Abstract
Pseudoxanthoma elasticum (PXE), a heritable multisystem disorder, is caused by mutations in the ABCC6 gene. We have developed a murine model for PXE by targeted inactivation of the corresponding mouse gene. A feature of this mouse model is ectopic mineralization of connective tissue capsule surrounding the bulb of vibrissae. This study was designed to investigate the effect of dietary sevelamer hydrochloride (Renagel®), a phosphate binder, and specific mineral modifications on ectopic mineralization of connective tissue in Abcc6−/− mice. Three groups were fed a specific diet: (1) a standard rodent diet, (2) a standard rodent diet supplemented with sevelamer hydrochloride, and (3) a custom experimental diet with specific mineral modifications (high phosphorus, low calcium and low magnesium). The degree of mineralization was determined in H&E stained sections using computerized morphometric analysis and by chemical assays to measure the calcium and phosphorus content of the vibrissae. The results indicated increased mineralization in the Abcc6−/− mice fed a standard diet or a diet with mineral modifications as compared to control mice fed a standard diet. Furthermore, feeding Abcc6−/− mice with diet supplemented with sevelamer hydrochloride did not improve mineralization, in comparison to mice fed with normal diet. Collectively, these results suggest that the mineralization process in PXE may be exacerbated by changes in mineral intake. The role of dietary minerals, and phosphorus in particular,, as well as that of phosphate binders, in ectopic mineralization of PXE, merits further investigation.
Keywords: Pseudoxanthoma elasticum, heritable skin diseases, ectopic mineralization, phosphate binders
Introduction
Pseudoxanthoma elasticum (PXE; OMIM #264800) is an autosomal recessive systemic disorder characterized by progressive mineralization of connective tissues primarily within the dermis of the skin, Bruch’s membrane of the retina, and the mid layers of the arterial blood vessels (1). The characteristic histopathologic finding in the skin in PXE is accumulation of pleiomorphic elastotic structures which become progressively mineralized. PXE results from mutations in the ABCC6 gene, a member of the ATP-binding cassette family, encoding multidrug resistance-associated protein 6 (MRP6), a putative membrane transporter (2,3). The ABCC6 gene is expressed primarily in the baso-lateral surface of the liver, to a lower extent in the proximal tubules of the kidneys, and at very low level, if at all, in resident cells, such as fibroblasts and smooth muscle cells, in tissues affected by PXE (4,5). These observations have allowed us to develop the hypothesis that PXE is a “metabolic disease” (6).
PXE presents a diagnostic dilemma due to late onset of manifestations and considerable both intra- and interfamilial heterogeneity. Specifically, there is variability as to the major organ systems being affected, so that in some families, eye manifestations predominate leading to loss of visual acuity, in other families skin manifestations may be the major problem, while yet in other families cardiovascular involvement can lead to considerable morbidity and even mortality (1). The reasons for this phenotypic variability are currently unknown. Mouse genetic studies (7,8) have suggested that modifier genes may alter the degree of mineralization, and recent human genetic observations (9,10) similarly suggest that modifier genes also contribute to the phenotypic heterogeneity in humans.. At the same time, early epidemiologic studies have suggested that high intake of dairy products (rich in calcium and phosphorus) during childhood or adolescence correlates with the severity of phenotypic presentation of PXE (1,11). Furthermore, recent preliminary studies have suggested that treatment with aluminum hydroxide, an oral phosphate binder, of patients with PXE can result in significant clinical improvement of skin lesions, and no deterioration of eye disease was noted in any of the six patients examined at one-year follow-up (12). Collectively, the latter findings suggest that abnormalities in calcium/phosphate metabolism may contribute to the aberrant mineralization process in PXE.
We have recently developed a mouse model, Abcc6−/−, by targeted ablation of the corresponding gene (13). These mice recapitulate the histopathologic, ultrastructural, and genetic features of human PXE, serving as a reliable animal model to study this disease. An intriguing feature of the Abcc6−/− mice is ectopic mineralization of connective tissue capsules surrounding the bulb of vibrissae, which can be observed as early as 5–6 weeks of postnatal development; it has been suggested that this mineralization process serves as an early biomarker of the development of PXE in these mice (13,14). In the present study we have examined the effect of changes in dietary calcium, phosphate and magnesium as well as the effect of feeding the Abcc6−/− mice a phosphate binder, on the mineralization of vibrissae.
