Abstract
The directional migration of many cell populations occurs as a coherent group. An amenable model is provided by the posterior lateral line in zebrafish, which is formed by a cohesive primordium that migrates from head to tail and deposits future neuromasts at intervals. We found that prior to the onset of migration, the compact state of the primordium is not fully established, as isolated cells with lateral line identity are present caudal to the main primordium. These isolated cells are retained in position such that they fuse with the migrating primordium as it advances, and later contribute to the leading zone and terminal neuromasts. We found that the isolated lateral line cells are positioned by two antagonistic cues: Fgf signalling attracts them towards the primordium, which counteracts Sdf1α/Cxcr4b-mediated caudal attraction. These findings reveal a novel chemotactic role for Fgf signalling in which it enables the coalescence of the lateral line primordium from an initial fuzzy pattern into a compact group of migrating cells.
Keywords: Fgf, Cxcr4b, Sdf1-α, Lateral line primordium, Zebrafish
INTRODUCTION
Collective cell migration is a crucial aspect of tissue morphogenesis, repair and cancer metastasis, in which cells retain contacts with each other and move as a coherent assembly with front and rear properties (Friedl and Gilmour, 2009). The path taken by the migrating cells is controlled by the coordinated effects of multiple attractive and repulsive cues. Among many guidance signals, the best known are chemokines, such as stromal cell-derived factor-1 (Sdf1α; Cxcl12), which acts as a chemoattractant for many cell types through activation of its receptors Cxcr4b and Cxcr7b (Tiveron and Cremer, 2008; Sun et al., 2010).
The posterior lateral line in zebrafish has emerged as a powerful paradigm to study directional collective cell migration. The posterior lateral line arises from a cranial placode that forms sensory neurons and a primordium that migrates as a cohesive group towards the tail, depositing the future neuromasts at intervals. The directional migration requires Cxcr4b, expressed in the primordium; Sdf1α, which is present along its path (David et al., 2002; Li et al., 2004; Haas and Gilmour, 2006); and Cxcr7b, which is expressed in the trailing part of the primordium and possibly acts as a sink for ligand (Dambly-Chaudiere et al., 2007; Valentin et al., 2007; Boldajipour et al., 2008). Fibroblast growth factor (Fgf) signalling is required for epithelial morphogenesis and patterning within the primordium (Nechiporuk and Raible, 2008; Lecaudey et al., 2008; Aman and Piotrowski, 2008). The canonical Wnt pathway in the leading primordium induces and restricts Fgf signalling to the trailing zone, which in turn restricts Wnt signalling to the front. This double-feedback cross-talk underlies the polarised expression of chemokine receptors that is required for collective migration of the primordium (Aman and Piotrowski, 2008).
Although previous studies have uncovered key mechanisms required for the migration of the primordium and for neuromast formation, the early steps in which migration is initiated are poorly understood. We find that prior to the onset of migration, there are clusters of isolated cells caudal to the main primordium that express markers of the lateral line primordium. These cells are retained in position such that they fuse with the migrating primordium and contribute to the leading zone and later to the terminal neuromasts. We show that this retention of the isolated cells requires Fgf signalling that mediates attraction towards the primordium, thus counteracting caudal chemoattraction by Sdf1α. The formation of a single cohesive primordium is thus established by opposing chemoattractive signals that act on the initially isolated cell clusters.
MATERIALS AND METHODS
Strains
Cldnb:lyngfp and cxcr4b/odyseus(odyJI049) strains were provided by D. Gilmour (Haas and Gilmour, 2006). hsp70:dn-fgfr1 line was used as previously described (Gonzalez-Quevedo et al., 2010).
In situ hybridisation and immunostaining
Whole-mount in situ hybridisation was performed as described (Xu and Wilkinson, 1998) with digoxigenin-labelled RNA probes synthesised according to the manufacturer’s instructions (Roche, Boehringer Mannheim). Detection with fluorescent substrate was performed using the Tyramide TSA-Plus Palette System (Perkin Elmer). GFP was detected with anti-GFP (Torrey Pines Biolabs) and anti-rabbit FITC-conjugated antibodies (Jackson ImmunoResearch).
Morpholino knockdowns
Morpholinos were obtained from Gene Tools LLC, Oregon. The Cxcr4b and Cxcr7b morpholinos were used as described (Li et al., 2004; Valentin et al., 2007; Boldajipour et al., 2008). The sequence of the additional Cxcr7b morpholino used is 5′-GGTAATGAGATTCCGATGCCTGGAG-3′. Control morphants correspond to sibling embryos injected with equal doses of the standard control morpholino provided by Gene Tools. To avoid any toxicity effect, 4 ng of morpholino was co-injected with 8 ng of p53 morpholino (Robu et al., 2007; Gerety and Wilkinson, 2011).
