Abstract
Trans-epithelial migration describes the ability of migrating cells to cross epithelial tissues and occurs during development, infection, inflammation, immune surveillance, wound healing and cancer metastasis. Here we investigate Drosophila primordial germ cells (PGCs), which migrate through the endodermal epithelium. Through live imaging and genetic experimentation we demonstrate that PGCs take advantage of endodermal tissue remodeling to gain access to the gonadal mesoderm and are unable to migrate through intact epithelial tissues. These results are in contrast to the behavior of leukocytes, which actively loosen epithelial junctions to migrate, and raise the possibility that in other contexts in which migrating cells appear to breach tissue barriers, they are actually exploiting existing tissue permeability. Therefore, the use of active invasive programs is not the sole mechanism to infiltrate tissues.
Keywords: Primordial germ cells, Cell migration, Epithelial to mesenchymal transition
INTRODUCTION
Cells undergoing trans-epithelial migration (TEM) must overcome the specialized cell-cell junctions of epithelial cells that form occluding barriers and obstruct cell migration. During leukocyte extravasation, a well-studied model of TEM, interactions between leukocytes and endothelial cells through ligand-receptor binding trigger a loosening of endothelial cell-cell junctions, allowing leukocytes to squeeze through these sites of reduced endothelial contact (Muller, 2011; Nourshargh et al., 2010). Although the active remodeling of cell-cell contacts by migrating cells is an established mechanism of TEM, migrating cells in other systems might employ additional mechanisms.
In many species, primordial germ cells (PGCs), which are responsible for producing gametes, migrate through several tissues within the embryo as they travel to the site where the gonad will form (Richardson and Lehmann, 2010). In Drosophila, PGCs start their active migration by penetrating an epithelium comprising endodermal cells. After crossing the endoderm, PGCs reorganize on the basal surface of the future midgut and migrate into the mesoderm where they contact and adhere to the somatic gonadal precursor cells (Moore et al., 1998; Warrior, 1994). Migration through the endoderm requires activation of the G protein-coupled receptor (GPCR) Trapped in endoderm 1 (Tre1) within PGCs (Kunwar et al., 2008; Kunwar et al., 2003). However, the signal that activates the Tre1 receptor is currently unknown. Transplantation studies indicate that the timing of PGC migration is dictated by the developmental stage of the endoderm, not by the developmental stage of the PGCs (Jaglarz and Howard, 1994), consistent with a model in which the endoderm produces the Tre1 signal leading to PGC migration. PGCs do not initiate migration in embryos mutant for serpent (srp), a GATA transcription factor required for endoderm specification, further supporting this model (Moore et al., 1998; Reuter, 1994; Warrior, 1994).
Concomitant with PGC migration, the endoderm undergoes epithelial remodeling as part of an epithelial to mesenchymal transition (EMT) (Campbell et al., 2011; Campos-Ortega and Hartenstein, 1985). Discontinuities in circumferential adherens junctions and intercellular spaces between endodermal cells observed by electron microscopy have been postulated to act as exit sites for PGCs, although their functional role has never been tested (Callaini et al., 1995; Jaglarz and Howard, 1995). In srp mutants EMT does not occur, nor does the epithelium display intercellular gaps (Campbell et al., 2011; Reuter, 1994). Therefore, it is unknown whether the physical transformation of the endoderm from epithelium to mesenchyme is required for PGC migration or if the endoderm is responsible for generating a Tre1 signal leading to PGC migration.
To determine the role of the endoderm during PGC migration we assessed the ability of PGCs to migrate in genetic backgrounds that perturb epithelial remodeling, endodermal specification or epithelial maintenance. Our results indicate that PGCs are incapable of migrating through intact epithelial tissues and are dependent on developmentally regulated epithelial remodeling, which causes discontinuities in the endoderm that allow PGCs to migrate. We find that two independent programs are required for PGC migration through the endoderm: first, activation of Tre1 within PGCs as part of an autonomous migratory program; and second, disruption of the endodermal epithelium, which generates spaces within the tissue for PGC migration.