Materials and Methods
The PXE mouse model was developed by targeted inactivation of the Abcc6 gene as described (13). Heterozygous offspring were backcrossed with C57BL/6J mice for eight generations and were interbred to generate Abcc6+/+ and Abcc6−/− mice. These mice were fostered in the Animal Facility at Thomas Jefferson University and maintained in a temperature and moisture controlled environment utilizing 12-h light/dark cycle. Female Abcc6−/− mice were divided into three groups and fed a specific diet. All groups had free access to water. In the first group, Abcc6−/− and Abcc6+/+ mice were fed a standard rodent diet (Laboratory Rodent Diet 5010; PMI Nutrition, Brentwood, MO). A second group of Abcc6−/− mice were fed a standard meal rodent diet (ground pellets) (Lab Diet 5010) supplemented with sevelamer hydrochloride (Renagel®). Based on PDR recommendations for a 75 kg adult, the equivalent dose of sevelamer hydrochloride for a 25 g mouse is 1.6 mg daily. One 400 mg tablet of Renagel was pulverized into a powder and mixed with 325 g of standard meal rodent diet. The third group of Abcc6−/− mice was given a custom diet with specific mineral modulations (Harlan Teklad; Madison, WI). In comparison to the standard rodent diet, the percentage of absorbable phosphorus (non-phytate) was increased by two-fold (from 0.43 to 0.85%). In addition, there was a decrease in calcium (from 1.0 to 0.4%) and magnesium (0.2 to 0.04%). Mice were weighed weekly and food was checked daily to monitor proper intake and subsequent weight gain.
Histology
For histological substantiation of mineralization of vibrissae, muzzle skin was fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned (5 µm), and stained with hematoxylin-eosin (H & E) utilizing standard techniques. Similarly, organ specimens from eye, heart, and kidney were processed and stained using the same techniques.
Computerized morphometric quantitation of tissue mineralization
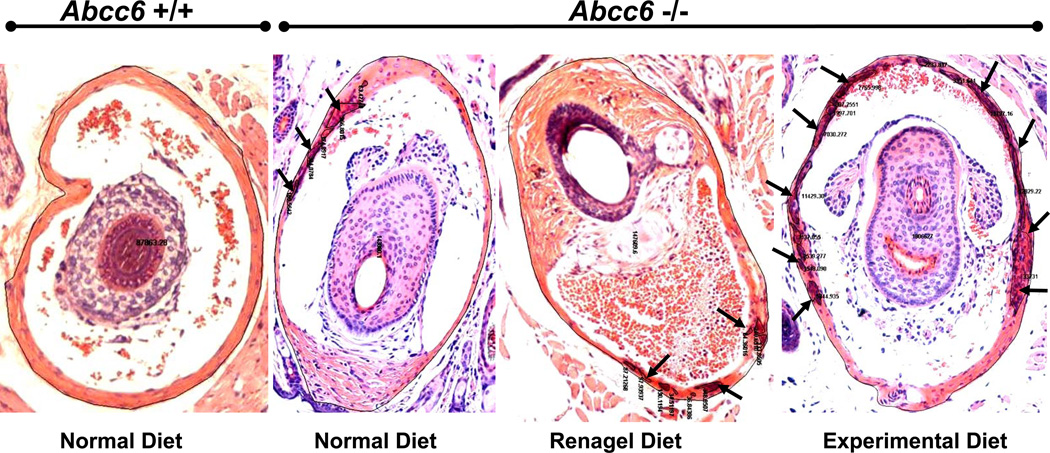
Examination of H & E stained sections of muzzle skin was performed using computerized morphometric analysis (14). The Nikon model Te2000 microscope (at a magnification of 10 ×), equipped with an Auto Quant Imaging system, was employed to examine the sections (Watervliet, New York, N.Y.). The number of vibrissae, including those with perceptible mineralization and those with an absence of mineralization, was established in all sections, and the magnitude of mineralization was expressed as both the percentage of the mineralized vibrissae and the percentage of area of mineralization per area of vibrissae (see Fig. 1).
Figure 1.
Computerized morphometric analysis of mineralization of the connective tissue capsule surrounding the bulb of vibrissae in Abcc6+/+ (left panel) and Abcc6−/− (right panels) mice fed either with normal diet, diet supplemented with sevelamer hydrochloride (Renagel®), or experimental diet, high in phosphorous and low in calcium and magnesium (for details see Materials and Methods). Muzzle skin was biopsied and histopathologic sections stained with Hematoxylin-Eosin. The arrows indicate foci of mineralization, and thin black lines outline either the total area of vibrissae or the areas of mineralization, allowing computerized quantitation of the mineralization process.
