Abstract
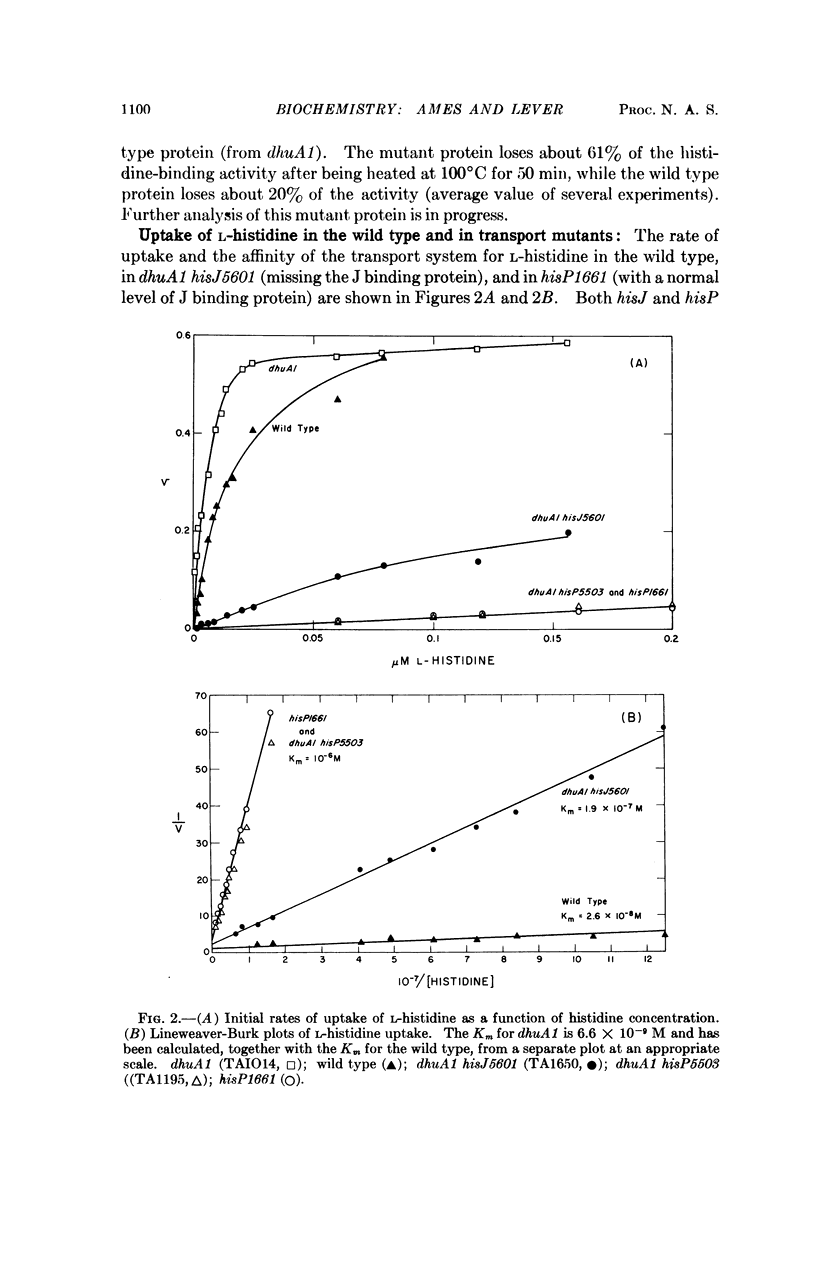
The high-affinity (Km = 3 × 10-8 M) transport system for histidine in Salmonella typhimurium has been resolved into three components: J, K, and P. J, which is a histidine-binding protein released by osmotic shock, is specified by the hisJ gene: hisJ mutants lack the binding protein and are defective in histidine transport. Another class of mutants—dhuA, which is closely linked to hisJ—has five times the normal level of binding protein and has an increased rate of histidine transport. P, which is a protein specified by the hisP gene, is required for J binding protein to be operative in transport. hisP mutants, though defective in transport, have normal levels of J binding protein. K, a third transport component, works in parallel to J, and also requires the P protein in order to be operative in transport. A second histidine-binding protein has been found but its relation to K is unclear. hisJ, dhuA, and hisP have been mapped and are in a cluster (near purF) on the S. typhimurium chromosome.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Oeschger N. S., Hartman P. E. ICR-induced frameshift mutations in the histidine operon of Salmonella. J Bacteriol. 1970 Feb;101(2):490–504. doi: 10.1128/jb.101.2.490-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S. Cell-free activity of a sulfate binding site involved in active transport. Proc Natl Acad Sci U S A. 1966 Jan;55(1):189–191. doi: 10.1073/pnas.55.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Shifrin S., Ames B. N., FerroLuzzi-Ames G. Effect of the alpha-hydrazino analogue of histidine on histidine transport and arginine biosynthesis. J Biol Chem. 1966 Jul 25;241(14):3424–3429. [PubMed] [Google Scholar]