Confocal time-lapse imaging
Cldnb:lyngfp embryos were dechorionated, anaesthetised in 0.01% tricaine (Sigma) and mounted in 3% methylcellulose in 0.65× Danieau’s solution. When needed, embryos were injected with 80 pg H2B-RFP mRNA. Movies were recorded at 24°C under a Leica DMIRE2 SP2 upright confocal microscope. Cells were manually tracked using the Fiji software.
Heat-shock and SU5402 treatments
hsp70:dn-fgfr1 embryos were heat-shocked at the 16 somite (s) stage for 45 minutes at 37°C and fixed at 24s for analysis. Embryos were treated with 50 μM SU5402 or 0.05% DMSO from 16s to 24s. For live imaging during drug treatment, embryos were pre-treated with 50 μM SU5402 for 2 hours and mounted in the same solution. pea3 expression in the primordium (Lecaudey et al., 2008) or hmx3 expression in the otic placode (Feng and Xu, 2010) were used to assess efficiency of the inhibition.
Cell transplantation
Donor embryos were co-injected with LFD (lysine-fluorescein-dextran, Invitrogen) and 50 pg of capped mRNA encoding Fgf10 or tdTomato. Cells were transplanted into wild-type embryos at 50% epiboly stage. The embryos were analysed at 24s by in situ hybridisation for hmx3 followed by anti-fluorescein (Roche) immunostaining. Embryos showing transplanted cells in the neural tube and/or skin dorsal to the primordium region were selected for further analysis.
Kaede photoconversion
Embryos were injected with 80 pg Kaede mRNA, mounted as described above and photoactivation performed using 30 successive scans with a DPSS 405 nm laser on a Leica DMIRE2 SP2 upright confocal microscope.
Laser ablation
Cldnb:lyngfp embryos were injected with 80 pg H2B-RFP mRNA and mounted as described above. Laser ablation was performed with a VSL-337ND-S nitrogen laser (Spectra-Physics) on a Carl Zeiss Axiovision microscope. Pulses lasting 5-10 seconds were applied on the cells. The primordium region was analysed 2 hours after ablation under a Leica DMIRE2 SP2 upright confocal microscope to select embryos in which separate primordial cells (SPCs) showed fragmented DNA labelled with H2B-RFP.
Statistical analysis
All graphs show means ± s.e.m. SPC frequency graphs represent data collected from at least four independent experiments. P values correspond to two-tailed Student’s t-test analysis, except in Fig. 5D, where the ANOVA and Tukey’s post-hoc test were applied.
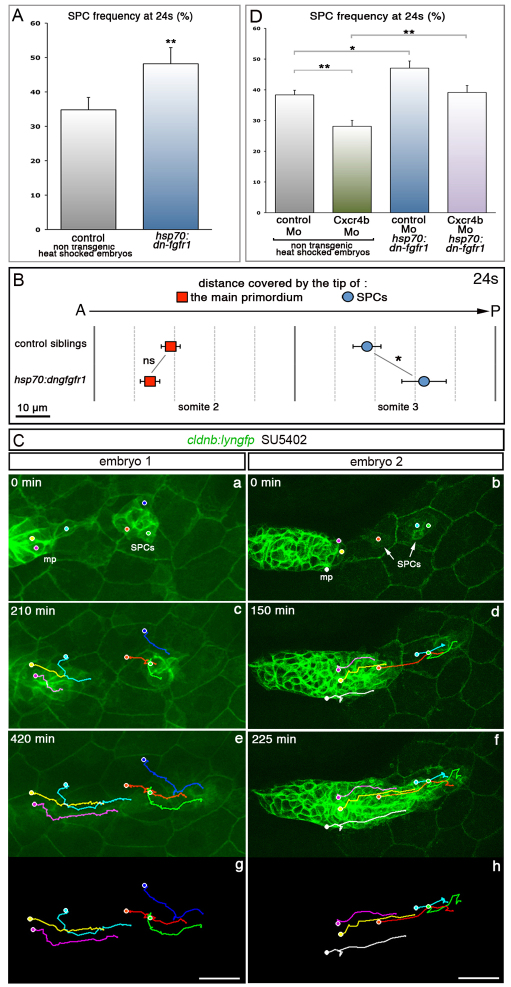
Fig. 5.
Fgf signalling attracts SPCs towards the primordium. (A) SPC frequency in hsp70:dn-fgfr1 embryos, heat-shocked at 16s and fixed at 24s, compared with heat-shocked siblings. **P=0.008. (B) Average distances covered by the tip of the main primordium (red) and SPCs (blue) in hsp70:dn-fgfr1 embryos (n=94) and control siblings (n=87) at 24s. See also supplementary material Fig. S3 and Materials and methods. *P=0.034, ns, non significant (P=0.13). (C) Tracking of SPCs and primordium tip cells in two SU5402-treated embryos (supplementary material Movie 6). Dots show initial positions of tracked cells in a and b. c and d show intermediate time points and e-h show final time points. (D) SPC frequency in embryos injected with control or Cxcr4b morpholinos in hsp70:dn-fgfr1 embryos and heat-shocked siblings. Embryos were heat-shocked at 16s and fixed at 24s. *P<0.05, **P<0.01 (ANOVA followed by Tukey’s test). Error bars represent s.e.m.