MATERIALS AND METHODS
Drosophila strains
The following Drosophila strains were used: crb8F105 (FBal0001816) and UAS-crb (FBst0005544) acquired from the Bloomington Stock Center; srp6G (FBal0016081; R. Reuter, University of Tuebingen, Germany); nullo-Gal4 (FBtp0018484; W. Gehring, University of Basel, Switzerland and E. Wieschaus, Princeton University, NJ, USA); UAS-Nact (FBal0090559; S. Artavanis-Tsakonis, Harvard Medical School, MA, USA); P(lacZ)inscAB44 (FBal0049666; G. Somers, La Trobe University, Melbourne, Australia); osk301 (FBal0013310) and osk54 (FBal0013303) (Ephrussi et al., 1991); tre1ΔEP5 (FBal0127254) (Kunwar et al., 2003); and Pnos::egfp-moe::nos 3′UTR (FBtp0040584) (Sano et al., 2005).
Immunohistochemistry
The following primary antibodies were used: rabbit aPKC (1:250; Santa Cruz); rat DECad (1:50; DSHB); mouse β-gal (1:250; Promega); rabbit β-gal (1:10,000; Cappel); rabbit Vasa (1:2500; Lehmann lab); chicken GFP (1:250; Aves); and guinea pig DHB9 (1:500; J. Skeath, Washington University School of Medicine, MO, USA). Secondary antibodies were obtained from Jackson ImmunoResearch and Invitrogen. Phalloidin-Alexa Fluor 488 (1:250; Invitrogen) and phalloidin-TRITC (1:250; Sigma) were also used.
Embryos were dechorionated using 50% bleach in water for 5 minutes and fixed using 1:1 10% methanol-free formaldehyde:heptane for 45 minutes. Embryos were hand devitellinized with 28G 1/2 needles (Becton Dickinson) in PBS containing 1% Triton X-100 and 1% BSA and were mounted in Vectashield (Vector Laboratories).
Quantification and statistical analysis
Adult flies were placed in 25°C incubators and embryos were collected at 45-minute intervals. Stage 9 embryos were fixed after 4 hours of incubation at 25°C. Stage 10 embryos were fixed after 5 hours of incubation at 25°C. Staging was determined by the percent of germ band extension and budding of the malpighian tubules, except in nullo-Gal4,UAS-Nact, where the anterior stomodeal invagination was used because malpighian tubule formation is disrupted.
Fisher’s exact test was performed via the VassarStats.net website, provided by Dr Richard Lowry (Vassar College, NY, USA).
Image acquisition
Fixed fluorescent images were acquired using a Zeiss LSM-510 confocal microscope and 20× (Zeiss, Plan Apochromat, air, NA 0.8) and 63× (Zeiss, Plan Neufluor, water immersion, NA 1.3) objectives. Images were processed using Photoshop software (Adobe).
Live images were acquired using a Prairie Technologies Ultima Multiphoton system with an Olympus BX51 W1 microscope and a Coherent Chameleon Ultra 1 80 MHz Ti:Saph laser controlled by Prairie View software. A 40× objective (Olympus, UPlan FL, oil immersion, NA 1.3) was used. Movie files were generated using Imaris software (Bitplane). Still images from movies were extracted using ImageJ. Embryos were prepared for live imaging using an established protocol (Seifert and Lehmann, 2011).
RESULTS AND DISCUSSION
PGC migration coincides with extensive endodermal remodeling
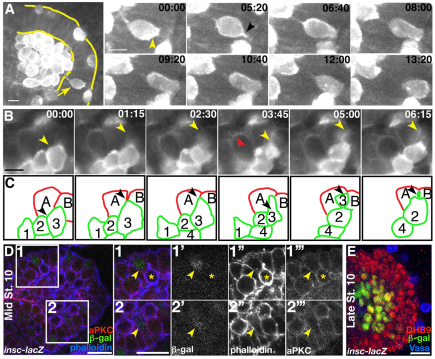
We used multiphoton live imaging to visualize interactions between PGCs and the endoderm during TEM. To mark PGCs, the actin-binding domain of Moesin was fused to eGFP under the control of nanos regulatory sequences (Pnos::egfp-moe::nos 3′UTR), which allows the visualization of F-actin accumulation and cell dynamics (Sano et al., 2005). PGCs rarely appeared extended or spindle shaped, as would be expected if they needed to squeeze through an epithelial barrier (Fig. 1A; supplementary material Movies 1, 2, 4). Because low levels of eGFP expression were detectable within the endoderm, epithelial remodeling could be visualized and was seen to occur simultaneously with PGC migration (Fig. 1B; supplementary material Movies 1, 3). eGFP became cortically enriched in endodermal cells as they lost their columnar appearance and delaminated from the epithelium. PGCs extended F-actin-rich protrusions toward these delaminating cells, possibly gauging the extent of epithelial integrity within the tissue, and migrated at sites of delamination (Fig. 1B,C, yellow arrowheads). To extend these live observations, we visualized PGCs and atypical protein kinase C (aPKC) localization in fixed tissue (supplementary material Fig. S1). While the epithelium remained intact, PGCs appeared clustered and non-migratory (supplementary material Fig. S1A). However, once epithelial integrity appeared disrupted, as marked by a loss of aPKC localization, PGCs appeared migratory (supplementary material Fig. S1B). These results support the hypothesis that a loss of epithelial integrity provides spaces within the endoderm, allowing PGCs to migrate through the tissue.