Chemical quantification of calcium and phosphate deposition
Specimens of muzzle skin, which embodies the vibrissae, were harvested and decalcified with 0.15 N HCl for 48 hours. The o-cresolphalein complexone method was utilized to measure the calcium content in a colorimetric fashion (Calcium (CPC) Liquicolor ®, Stanbio Laboratory, Boerne, TX, USA). The content of phosphate was determined with the Malachite Green Phosphate Assay kit (Bioassay Systems, Hayward, CA, USA). The values for calcium and phosphate were standardized to tissue weight.
Statistical analysis
The statistical differences between the means in groups of mice receiving different diets were computed by the Student’s two-tailed t-test.
Results and Discussion
Mineralization of connective tissue capsule of vibrissae in Abcc6−/− mice
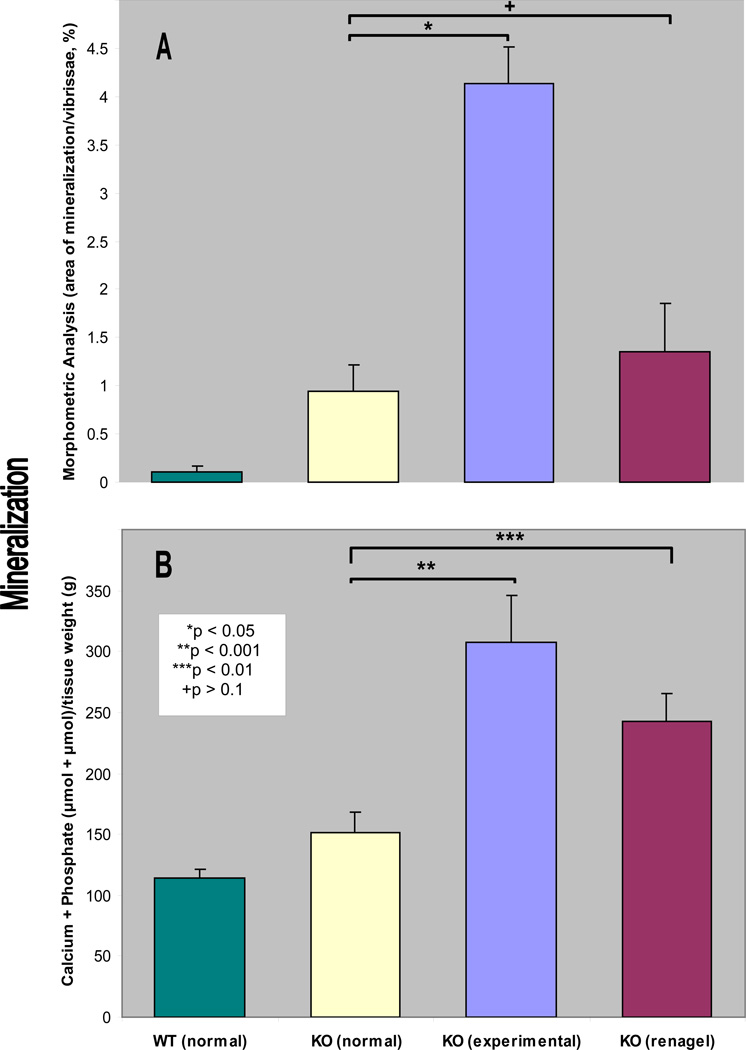
We have recently developed an Abcc6−/− mouse that serves as a model recapitulating features of PXE (13). A feature of this mouse model is mineralization of the connective tissues capsules surrounding the vibrissae, a finding that has been suggested to serve as an early biomarker of the mineralization process. In this study, we examined these PXE mice, first, by determining the mineralization in vibrissae by computerized morphometric analysis. As reported previously (13,14), these mice fed normal diet at the age of 3 months demonstrate distinct foci of mineralization visualized by H&E stain (Fig. 1 and 2A). At the same time, wild-type Abcc6+/+ littermates fed normal diet did not show any evidence of mineralization (Fig. 1). Secondly, we performed specific quantitation of mineralization in the vibrissae by chemical assays of calcium and phosphorus in the muzzle skin containing the vibrissae. The chemical assays revealed significant increase of mineralization in Abcc6−/− mice in comparison to their wild-type littermates (Fig. 2B). These results confirmed the observations on early mineralization of vibrissae in Abcc6−/− mice and demonstrate the utility of these mice as a model of PXE.