Quantification
The distance covered by the primordium tip and SPCs in hsp70:dn-fgfr1, SU5402-treated and control embryos was quantified after hmx3 in situ hybridisation at 24s. Each somite was sub-divided into five portions in order to assign a precise position for the tip of main primordium and SPCs in each embryo. The average position represents the distance travelled by these two groups of cells (Fig. 5B; supplementary material Fig. S3).
To quantify the effect of Fgf10 in transplantation experiments, the distance between SPC clusters and the horizontal somitic midline was measured in each embryo using the Fiji software. Distances were transformed into positive and negative values when SPCs were dorsal and ventral to the midline, respectively. The resulting graph is shown in Fig. 6B.
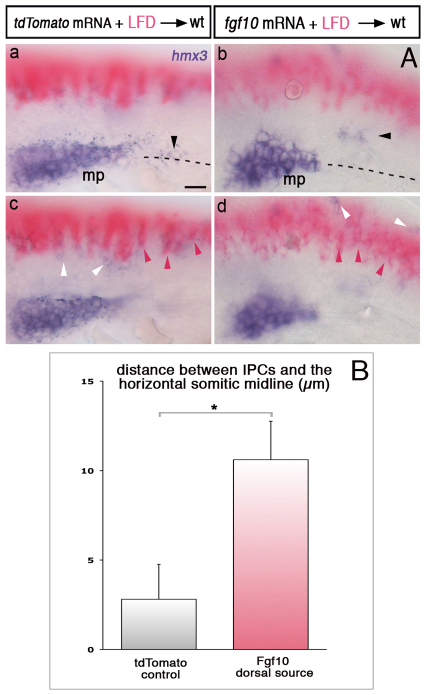
Fig. 6.
Ectopic sources of Fgf10 attract SPCs. (A) hmx3 expression (purple) in embryos transplanted with Fgf10- or tdTomato-expressing cells (pink). Black, pink and white arrowheads show SPCs, transplanted cells and hmx3-expressing spinal cord neurons, respectively. Dashed lines represent the somitic midlines. z-focus made on the primordium and SPCs (a,b) and on the transplanted cells in the dorsal neural tube (c,d). (B) Distance between SPCs and the horizontal somitic midline in embryos with transplanted cells expressing Fgf10 (n=46) or tdTomato (n=38) dorsal to the primordium region. *P=0.010. See also supplementary material Fig. S3 and Materials and methods. Error bars represent s.e.m. Scale bars: 25 μm.
RESULTS
Identification of isolated cells that later fuse with the main primordium
The posterior lateral line placode can be first recognised under Nomarski optics by the 20-somite (s) stage and the primordium starts its migration at 22s (Kimmel et al., 1995; Ghysen and Dambly-Chaudiere, 2007). To understand better the events that precede the onset of migration, we analysed expression of markers of the posterior lateral line placode and migrating primordium, hmx3 (Adamska et al., 2000; Feng and Xu, 2010), eya1 (Kozlowski et al., 2005), and the cldnb:lyngfp transgenic line (Haas and Gilmour, 2006). This revealed the presence of one or more small clusters of cells expressing hmx3 that are located posterior to and clearly separate from the main body of the primordium between 10s and 26s (Fig. 1A,B; supplementary material Fig. S1). These cell clusters express the placodal marker eya1 and are labelled by cldnb:lyngfp (Fig. 1C-F). These cells thus have a lateral line primordium identity, and we refer to them as separate primordial cells (SPCs).
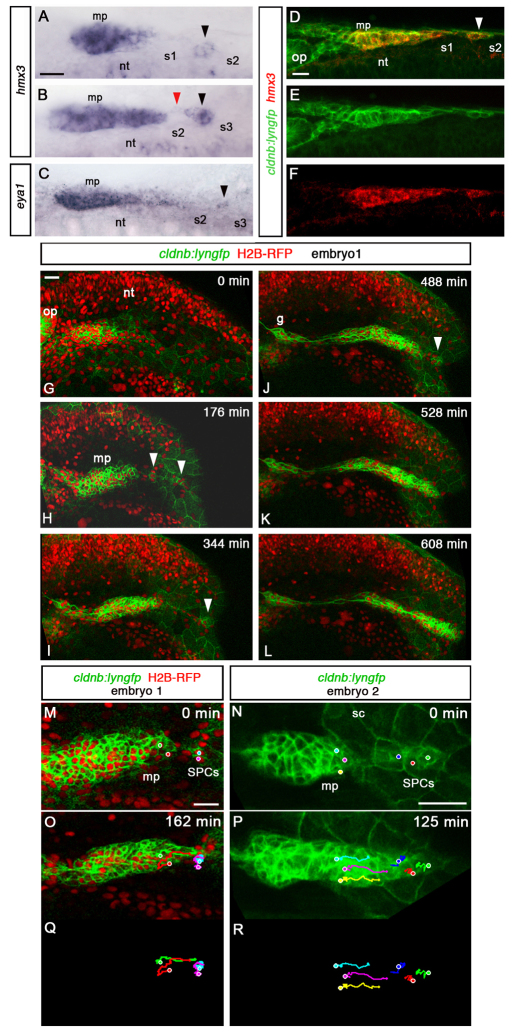
Fig. 1.