Fig. 1.
Primordial germ cell migration occurs during endodermal epithelial remodeling and ingression. Drosophila embryos imaged dorsally with anterior to the left. (A,B) Still images from a time series (supplementary material Movies 1-3). Embryos are derived from Pnos::egfp-moe::nos 3′UTR-expressing mothers and carry zygotic copies of this transgene. (A) Endodermal epithelium is outlined in yellow. Arrow highlights a migrating primordial germ cell (PGC). Still images demonstrate that PGCs do not deform as they migrate. (B) During endodermal remodeling, cells acquire a rounded-up phenotype and delaminate from the epithelium (red arrowhead). Time is shown in minutes:seconds (C) Illustration of cell identity and position in time series in B. PGCs (1-4) are outlined in green. Black arrowheads highlight PGC 3 (same as yellow arrowhead in B), which migrates between endodermal cells A and B (red). (D) Mid-stage 10 insc-lacZ embryo. The boxed regions 1 and 2 are magnified on the right. β-gal-positive cells (green in D, grayscale in 1′ and 2′) are ingressing and appear rounded or spindle shaped (yellow arrowhead in 2; phalloidin is blue in D, grayscale in 1″ and 2″). aPKC antibody staining appears discontinuous (aPKC is red in D, grayscale in 1″′ and 2″′). A migrating PGC is identifiable by enriched phalloidin staining (yellow asterisk). (E) By late stage 10, cells expressing β-gal (green) have ingressed (endoderm marked with DHB9 antibody, red). PGCs have migrated out of the endoderm (Vasa antibody in blue). Scale bars: 10 μm in A,B; 20 μm in D.
Delaying endodermal remodeling delays PGC migration
During endodermal epithelial remodeling two subpopulations of endodermal cells are specified: the interstitial cell precursors (ICPs) and the adult midgut precursors (AMPs) (Tepass and Hartenstein, 1995). ICP and AMP cell fate is determined by the expression of proneural genes and is inhibited in remaining endodermal cells by the Notch pathway (Tepass and Hartenstein, 1995). Using the enhancer trap line inscuteable-lacZ (insc-lacZ), which labels both ICP and AMP cells, we observed the apical ingression of ICPs at stage 10, which correlated with the onset of PGC migration (Fig. 1D,E). ICPs ingress apically from the surrounding epithelium, in contrast to other ingressing cells, which constrict their apical membrane and extrude basally allowing the neighboring epithelial cells to maintain epithelial integrity (Shook and Keller, 2003).
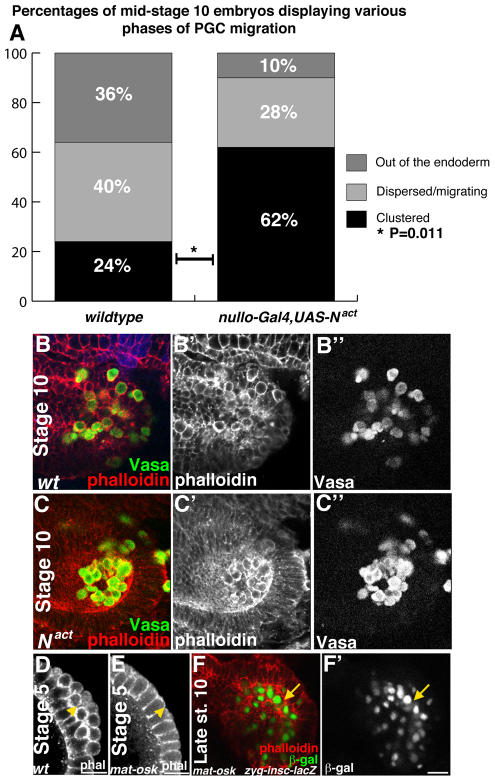
To test whether the ingression of ICPs disrupts epithelial integrity, allowing PGCs to migrate through the endodermal epithelium, we blocked ICP and AMP specification by overexpressing an activated form of the Notch receptor Nact using nullo-Gal4 (Go et al., 1998; Simpson and Wieschaus, 1990; Tepass and Hartenstein, 1995). Expression of Nact blocked the specification of ICP and AMP cells, as determined by loss of β-gal expression in insc-lacZ,nullo-Gal4,UAS-Nact embryos (supplementary material Fig. S2). Embryos expressing Nact display a statistically significant delay in the initiation of migration compared with sibling controls (Fig. 2A). Although Nact blocked cell ingression and delayed epithelial remodeling, EMT still occurred by stage 11 (supplementary material Fig. S2) (Tepass and Hartenstein, 1995), which was sufficient for PGCs to migrate out of the endoderm. These results suggest that PGCs exploit ICP ingression in order to migrate through the endoderm while the remaining endodermal tissue is still epithelial. However, ingression does not appear to be absolutely necessary for PGC migration, as PGCs are able to migrate once endodermal-mesenchymal transition occurs.
