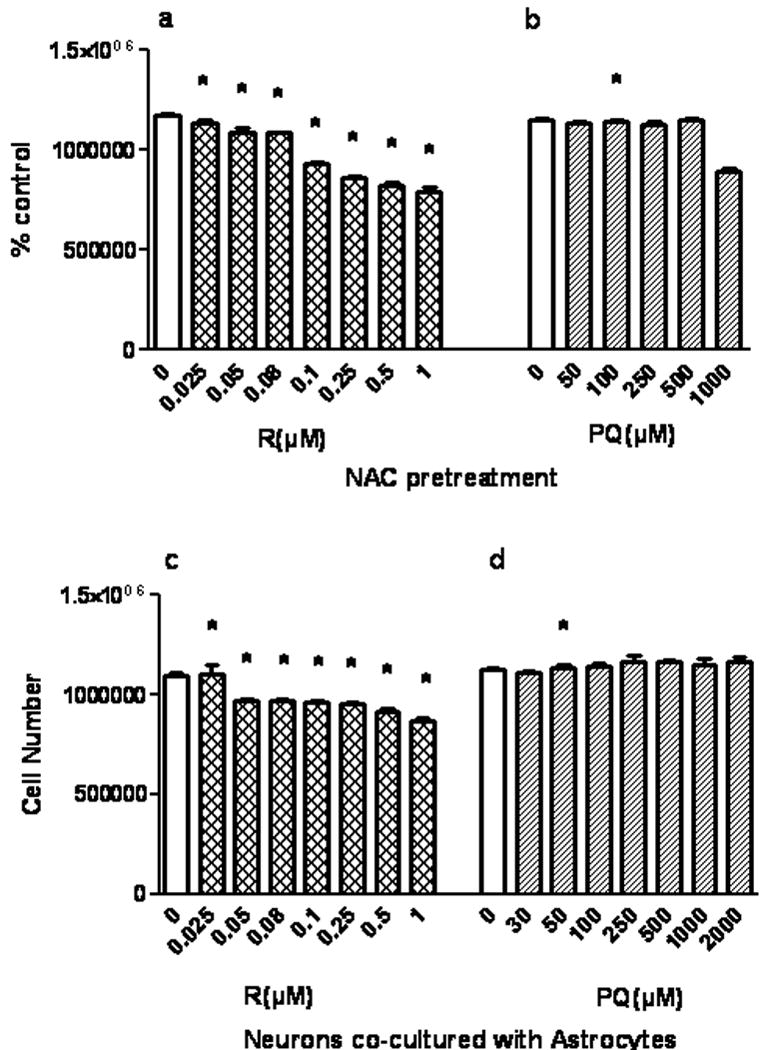
Protection of neurons from rotenone and paraquat by GSH augmentation via pretreatment with NAC or by co-culture with astrocytes. Neurons were pretreated with 5mM NAC (fig. 5a and b) or co-cultured with astrocytes (fig. 5c and d) in the presence of varying concentrations of rotenone or paraquat for 4 h. % control refers to the number of trypan blue negative cells as a % on control values (0). Values are expressed as the mean±SEM. n=3. * denotes P<0.05, treated cells compared to control in fig 1a.
3.4. γ-GT and ApN are required for Astrocyte- Mediated Neuroprotection
Maintaining astrocyte GSH content and its efflux are initial steps in astrocytic maintenance of neuronal GSH homeostasis (Rathinam et al. 2006). The second step in this neuroprotective pathway is hydrolysis of the effluxed GSH to the dipeptide, Cysgly, by γ-GT which is highly concentrated on the astrocyte plasma membrane, and ultimately to Cys by ApN at the neuronal plasma membrane. Fig. 6, illustrate the importance of this three step generation of Cys for protection from rotenone and paraquat. In Fig. 6, cell death was assessed as a measure of Annexin CY 3. Illustrated in panel (a), are 8.3 (P<0.05) and 13.7(P<0.05) fold increases in early apoptotic neuron death with rotenone and paraquat respectively. These were reduced by 5.5 fold (P<0.05) and 11.6 fold (P<0.05) when co-cultured with cortical astrocytes (6b). In group (c), astrocyte γ-GT activity was inhibited with 0.5mM acivicin prior to co-culture. This inhibition, albeit not complete (63%) increased the percent of annexin Cy3 positive cells by 2.7 fold (P<0.05) and 3.3 fold (P<0.05) with rotenone and paraquat, respectively. This effect was confirmed by experiments in which γ-GT was knocked down to a similar degree by siRNA treatment.
Fig.6.