SPCs have placode identity and fuse with the main primordium. All images are lateral views, anterior to the left, except A-F which show dorsal views. (A,B) hmx3 expression in the primordium at 22-24s. Black arrowheads indicate SPCs; the red arrowhead indicates fusion between SPCs and main primordium. (C) eya1 expression in SPCs (black arrowhead) at 20s. (D-F) Fluorescence detection of hmx3 mRNA (red) in cldnb:lyngfp (green) embryos at 20s. White arrowhead indicates SPCs. (G-L) Images from time-lapse microscopy performed from 18s on a cldnb:lyngfp embryo injected with H2B-RFP mRNA (supplementary material Movie 1). White arrowheads indicate SPCs. (M-R) Tracking of SPCs and primordium tip cells in embryo 1 (supplementary material Movie 1) and embryo 2 (supplementary material Movie 2) before the fusion. Dots in M and N indicate initial positions of tracked cells. O-R show final time points. g, posterior lateral line ganglion; mp, main primordium; nt, neural tube; op, otic placode; s, somite, sc, skin cells. Scale bars: 25 μm.
SPCs were rarely seen after 24s, suggesting that they either die, fuse with the main primordium or migrate to another location. No apoptotic cells were detected in this region (not shown), whereas the pattern of hmx3 expression suggested that SPCs are fusing with the primordium at ∼24s, supporting the coalescence hypothesis (Fig. 1B). To distinguish these possibilities unequivocally, we performed time-lapse imaging on cldnb:lyngfp embryos (n=7). At 18s, the primordium could be identified as a large cluster of cells extending to somite 1 (Fig. 1G). Time-lapse studies revealed the existence of small groups of Gfp-positive cells located caudal to the primordium (Fig. 1H-J; supplementary material Movies 1, 2). The primordium sequentially coalesced with these clusters and continued its migration (Fig. 1G-L; supplementary material Movies 1, 2), confirming the fusion hypothesis. Tracking analysis of the SPCs and primordium tip cells indicated that the fusion is a consequence of the forward (caudal) movement of the main primordium, rather than backward (rostral) movement of SPCs (Fig. 1M-R; supplementary material Movies 1, 2). To analyse the effect of fusion further, we tracked cells in three regions of the main primordium (leading edge, middle and trailing edge) for 1 hour before and after the fusion. The coalescence with the proximal clusters of SPCs correlated with an increase in speed in the leading two-thirds of the primordium (supplementary material Fig. S1).
To examine in more detail the behaviour of SPCs, we took advantage of the Cxcr7b morpholino (Mo)-mediated knockdown condition, in which the primordium is unable to initiate forward movement (Valentin et al., 2007; Dambly-Chaudiere et al., 2007). We observed variable phenotypes with delayed or ventral migration in Cxcr7b morphants (not shown), as previously described (Valentin et al., 2007). At 28 hours post-fertilisation (hpf), 10% of Cxcr7b morphants (n=206) showed a split phenotype, with a complete migration block and an SPC cluster caudal to the main primordium (supplementary material Fig. S2), whereas control embryos never have a split primordium at this stage. We interpret this phenotype as resulting from an absence of coalescence between the main primordium and SPCs. Live imaging in Cxcr7b morphants (n=6) showed that SPCs are highly protrusive and motile, but fail to make significant forward or backward movements (supplementary material Fig. S2 and Movies 3, 4).
Cxcr4b mediates caudal attraction of SPCs
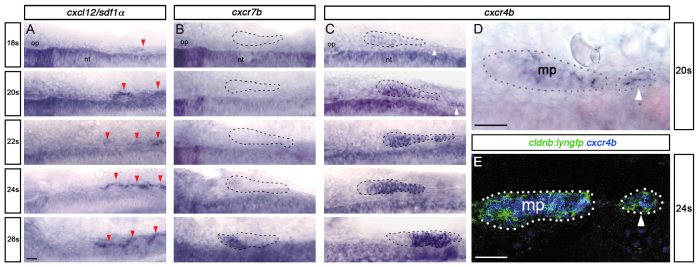
Directional caudal migration of the primordium is controlled by the guidance receptors Cxcr4b and Cxcr7b. As SPCs express markers of primordial cell identity, this raises the possibility that they too are subject to Sdf1α-mediated chemoattraction. We therefore analysed the expression of the chemokine receptors and ligand Sdf1α at early stages. SPCs express cxcr4b from 18s (Fig. 2C-E), sdf1α expression in the somites starts at the same stage (Fig. 2A), but cxcr7b is only detected at 26s in the trailing part of the primordium and is never seen in the location of SPCs (Fig. 2B).