Fig. 2.
Delaying epithelial remodeling delays PGC migration. Drosophila embryos imaged dorsally (B,C), posteriorly (D,E) or laterally (F) with anterior to the left. (A) Analysis of PGC migration at stage 10 in wild-type embryos versus nullo-Gal4,UAS-Nact embryos, in which interstitial cell precursors (ICPs) are not specified. Twenty-four percent (6/25) of wild-type embryos show PGCs inside the endoderm as compared with 62% (18/29) of nullo-Gal4,UAS-Nact embryos, a statistically significant difference (*P=0.011, two-tailed Fisher’s exact test). (B-B″) A stage 10 wild-type (wt) control embryo. PGCs labeled with an antibody to Vasa (green in B, grayscale in B″) have initiated migration and the endoderm is no longer epithelial (phalloidin is red in B, grayscale in B′). (C-C″) A stage 10 nullo-Gal4,UAS-Nact embryo. PGCs (labeled with Vasa antibody, green in C, grayscale in C″) have not initiated migration and the endoderm retains epithelial character (phalloidin is red in C, grayscale in C′). (D) PGCs (arrowhead) at the posterior pole of a stage 5 embryo stained with phalloidin. (E) A stage 5 embryo from an osk54/osk301 mother lacks PGCs (arrowhead; stained with phalloidin). (F,F′) A late stage 10 embryo laid by an osk54/osk301 mother. In the absence of PGCs, insc-lacZ cells ingress (arrow; β-gal antibody, green in F, grayscale in F′; phalloidin in red). Scale bars: 20 μm.
PGCs do not regulate epithelial remodeling
During leukocyte TEM, the integrity of the endothelium is disrupted by ligand-receptor interactions between migrating immune cells and endothelial cells (Schnoor and Parkos, 2008). Although previous work demonstrated that endodermal EMT is a developmentally programmed process that occurs in the absence of PGCs, it is unknown whether ICP ingression is regulated by PGCs (Callaini et al., 1995; Jaglarz and Howard, 1995; Tepass and Hartenstein, 1995). To determine whether PGCs facilitate ingression of insc-lacZ cells, we analyzed insc-lacZ expression in embryos derived from oskar (osk) mutant females, which lay eggs devoid of PGCs (Lehmann and Nusslein-Volhard, 1986) (Fig. 2D-F′). In embryos laid from osk54/301 mutant females, we observed no obvious defects in cell ingression or later endoderm morphogenesis (Fig. 2F,F′). These results indicate that epithelial remodeling is a consequence of an endoderm-specific developmental program and occurs independently of the germ line. This highlights a significant difference between TEM of leukocytes, which actively reorganize cell-cell junctions, and TEM of PGCs, which exploit developmentally programmed remodeling of the epithelium.