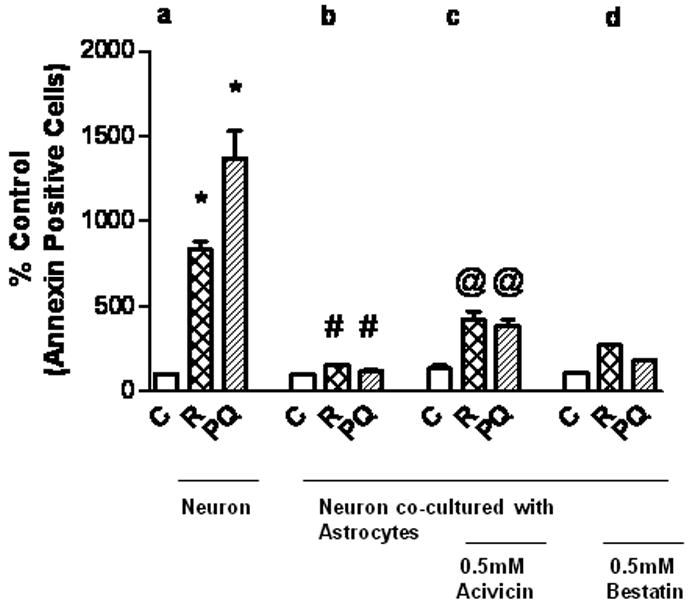
Importance of the γ-glutamyl cycle components γ-GT and ApN for astrocyte-mediated neuroprotection. Neurons were exposed to 30nM rotenone or 30μM paraquat for 24 h either alone (a) or co-cultured with astrocytes (b, c, d). Two sets of exposures were also treated with either 0.5 mM acivicin (c) to inhibit γ-GT or with 0.5 mM bestatin (d) to inhibit ApN. The percentage of Annexin Cy3 positive cells is taken as a measure of apoptotic death.
4. Discussion
Rotenone and paraquat are toxic to the developing brain (Choi et al. 2010). In our laboratory, we have shown that oxidative stress which can ultimately enhance apoptotic death of cortical neurons can be mitigated by the presence of astrocytes and that the underlying mechanism is maintenance of neuronal GSH homeostasis via the γ-glutamyl cycle (Rathinam et al. 2006; Watts et al. 2005). In the present study, we report that in a neuron-astrocyte co-culture setting, the toxic effects of rotenone and paraquat can also be strikingly reduced, through such a GSH dependent mechanism. This initial report addresses a setting relevant to a later in utero stage of brain development during which the mature astrocytes that can provide this protection are increasingly expressed (Bandeira et al. 2009). It illustrates the importance of maintenance of neuron GSH homeostasis in this developmental setting.
4.1. Rotenone and Paraquat Toxicity towards Fetal Cerebral Cortical Neurons and Astrocytes
The above experiments show that viability of cortical neurons and astrocytes were reduced significantly by rotenone and paraquat, in a time and dose-dependent fashion. Both toxins have a higher impact on neurons than astrocytes as determined by measures of plasma membrane integrity and the MTT test, which is consistent with other reports, at least with paraquat (Schmuck et al. 2002). For neurons, a rotenone concentration of about 0.1 uM elicited a 50% increase in trypan blue internalization (Fig. 1a), while a concentration of greater than 0.25 uM was required for the same response in astrocytes (Fig. 2a). Both cell types were less sensitive to paraquat (Figs 1 c, d and 2 c, d) than to rotenone yet astrocytes were clearly the least sensitive. A 24 h exposure to 100 uM paraquat was required to elicit a significant decrease in trypan blue exclusion by astrocytes (Fig. 2 d). A likely explanation for the lesser astrocyte sensitivity to these two pro-oxidants is that cerebral cortical astrocytes have a more robust GSH homeostasis system which enables a more proficient oxygen radical detoxification and higher steady state levels of GSH than neurons (Sagara et al. 1993; Wilson 1997). However, astrocytes can be damaged by rotenone especially for prolonged exposures (Fig. 2 and 4) as well as by paraquat, albeit to a much lesser degree. Thus, it is clear that one target for these toxins could be damage to the neuroprotective capacity of astrocytes, a topic that will be addressed in detail by future experiments.