Fig. 2.
SPCs express Cxcr4b, but not Cxcr7b. (A-C) Time course of cxcl12/sdf1α (A), cxcr7b (B) and cxcr4b (C) expression at 18s-26s. Red arrowheads indicate sdf1α expression in somites; white arrowheads indicate cxcr4b expression in SPCs. The main primordium (18 and 20s) and primordium fused with SPCs (22 to 26s) are outlined with dashed lines. (D,E) High magnification views of cxcr4b expression at 20s (D) and cxcr4b (blue) in cldnb:lyngfp embryos (green) at 24s (E). Main primordium (mp) and SPCs are outlined. Scale bars: 25 μm.
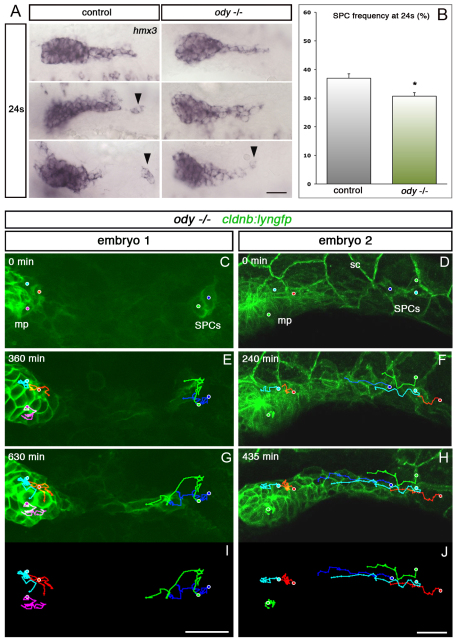
We addressed next the effect of loss-of-function of Cxcr4b on SPCs. We analysed hmx3 expression at 24s in homozygous ody (cxcr4b) mutants and stage-matched controls (Fig. 3A), and calculated the frequency of SPCs, defined as the proportion of embryo sides in which SPCs could be identified at this stage. SPC frequency was significantly decreased in ody mutants (Fig. 3B). This effect is not due to the death of Cxcr4b-deficient SPCs, as no increase in apoptosis was detected in ody embryos (not shown). Live imaging of ody mutants (n=5) and cell tracking revealed that the SPCs move backwards and fuse with the immobile main primordium (Fig. 3C-J; supplementary material Movie 5). A simple model to explain these findings is that, as for the main primordium, Cxcr4b mediates a caudal chemoattraction of SPCs, probably through the sensing of Sdf1α; however, this effect is normally counter-balanced by attraction towards the main primordium, which is revealed only when Cxcr4b signalling is impaired.
Fig. 3.
Cxcr4b attracts SPCs caudally. (A) hmx3 expression at 24s in control and ody–/– embryos. Black arrowheads indicate SPCs. (B) SPC frequency analysed by hmx3 expression in ody–/– and stage-matched controls at 24s. *P=0.013. Error bars indicate s.e.m. (C-J) Tracking of the SPCs and primordium tip cells in two ody–/– embryos (supplementary material Movie 5). In C and D, dots show initial positions of tracked cells. E and F show intermediate time points and G-J show final time points. Scale bars: 25 μm.
Fgf signalling attracts SPCs towards the main primordium
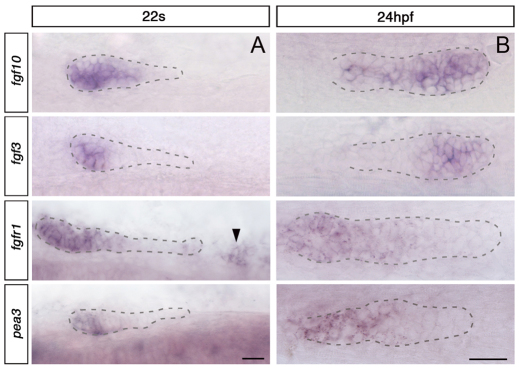
A previous study revealed a patterning role for Fgf signalling within the migrating primordium, in which it restricts Wnt activity to the leading zone, thereby indirectly regulating the asymmetric expression of chemokine receptors required for cell migration (Aman and Piotrowski, 2008). Because in other tissues Fgfs act as chemoattractive signals (Sutherland et al., 1996; Sato et al., 2011; Kadam et al., 2012), we considered the possibility that Fgfs also have a more direct role in lateral line cell migration. We analysed the expression of Fgf signalling components and found that after the fusion between the SPCs and primordium, fgf3 and fgf10 are expressed in the leading zone, and fgfr1 in the trailing zone, as previously reported (Fig. 4B) (Nechiporuk and Raible, 2008; Lecaudey et al., 2008; Aman and Piotrowski, 2008). However, the expression pattern is strikingly different before fusion, when fgf3 and fgf10 are expressed in the back of and throughout the main primordium, respectively, but barely detectable in SPCs, and the receptor fgfr1 is expressed in the main primordium and in SPCs (Fig. 4A). Thus, the expression of Fgf signalling components is dynamic and switches during the fusion.