PGC migration requires disruption of epithelial architecture but not endoderm specification
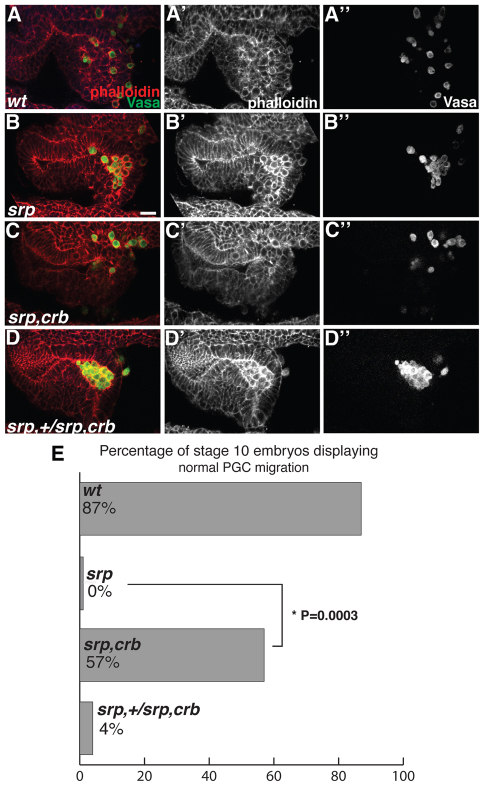
Although our results suggest that PGCs exploit endodermal remodeling for their migration, it is also possible that the endoderm is responsible for providing the Tre1 signal that allows PGC migration to occur. Endodermal fate is lost in srp mutants and the tissue fails to undergo EMT (Abel et al., 1993; Campbell et al., 2011; Reuter, 1994). Consistent with a cell fate transformation, insc-lacZ cells are not specified and no cell ingression occurs (supplementary material Fig. S3). To determine whether the PGC migration defects in srp mutants are due to the persistence of epithelial integrity or are caused by loss of endodermal fate, we used crumbs (crb) mutants as a means to disrupt epithelial integrity. crb encodes a transmembrane protein that acts as an apical determinant in epithelial cells (Tepass et al., 1990; Wodarz et al., 1995). In zygotic crb mutants the blastoderm epithelium is initially established; however, as epithelial tissues undergo morphogenesis they lose polarity and adhesion (Campbell et al., 2009).
We compared the ability of PGCs to migrate in three conditions: wild-type; srp mutants, in which endodermal fate is lost and the epithelium is maintained; and srp,crb double mutants, in which endoderm fate is lost but epithelial integrity is disrupted (Fig. 3A-C″). We scored stage 10 embryos for normal migration and found that srp,crb double mutants significantly rescued the srp block in PGC migration (P=0.0003, two-tailed Fisher’s exact test; Fig. 3B-C″,E). The rescue of PGC migration in srp,crb double mutants was not due to restored endodermal fate (supplementary material Fig. S4). Therefore, PGCs are able to migrate in the absence of endoderm and, by extension, of any endoderm-specific signal, as long as epithelial integrity is disrupted. This indicates that the role of the endoderm during PGC migration is to instruct the remodeling of the epithelium, which generates spaces for PGCs to migrate.
Fig. 3.
Epithelial disruption in the absence of endodermal specification is sufficient for PGC migration. (A-D″) Drosophila embryos imaged laterally with anterior to the left. Late stage 10 embryos stained with phalloidin (red) and Vasa antibody (green). (A-A″) In wild-type embryos the endoderm has lost epithelial character and PGCs have initiated migration. (B-B″) In srp mutants PGCs are unable to migrate and are surrounded by epithelial tissue. (C-C″) In srp,crb double mutants PGCs appear to migrate and are surrounded by tissue that has lost epithelial character. (D-D″) Embryos carrying the original srp mutation over the srp,crb recombinant chromosome phenocopy srp mutants (C) in both the epithelial character of the tissue surrounding PGCs and the inability of PGCs to migrate. (E) The percentage of embryos displaying normal migration, as characterized by the absence of clusters of eight or more PGCs, was 87% (55/63) for wild type, 0% (0/16) for srp and 57% (13/23) for the srp,crb double mutant. The difference between srp and srp,crb was significant (*P=0.0003, two-tailed Fisher’s exact test). The percentage for the original srp mutation over the srp,crb recombinant chromosome was 4% (1/23). Scale bar: 20 μm.
Premature disruption of epithelium leads to precocious migration
Our results suggest that the integrity of the epithelium surrounding PGCs influences their ability to migrate. To examine whether precocious disruption of the epithelium affects PGC migration we analyzed crb mutants, in which the endodermal epithelium is disrupted by stage 9 (Fig. 4A-B″; supplementary material Fig. S5). Concomitant with the loss of epithelial organization, PGCs disperse prematurely in crb mutant embryos (Fig. 4B-B″,E). Live imaging of stage 9 crb mutant embryos demonstrated that PGCs were actively migrating (supplementary material Movie 5). These results indicate that the timing of epithelial remodeling determines the initiation of PGC migration.
Fig. 4.