4.2. Neuroprotection by Astrocytes
Cellular GSH is a vital line of defense against pro-oxidants and it plays a cytoprotective role. Thus, administration of NAC to adult rats prior to paraquat challenge protects against subsequent toxicity and this can be reproduced in vitro with cultured alveolar cells (Suntres 2002). Augmenting neuronal GSH content by NAC pretreatment, protected neurons from rotenone and paraquat toxicity in our current study (Fig.4). Similar observations have been reported by Watts et al (2005) and Ramachandran et al (2003) for another pro-oxidant. We have also found that exposure to mild pro-oxidants elicits oxidative stress-mediated apoptotic death of a select subpopulation of cerebral cortical neurons that contain low GSH content (Maffi et al. 2008) and that this can be blocked by overexpression of Nrf2 which enhances activation of components of the γ-glutamyl pathway as well as other elements of the neuronal GSH homeostasis machinery (Narasimhan et al, 2011). This is a key function of the astrocyte, to augment the availability of cysteine which is a determinant of GSH synthesis (Kranich et al. 1998; Kranich et al. 1996; Wang and Cynader 2000). The experiments presented above document the relevance of astrocyte-mediated neuroprotection from rotenone and paraquat and supply one layer of mechanism underlying this. They illustrate that when astrocytes are present in the neuronal environment, the toxic effects of rotenone and paraquat are mitigated (Fig. 5 and 6). However, they also demonstrate that at early in utero stages of brain development, the cerebral cortical neurons can be more susceptible to damage by these two environmental toxins. The patterns of protection (Fig. 5 a and c) are almost identical to those seen with NAC pretreatment, both of which equally increase neuron GSH content (Fig. 4). That there is a functional connection between this GSH augmentation and astrocyte-mediated neuroprotection is confirmed by the experiments illustrating that full protection requires an intact γ-glutamyl pathway with functional γ-glutamyl transpeptidase and aminopeptidase N (Fig. 6).
A significance of an understanding of this astrocyte role is that mature multistellate astrocytes are only found in the brain in considerable numbers following the human second trimester. Thus, components of this neuroprotective cycle are absent during the early stages of brain development (data not shown), a period of high brain vulnerability to such toxins (Bandeira et al. 2009). This dictates that interventions aimed at astrocytes will primarily be of value subsequent to the second trimester of brain development, while during the earlier stages, such intervention measures must address neuronal components of GSH homeostasis machinery. Work is ongoing in our lab addressing manipulations of GSH homeostasis machinery in primary fetal cortical neurons.
In conclusion, these studies clearly illustrate the striking neuroprotective capacity of astrocytes against rotenone and paraquat and link this to the activity of the γ-glutamyl cycle and its ability to augment neuronal GSH homeostasis.
Highlights for the paper.
The developing brain can be damaged by rotenone (R) and paraquat (P).
Fetal neurons are more sensitive to rotenone and paraquat than astrocytes.
Mature astrocytes can augment neuron GSH homeostasis via the γ-glutamyl cycle.
Co-culturing fetal neurons with astrocytes protects from R and P-related damage and death.
This protection is provided by the γ-glutamyl cycle.
Acknowledgments
This work was supported by R01 ES015700. We would like to thank Dr. Patrick Reynolds, Director of the TTUHSC Cancer Center Flow Cytometry Core Facility for use of the core, and Dr Malkanthi Mudannayake and Matthew V. Wilkin for helping us generate the FACS data.
Footnotes
Conflicts of interest: All the authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A. 2009;106(33):14108–14113. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823(1-2):1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Choi WS, Abel G, Klintworth H, Flavell RA, Xia Z. JNK3 mediates paraquat- and rotenone-induced dopaminergic neuron death. J Neuropathol Exp Neurol. 2010;69(5):511–520. doi: 10.1097/NEN.0b013e3181db8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. Glutathione in the brain: disorders of glutathione metabolism. In: Rosenberg RNPS, DiMauro S, Barchi RI, Kunk LM, editors. The molecular and genetic basis of neurological disease. Boston: Butterworth- Heinemann; 1997. pp. 1195–1230. [Google Scholar]
- Corasaniti MT, Strongoli MC, Rotiroti D, Bagetta G, Nistico G. Paraquat: a useful tool for the in vivo study of mechanisms of neuronal cell death. Pharmacol Toxicol. 1998;83(1):1–7. doi: 10.1111/j.1600-0773.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267(16):4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Kussmaul L, Gutterer JM, Hirrlinger J, Hamprecht B. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J Neurochem. 1999a;72(6):2523–2530. doi: 10.1046/j.1471-4159.1999.0722523.x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999b;19(2):562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton G. Isolation, culture and use of viable central nervous system perikarya. In: Conn VS, editor. Methods in Neuroscience. New York: Academic Press; 1990. pp. 87–102. [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990;190(2):212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Schulz JB, Kowall NW, Beal MF. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997;753(1):157–162. doi: 10.1016/s0006-8993(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem. 2002;81(6):1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- Ju C, Yoon KN, Oh YK, Kim HC, Shin CY, Ryu JR, Ko KH, Kim WK. Synergistic depletion of astrocytic glutathione by glucose deprivation and peroxynitrite: correlation with mitochondrial dysfunction and subsequent cell death. J Neurochem. 2000;74(5):1989–1998. doi: 10.1046/j.1471-4159.2000.0741989.x. [DOI] [PubMed] [Google Scholar]