Fig. 4.
Expression pattern of Fgf signalling components before and after the fusion. (A) Expression of Fgf signalling components at 22s, before fusion. Main primordium is outlined with dashed lines; the black arrowhead indicates SPCs. Dorsal view. (B) Expression of Fgf components at 24 hpf, after fusion. The primordium fused with SPCs is outlined with dashed lines. Side view. Scale bar: 25 μm.
We hypothesised that Fgf ligands produced by the main primordium could act as chemoattractive cues for the Fgfr1-expressing SPCs, thus mediating the backward migration in Cxcr4b-deficient embryos. To test this hypothesis, we utilised the hsp70:dn-fgfr1 line which allows heat shock-induced expression of a dominant-negative form of Fgfr1 (dn-fgfr1) (Lee et al., 2005). Blocking Fgfr activation from 16s led to a higher SPC frequency at 24s (Fig. 5A). Quantification of the distance covered by the tip of the main primordium and SPCs showed that the increased SPC frequency in hsp70:dn-fgfr1 embryos is mainly due to a forward shift in SPC position, rather than a defect in migration of the main primordium (Fig. 5B; supplementary material Fig. S3).
As the dn-fgfr1 protein is fused with Gfp, we could not use this approach to visualise effects on the dynamic behaviour of cells, and therefore used the Fgfr inhibitor SU5402 in cldnb:lyngfp embryos. Treated embryos showed an increase in SPC frequency at 24s, although milder than when the hsp70:dn-fgfr1 line is used (supplementary material Fig. S3). Quantification of the distance travelled by the main primordium and SPCs at 24s revealed a forward shift in SPC position upon SU5402 treatment, with no significant change in main primordium migration at this stage, as also observed in hsp70:dn-fgfr1 embryos (supplementary material Fig. S3). Live imaging of SU5402-treated embryos (n=3) showed that SPCs underwent a forward movement before being eventually caught up by the main primordium (Fig. 5C; supplementary material Movie 6). After normal initiation of the migration, the primordium stopped its movement at around 24 hpf (data not shown), consistent with the migration defect previously reported (Nechiporuk and Raible, 2008; Lecaudey et al., 2008). As SPC clusters do not express Cxcr7b, their forward migration might not be sustained, but the fusion with the main primordium precludes analysis of their longer term behaviour. We therefore blocked Fgf signalling with SU5402 in Cxcr7b morphants, in which fusion is prevented. Live imaging revealed that SPCs migrate a short distance forward but then stop their progression (data not shown), consistent with a requirement for Cxcr7b for persistent directional migration.
We analysed next whether the backward migration of SPCs observed in Cxcr4b-deficient embryos is mediated by the Fgf pathway. To inactivate Fgf signalling in Cxcr4b-deficient embryos, we used hsp70:dn-fgfr1 embryos which were injected with Cxcr4b Mo. As observed in ody mutants, Cxcr4b morphants displayed a reduced SPC frequency, confirming the efficiency and specificity of the Cxcr4b Mo (Fig. 5D). Blocking of Fgfr activation prevented the backward migration of SPCs seen in Cxcr4b morphants (Fig. 5D).
To investigate whether Fgf ligands are sufficient to mediate SPC attraction, we transplanted Fgf10- or control tdTomato-expressing cells into wild-type hosts and analysed the position of SPCs. SPCs were found to have deviated towards ectopic dorsal sources of Fgf10 (Fig. 6; supplementary material Fig. S3), indicating that Fgf10 is sufficient to attract SPCs. As primordium cells also express Fgfr1 (Fig. 4), it was possible that they too respond to ectopic Fgf expression. We occasionally observed cells of the main primordium that slightly deviated towards dorsal sources of Fgf10 (not shown), but most primordium cells stayed in place. Our interpretation of this result is that cells of the main primordium themselves produce high levels of Fgf ligands and are, therefore, less sensitive to ectopic sources of Fgf10 than the SPCs, which are located further away from endogenous sources of Fgf10 and Fgf3. Taken together, these results show that Fgf signalling from the primordium attracts SPCs, which, by counter-balancing the caudal attraction to Sdf1α, keeps them in place so that they fuse with the main primordium once it initiates migration.