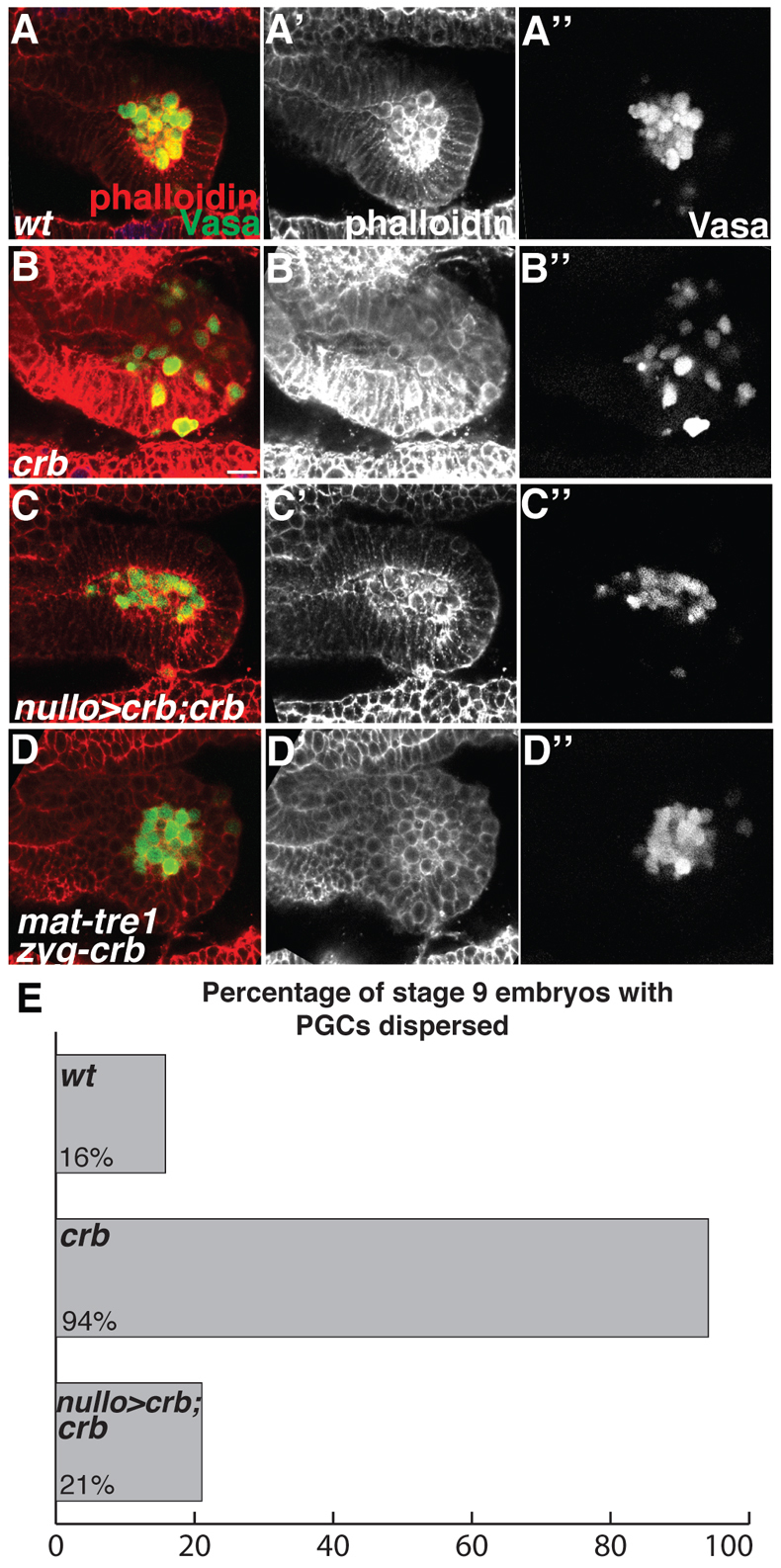
Premature epithelial disruption leads to precocious PGC migration. (A-D″) Stage 9 Drosophila embryos imaged laterally with anterior to the left and stained with phalloidin (red) and Vasa antibody (green). (A-A″) In wild-type embryos the tissue surrounding PGCs is epithelial and PGCs are clustered. (B-B″) In crb mutant embryos the tissue surrounding PGCs is not epithelial and PGCs appear to be migrating. (C-C″) Expressing UAS-crb in somatic tissue with nullo-Gal4 in a crb mutant background restores the epithelial character of the tissue and blocks premature migration. (D-D″) In crb mutant embryos derived from tre1 mutant females the tissue is not epithelial; however, PGCs do not migrate out of the endoderm. (E) The percentage of stage 9 embryos with dispersed PGCs (genotypes as in A-C). In 16% (3/19) of wild-type embryos PGCs were dispersed, whereas 94% (16/17) of crb embryos had PGCs dispersed. In nullo-Gal4,UAS-Crb,crb embryos, 21% (4/19) had PGCs dispersed. Scale bar: 20 μm.