- Jung Y, Surh Y. Oxidative DNA damage and cytotoxicity induced by copper-stimulated redox cycling of salsolinol, a neurotoxic tetrahydroisoquinoline alkaloid. Free Radic Biol Med. 2001;30(12):1407–1417. doi: 10.1016/s0891-5849(01)00548-2. [DOI] [PubMed] [Google Scholar]
- Kim DW, Kim SY, Lee SH, Lee YP, Lee MJ, Jeong MS, Jang SH, Park J, Lee KS, Kang TC, Won MH, Cho SW, Kwon OS, Eum WS, Choi SY. Protein transduction of an antioxidant enzyme: subcellular localization of superoxide dismutase fusion protein in cells. BMB Rep. 2008;41(2):170–175. doi: 10.5483/bmbrep.2008.41.2.170. [DOI] [PubMed] [Google Scholar]
- Kranich O, Dringen R, Sandberg M, Hamprecht B. Utilization of cysteine and cysteine precursors for the synthesis of glutathione in astroglial cultures: preference for cystine. Glia. 1998;22(1):11–18. [PubMed] [Google Scholar]
- Kranich O, Hamprecht B, Dringen R. Different preferences in the utilization of amino acids for glutathione synthesis in cultured neurons and astroglial cells derived from rat brain. Neurosci Lett. 1996;219(3):211–214. doi: 10.1016/s0304-3940(96)13217-1. [DOI] [PubMed] [Google Scholar]
- Lapointe N, St-Hilaire M, Martinoli MG, Blanchet J, Gould P, Rouillard C, Cicchetti F. Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J. 2004;18(6):717–719. doi: 10.1096/fj.03-0677fje. [DOI] [PubMed] [Google Scholar]
- Maffi SK, Rathinam ML, Cherian PP, Pate W, Hamby-Mason R, Schenker S, Henderson GI. Glutathione content as a potential mediator of the vulnerability of cultured fetal cortical neurons to ethanol-induced apoptosis. J Neurosci Res. 2008;86(5):1064–1076. doi: 10.1002/jnr.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277(3):1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23(8):3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and aligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem. 2003;85(1):82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- Mollace V, Iannone M, Muscoli C, Palma E, Granato T, Rispoli V, Nistico R, Rotiroti D, Salvemini D. The role of oxidative stress in paraquat-induced neurotoxicity in rats: protection by non peptidyl superoxide dismutase mimetic. Neurosci Lett. 2003;335(3):163–166. doi: 10.1016/s0304-3940(02)01168-0. [DOI] [PubMed] [Google Scholar]
- Narasimhan M, Mahimainathan L, Rathinam ML, Riar AK, Henderson GI. Overexpression of Nrf2 Protects Cerebral Cortical Neurons from Ethanol-induced Apoptotic Death. Mol Pharmacol. 2011;80(6):1–12. doi: 10.1124/mol.111.073262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74(4):577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Rathinam ML, Watts LT, Stark AA, Mahimainathan L, Stewart J, Schenker S, Henderson GI. Astrocyte control of fetal cortical neuron glutathione homeostasis: up-regulation by ethanol. J Neurochem. 2006;96(5):1289–1300. doi: 10.1111/j.1471-4159.2006.03674.x. [DOI] [PubMed] [Google Scholar]
- Sagara JI, Miura K, Bannai S. Maintenance of neuronal glutathione by glial cells. J Neurochem. 1993;61(5):1672–1676. doi: 10.1111/j.1471-4159.1993.tb09802.x. [DOI] [PubMed] [Google Scholar]
- Schmuck G, Rohrdanz E, Tran-Thi QH, Kahl R, Schluter G. Oxidative stress in rat cortical neurons and astrocytes induced by paraquat in vitro. Neurotox Res. 2002;4(1):1–13. doi: 10.1080/10298420290007574. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Yuwiler A, Markham CH. Selective loss of subpopulations of ventral mesencephalic dopaminergic neurons in the monkey following exposure to MPTP. Brain Res. 1987;411(1):144–150. doi: 10.1016/0006-8993(87)90691-3. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267(16):4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003a;23(34):10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003b;179(1):9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23(8):3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180(1):65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci. 2000;20(24):9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 2000;74(4):1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- Watts LT, Rathinam ML, Schenker S, Henderson GI. Astrocytes protect neurons from ethanol-induced oxidative stress and apoptotic death. J Neurosci Res. 2005;80(5):655–666. doi: 10.1002/jnr.20502. [DOI] [PubMed] [Google Scholar]
- Weiss B, Amler S, Amler RW. Pesticides. Pediatrics. 2004;113(4 Suppl):1030–1036. [PubMed] [Google Scholar]
- Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75(10-11):1149–1163. [PubMed] [Google Scholar]
- Wolff ARaJ. Subcellular topography and plasticity of gap junction distribution on astrocytes. In: Dermietzel DSaR., editor. Neuroscience Intelligence. Austin: Springer; 1996. pp. 175–189. [Google Scholar]