SPCs remain in the leading zone and contribute to the terminal neuromasts
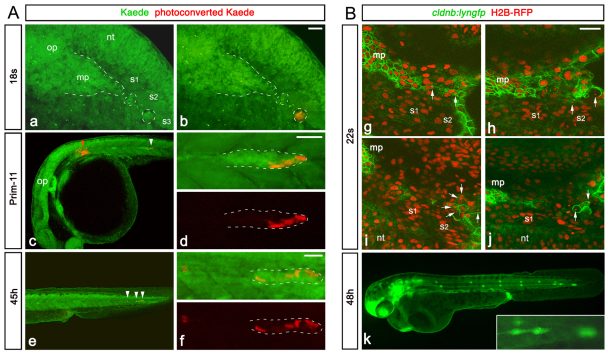
To analyse the contribution of SPCs to the lateral line, we conducted fate-mapping experiments using Kaede photoconversion (Hatta et al., 2006). Kaede-expressing SPCs (Fig. 7Aa,b) or a region containing SPCs (supplementary material Fig. S4) were photoconverted from green to red before the fusion. In most embryos (82%, n=17), red cells were found in the leading zone of the primordium (Fig. 7Ac,d; supplementary material Fig. S4) and later contributed to the last three neuromasts (Fig. 7Ae,f; supplementary material Fig. S4). Activation of a region containing all of the SPCs revealed that the leading zone contains cells derived both from SPCs and the initial main body of the primordium; these cell populations intermingle in the leading zone (supplementary material Fig. S4). We next performed laser-ablation experiments. Owing to bleaching of Gfp fluorescence upon laser application, we were unable to carry out repeated ablations in the same embryo to remove the whole population of SPCs. However, partial ablation of SPCs before the fusion led to impairment of the terminal phase of migration (71% embryos, n=14), consistent with the results of fate mapping. Upon arriving in the tail region, the primordium was smaller, stopped before the normal end point and failed to split to form the terminal neuromasts (Fig. 7B). The formation and the deposition of more anterior neuromasts was not affected (Fig. 7B), and their shape and organisation appeared normal (not shown). Taken together, these data show that SPCs are crucial for formation of the terminal neuromasts.
Fig. 7.
Progeny of SPCs contributes to the terminal neuromasts. (Aa,Ab) Primordium region of 18s embryo injected with Kaede mRNA, before (a) and after (b) photoactivation in the most caudal SPC cluster. The main primordium and SPCs are outlined with dashed lines. (Ac) Low magnification of the same embryo at prim-11, showing red cells (white arrowhead) in the migrating primordium. No deposited red cell is observed between the region of activation (red arrowhead) and the primordium. (Ad) High magnification of the primordium shown in c, showing red cells in the leading part of the primordium. (Ae) Low magnification of the trunk at 45h, showing red cells (white arrowheads) in the last deposited neuromast and in the primordium reaching the tail. No red cell is deposited more anteriorly. (Af) High magnification of the primordium and the terminal neuromast shown in e. See also supplementary material Fig. S4. (Bg-Bj) Examples of confocal sections showing the primordium region of cldnb:lyngfp embryos at 22s, 2 hours after partial laser ablation of SPCs. H2B-RFP-labelled fragmented DNA of dying SPCs is indicated by white arrows. (Bk) Phenotype observed at 48h in embryos in which a subset of SPCs were laser-ablated on the left side. The primordium is small and unable to complete its journey, compared with the control non-ablated side. The inset shows a high magnification of the arrested primordium. mp, main primordium; nt, neural tube; op, otic placode; s, somite. Scale bars: 25 μm.
DISCUSSION
The posterior lateral line primordium has been widely studied as a model for how a group of cells undergoes collective migration. We found that at early stages, prior to the onset of migration, cells with primordium identity are not organised in a single group. Rather, there is a subpopulation of future lateral line cells, SPCs, that are located caudal to the main primordium. Because SPCs express the Cxcr4b chemokine receptor, as for the cells in the main primordium, they are subject to chemoattraction that would cause them to migrate caudally. Such caudal migration would prevent or delay the SPCs from meeting the main primordium such that they can fuse to form a single cluster, a necessary step for the coordinated collective migration. We show that the formation of single primordium is achieved by two distinct guidance cues that counteract each other to position the SPCs.