We confirmed that premature migration was due to the loss of crb in somatic tissues leading to a defect in epithelial organization and not due to a role of crb in PGCs by expressing UAS-crb using nullo-Gal4 in an otherwise crb mutant embryo (Fig. 4C-C″). Suppression of the premature PGC migration phenotype correlated with rescue of the epithelial organization of the endoderm (Fig. 4C-C″,E). We also confirmed that endoderm specification was normal (supplementary material Fig. S5). Interestingly, insc-lacZ-expressing cells were not found within the endoderm at stage 9 when PGCs initiate premature migration in crb mutants, indicating that ingressing cells are not required for PGC migration if the epithelium is otherwise disrupted (supplementary material Fig. S5).
To determine whether the PGC autonomous Tre1 signaling pathway is required for premature PGC migration, we analyzed crb mutant embryos that also lack maternal tre1. In these embryos, PGCs were unable to migrate, similar to the tre1 mutant phenotype (Fig. 4D-D″). Thus, premature migration is dependent on the activity of the Tre1 GPCR. This result suggests that the Tre1 receptor is active in PGCs prior to the normal initiation of migration. PGCs are therefore competent and poised to migrate but await epithelial remodeling for initiation of their migration. Analysis of crb and crb,srp mutants clearly demonstrates that PGCs are able to migrate precociously and independently of endoderm specification. We propose that the Tre1 GPCR is active by stage 9, and possibly earlier, and that its activation does not require endoderm specification. These results suggest that two independent pathways regulate PGC migration through the endoderm. One pathway, requiring the activity of the Tre1 GPCR, acts in PGCs and controls the migratory program. A second, independent pathway under the control of the GATA transcription factor Srp specifies the endoderm program and provides a temporal cue for PGC migration by remodeling the endodermal epithelium.
PGCs can be regarded as invasive cells owing to their ability to migrate through many tissues during embryogenesis. In this study, we establish that in order to reach the somatic gonad, PGC migration depends on the epithelial remodeling of the endoderm. Our results suggest that Drosophila PGCs lack the ability to induce remodeling in epithelial tissues. Instead, PGCs exploit developmentally programmed epithelial remodeling to successfully reach the gonad. Several recent findings hint that migrating cells in other systems might also exploit existing permeability to access new tissue environments. Mouse dendritic cells and Drosophila hemocytes appear to rely on tissue permeability and tissue remodeling, respectively, during migration (Baluk et al., 2007; Evans et al., 2010; Pflicke and Sixt, 2009). Additionally, the idea has been raised that because the vasculature within tumors is grossly compromised, it might not present an efficient barrier for cancer cell migration (Chung et al., 2010; Madsen and Sahai, 2010). As cancer cells migrate through the interstitial tissue, they might actually encounter unimpeded paths existing within the tissue (Friedl and Alexander, 2011). These observations combined with our work indicate that cells do not necessarily need to actively remodel tissue barriers, but instead can exploit existing permeability within one tissue to reach another.
Supplementary Material
Acknowledgments
We thank members of the R.L. laboratory for critically reading the manuscript and our colleagues at the Skirball Institute for helpful suggestions. We also thank the Developmental Studies Hybridoma Bank, the Bloomington Stock Center and colleagues for sharing reagents.