Figure 2.
Quantitation of the mineralization either by using computerized morphometric analysis, as shown in Fig. 1 (A), or by chemical determination of calcium plus phosphate content in the muzzle tissue containing the vibrissae (B). The bars represent mean ± S.E., n = 4–5 in each group. Note the statistical significance, as indicated in an inset in B.
Modulation of mineralization by changes in diet
Previous studies have suggested that high intake of dairy products rich in calcium and phosphorus can exacerbate PXE in humans (1,11). To examine the effects of diet in the animal model of PXE, we placed Abcc6−/− mice on experimental diet enriched in phosphorus and containing decreased amounts of calcium and magnesium (see Materials and Methods). The mice were placed on experimental diet at three weeks of age and continued on this regiment for an additional eight weeks; a control group of mice continued to receive the normal diet. Examination of the vibrissae by histopathology coupled with quantitative morphometric analysis revealed a marked, ~4.4-fold increase of mineralization in Abcc6−/− mice fed experimental diet, as compared to the corresponding mice on normal diet (Fig. 2A; p<0.001). The mineralization was also quantified by determination of the calcium and phosphorus contents by chemical assays in the muzzle skin. Similar to morphometric analysis, the calcium plus phosphate content, corrected by the tissue weight, was increased by 2.0 fold (Fig. 2B; p<0.005). Thus, these two independent measurements indicate that modifications of the mineral content of the diet can alter the degree of mineralization of the connective tissue capsule of vibrissae in the Abcc6−/− mouse model of PXE. Survey of mineralization in other organs by histopathologic observations revealed significant mineral deposits also in the kidneys and heart of the Abcc6−/− mice fed the experimental diet, in comparison to the corresponding mice fed regular diet (Table 1). These findings seem to confirm the clinical observations made previously on patients with PXE. It should be noted, however, that previous reports (1,11) suggesting that excessive intake of dairy products during childhood or adolescence results in more severe clinical phenotype imply that the effects can be due to high calcium, high phosphorus and/or high magnesium or other nutrients, such as vitamin D, in the diet. Thus, the findings in our mouse model, although supporting the earlier clinical observations (1,11), do not necessarily identify the dietary factor(s) resulting in exacerbated mineralization. Furthermore, our study did not identify whether the changes are due to increased phosphorus or lower calcium or magnesium, or either one of them in the diet. This clearly requires further investigation with more defined diets.
Table 1.
Soft tissue mineralization of Abcc6−/− mice fed different diets
| DIET* | MINERALIZATION IN SOFT TISSUES† | |||
|---|---|---|---|---|
| VIBRISSAE | KIDNEYS | EYES | HEART | |
| Normal | 6/6 | 0/6 | 1/6 | 0/6 |
| Experimental | 6/6 | 6/6 | 1/6 | 3/6 |
| Renagel | 6/6 | 1/6 | 0/6 | 1/6 |
Abcc6−/− animals were fed the diet indicated (for details see Materials and Methods) for 8 weeks beginning at the age of 3 weeks.
Number of mice with tissue mineralization/total number of mice examined in each group, as determined by histopathologic examination of H&E stained tissue sections.
Preliminary study on a phosphate binder
Recent studies have suggested that phosphate binders may be effective in ameliorating severity of PXE (12). Specifically, a study consisting of six patients treated with aluminum hydroxide suggested that three of these patients showed significant improvement of skin lesions and revealed histopathologic regression of the disease. At the same time, no deterioration of eye disease was seen in any of the six patients at one-year follow up (12). The authors concluded that calcification seen in PXE may be reversible in some patients by treatment with phosphate binders, and this could provide a treatment option for patient with PXE.
In this study, we examined the effect of an oral phosphate binder, sevelamer hydrochloride (Renagel®), the mechanism of which has been shown to relate to reduction of phosphate absorption from the intestine. This phosphate binder has also been suggested to be as effective as calcium based phosphate binders but with less side effects than the aluminum based products. In fact, the aluminum based phosphate binders have been removed from the market due to their bone toxicity, renal osteodystrophy and encephalopathy. Feeding of Abcc6−/− mice with sevelamer hydrochloride containing diet for eight weeks revealed that the mineralization of vibrissae, as determined by computerized morphometric analysis, was not significantly altered in comparison to corresponding mice fed normal diet (Fig. 2A; p>0.1). Furthermore, chemical assay of calcium plus phosphate content in the knock-out mice fed with sevelamer hydrochloride demonstrated small, but statistically significant, increase (Fig. 2B; p<0.01), suggesting that this phosphate binder in the dose utilized was not effective in preventing the mineralization process in this experimental design in Abcc6−/− mice as target. It should be noted that the serum phosphorus levels in Abcc6−/− mice fed control diet and sevelamer hydrochloride containing diet for 8 weeks were 22.7 ± 3.4 and 33.2 ± 2.9 mg/dL (mean ± S.D.; n=6 and 3, respectively). This observation suggests a possible compensatory increase in serum phosphorus concentration as a result of impaired intestinal absorption of phosphate. These findings may serve as caution against premature clinical trials using novel phosphate binders in PXE.
Collectively, our findings suggest that the extent of the mineralization process in PXE can be altered by changes, potentially either elevation or reduction, in mineral homeostasis. These observations clearly suggest that the role of calcium, phosphorus and magnesium, as well as phosphate binders, in ectopic mineralization of PXE merits further investigation.
Acknowledgements
The authors thank Carol Kelly for assistance. Dr. Mark Lebwohl provided helpful suggestions. This study was supported by NIH/NIAMS grants R01AR28450 and R01AR52627.
Abbreviations
- PXE
pseudoxanthoma elasticum
References
- 1.Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J, Ringpfeil F. Heritable Disorders of Connective Tissue: The Paradigms of Ehlers-Danlos Syndrome and Pseudoxanthoma Elasticum. In: Runge MS, Patterson C, editors. Principles of Molecular Medicine. Second Ed. Totowa, NJ: Humana Press; 2006. pp. 1035–1042. [Google Scholar]
- 3.Miksch S, Lumsden A, Guenther UP, et al. Molecular genetics of pseudoxanthoma elasticum: Type and frequency of mutations in ABCC6. Hum Mutat. 2005;26:235–248. doi: 10.1002/humu.20206. [DOI] [PubMed] [Google Scholar]
- 4.Belinsky MG, Kruh GD. MOAT-E (ARA) is a full length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuzaki Y, Nakano A, Jiang Q-J, Pulkkinen L, Uitto J. Tissue-specific expression of the ABCC6 gene. J Invest Dermatol. 2005;125:900–905. doi: 10.1111/j.0022-202X.2005.23897.x. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q, Uitto J. Pseudoxanthoma elasticum: A metabolic disease? J Invest Dermatol. 2006;126:1440–1441. doi: 10.1038/sj.jid.5700267. [DOI] [PubMed] [Google Scholar]
- 7.Aherrahrou Z, Doehring LC, Kaczmarek PM, et al. Ultrafine mapping of Dyscalc1 to an 80 Kb chromosomal segment on chromosome 7 in mice susceptible for dystrophic calcification. Physiol Genomics. 2007;28:203–212. doi: 10.1152/physiolgenomics.00133.2006. [DOI] [PubMed] [Google Scholar]
- 8.Meng H, Vera I, Che N, et al. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc Natl Acad Sci U S A. 2007;13:4530–4535. doi: 10.1073/pnas.0607620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schön S, Schulz V, Prante C, et al. Polymorphisms in the xylosyltransferase genes cause higher serum XT-I activity in patients with pseudoxanthoma elasticum (PXE) and are involved in a severe disease course. J Med Genet. 2006;43:745–749. doi: 10.1136/jmg.2006.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendig D, Arndt M, Szliska C, Kleesiek K, Götting C. SPP1 promoter polymorphisms: Identification of the first modifier gene for pseudoxanthoma elasticum. Clin Chem. 2007;53:829–836. doi: 10.1373/clinchem.2006.083675. [DOI] [PubMed] [Google Scholar]
- 11.Renie WA, Pyeritz RE, Combs J, Fine SL. Pseudoxanthoma elasticum: High calcium intake in early life correlates with severity. Am J Med Genet. 1984;19:235–244. doi: 10.1002/ajmg.1320190205. [DOI] [PubMed] [Google Scholar]
- 12.Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610–615. doi: 10.1016/j.jaad.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Klement JF, Matsuzaki Y, Jiang Q-J, et al. Targeted ablation of the ABCC6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: Systemic and local regulatory factors. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]