Footnotes
Funding
This work was supported by the National Institutes of Health [F32 GM 82169 to J.R.K.S., R01 HD49100 to R.L.]. R.L. is a Howard Hughes Medical Institute Investigator. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.078949/-/DC1
References
- Abel T., Michelson A. M., Maniatis T. (1993). A Drosophila GATA family member that binds to Adh regulatory sequences is expressed in the developing fat body. Development 119, 623–633 [DOI] [PubMed] [Google Scholar]
- Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., et al. (2007). Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaini G., Riparbelli M. G., Dallai R. (1995). Pole cell migration through the gut wall of the Drosophila embryo: analysis of cell interactions. Dev. Biol. 170, 365–375 [DOI] [PubMed] [Google Scholar]
- Campbell K., Knust E., Skaer H. (2009). Crumbs stabilises epithelial polarity during tissue remodelling. J. Cell Sci. 122, 2604–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K., Whissell G., Franch-Marro X., Batlle E., Casanova J. (2011). Specific GATA factors act as conserved inducers of an endodermal-EMT. Dev. Cell 21, 1051–1061 [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J. A., Hartenstein V. (1985). The Embryonic Development of Drosophila melanogaster. Berlin, New York: Springer-Verlag; [Google Scholar]
- Chung A. S., Lee J., Ferrara N. (2010). Targeting the tumour vasculature: insights from physiological angiogenesis. Nat. Rev. Cancer 10, 505–514 [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Dickinson L. K., Lehmann R. (1991). Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66, 37–50 [DOI] [PubMed] [Google Scholar]
- Evans I. R., Hu N., Skaer H., Wood W. (2010). Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos. Development 137, 1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Alexander S. (2011). Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 [DOI] [PubMed] [Google Scholar]
- Go M. J., Eastman D. S., Artavanis-Tsakonas S. (1998). Cell proliferation control by Notch signaling in Drosophila development. Development 125, 2031–2040 [DOI] [PubMed] [Google Scholar]
- Jaglarz M. K., Howard K. R. (1994). Primordial germ cell migration in Drosophila melanogaster is controlled by somatic tissue. Development 120, 83–89 [DOI] [PubMed] [Google Scholar]
- Jaglarz M. K., Howard K. R. (1995). The active migration of Drosophila primordial germ cells. Development 121, 3495–3503 [DOI] [PubMed] [Google Scholar]
- Kunwar P. S., Starz-Gaiano M., Bainton R. J., Heberlein U., Lehmann R. (2003). Tre1, a G protein-coupled receptor, directs transepithelial migration of Drosophila germ cells. PLoS Biol. 1, E80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar P. S., Sano H., Renault A. D., Barbosa V., Fuse N., Lehmann R. (2008). Tre1 GPCR initiates germ cell transepithelial migration by regulating Drosophila melanogaster E-cadherin. J. Cell Biol. 183, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R., Nusslein-Volhard C. (1986). Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell 47, 141–152 [DOI] [PubMed] [Google Scholar]
- Madsen C. D., Sahai E. (2010). Cancer dissemination – lessons from leukocytes. Dev. Cell 19, 13–26 [DOI] [PubMed] [Google Scholar]
- Moore L. A., Broihier H. T., Van Doren M., Lunsford L. B., Lehmann R. (1998). Identification of genes controlling germ cell migration and embryonic gonad formation in Drosophila. Development 125, 667–678 [DOI] [PubMed] [Google Scholar]
- Muller W. A. (2011). Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 6, 323–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S., Hordijk P. L., Sixt M. (2010). Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378 [DOI] [PubMed] [Google Scholar]
- Pflicke H., Sixt M. (2009). Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 206, 2925–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter R. (1994). The gene serpent has homeotic properties and specifies endoderm versus ectoderm within the Drosophila gut. Development 120, 1123–1135 [DOI] [PubMed] [Google Scholar]
- Richardson B. E., Lehmann R. (2010). Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat. Rev. Mol. Cell Biol. 11, 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Renault A. D., Lehmann R. (2005). Control of lateral migration and germ cell elimination by the Drosophila melanogaster lipid phosphate phosphatases Wunen and Wunen 2. J. Cell Biol. 171, 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor M., Parkos C. A. (2008). Disassembly of endothelial and epithelial junctions during leukocyte transmigration. Front. Biosci. 13, 6638–6652 [DOI] [PubMed] [Google Scholar]
- Seifert J. R. K., Lehmann R. (2011). Live imaging of Drosophila development. In Imaging in Developmental Biology, vol. 3 (ed. Sharpe J., Wong R.), pp. 23–48 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Shook D., Keller R. (2003). Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev. 120, 1351–1383 [DOI] [PubMed] [Google Scholar]
- Simpson L., Wieschaus E. (1990). Zygotic activity of the nullo locus is required to stabilize the actin-myosin network during cellularization in Drosophila. Development 110, 851–863 [DOI] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. (1995). Neurogenic and proneural genes control cell fate specification in the Drosophila endoderm. Development 121, 393–405 [DOI] [PubMed] [Google Scholar]
- Tepass U., Theres C., Knust E. (1990). crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61, 787–799 [DOI] [PubMed] [Google Scholar]
- Warrior R. (1994). Primordial germ cell migration and the assembly of the Drosophila embryonic gonad. Dev. Biol. 166, 180–194 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Hinz U., Engelbert M., Knust E. (1995). Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67–76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.