Roles of Sdf1α and Fgf signalling
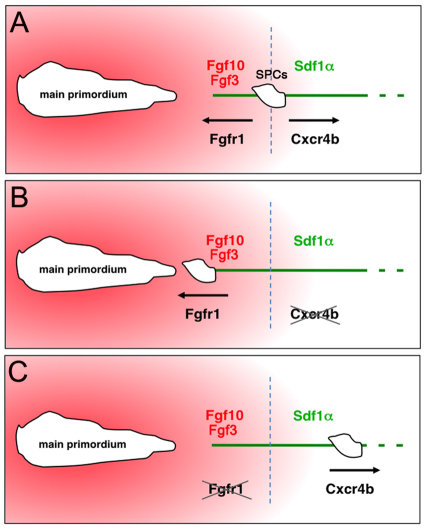
We find that disruption of Sdf1α/Cxcr4b or Fgf signalling elicits backward or forward migration of SPCs, respectively, and that inhibition of Fgf signalling blocks the backward migration that would otherwise occur in the absence of Cxcr4b. In addition, ectopic sources of Fgf ligands can attract SPCs. We therefore propose the following model (Fig. 8). The balance between the Sdf1α/Cxcr4b and Fgf3/10/Fgfr1 signalling normally leads to an absence of anteroposterior directional movement of SPCs, such that the migrating primordium meets and fuses with them. However, if SPCs are unable to sense Sdf1α, they migrate back towards the Fgf-producing main primordium in an Fgfr1-dependent manner. By contrast, compromising Fgf signalling releases the SPCs from Fgf-mediated chemoattraction, resulting in their Cxcr4b-dependent short distance forward migration on the Sdf1α-expressing path. Lack of Cxcr7b in SPCs prevents their long-term directional forward migration. These opposite movements reveal a tug of war between two guidance signals, which maintain the position of SPCs.
Fig. 8.
Model of opposing effects of Sdf1α/Cxcr4b and Fgf signalling on SPCs. (A) In the normal situation, a balance between Sdf1α/Cxcr4b and Fgf/Fgfr1 signalling leads to no net forward or backward movement of SPCs. (B) Cxcr4b-deficient SPCs are unable to sense the Sdf1α chemokine and therefore migrate towards the Fgf-secreting main primordium. (C) Inhibition of Fgf signalling releases the chemoattraction of SPCs, resulting in their Cxcr4b-dependent short distance forward migration on the Sdf1α path. These opposite movements reveal a tug of war between the two signalling systems, whereby Fgf-mediated chemoattraction retains SPCs in position to facilitate their fusion with the main primordium.
The classic transcriptional readout of the Fgf pathway, pea3, is expressed in the back of the primordium from around 20s, but could not be detected in SPCs (Fig. 4). It is possible that pea3 expression is too low in SPCs to be detected by in situ hybridisation. Alternatively, Fgfr1 activation in these cells could regulate migration independently of pea3 by acting more directly on cytoskeletal dynamics and small GTPases. The signalling events mediating Fgf chemotactic effects in other systems remain poorly understood (Dormann and Weijer, 2006; Dorey and Amaya, 2010) and might not involve transcriptional activation of target genes.
Origin and roles of SPCs
This is, to our knowledge, the first report of cells with placodal identity that are isolated from the main primordium and located beyond somite 1, which was thought to be the caudal limit of the primordium at the early stages (Kimmel et al., 1995; Dambly-Chaudiere et al., 2007; Sarrazin et al., 2010). SPCs are observed by hmx3 expression from 10s onwards, well before Cxcr4b and Sdf1α start to be expressed, and can also be detected in ody mutants at these early stages (supplementary material Fig. S1). Thus, SPCs do not come from a premature Cxcr4b-dependent caudal migration of posterior lateral line placodal cells. One possibility is that SPCs could have a developmental origin that is different from other cells of the posterior lateral line placode. A recent study uncovered the contribution of neuroepithelial cells to the otic placode (Freyer et al., 2011), showing that cells from distinct embryonic territories can contribute to placode formation and morphogenesis. However, it seems unlikely that SPCs have a distinct embryonic origin, as they express the same placodal markers as the main primordium. It is believed that cranial placodes arise from a horseshoe-shaped pan-placodal domain at the border between the anterior neural plate and non-neural ectoderm (Schlosser, 2010). The posterior lateral line placode is the most posterior cranial placode. The SPCs thus represent the most posterior placodal cells and could be a consequence of a fuzzy posterior boundary of the pan-placodal domain. Chemoattraction by Fgf signalling would thus represent a mechanism to ensure that an initial imprecise organisation is transformed into a single migrating primordium.
The migrating primordium is patterned along its anteroposterior axis such that it has a leading-trailing zone asymmetry in cell behaviour and gene expression (Nechiporuk and Raible, 2008; Lecaudey et al., 2008; Aman and Piotrowski, 2008). Of particular interest is the possibility that the leading zone cells could be similar to tip cells described in other migratory systems, including germ cells in C. elegans (Cinquin et al., 2010), and trachea in Drosophila (Sutherland et al., 1996; Uv et al., 2003). Tip cells located at the front exert an organisational effect over other cells that migrate in their wake and are essential for successful group migration to occur. The fusion of SPCs with the main primordium correlated with a speeding up of migration. The premature arrest of migration following partial SPC ablation could be due to the decreased number of progenitors in the leading zone of the primordium (McGraw et al., 2011; Valdivia et al., 2011), or a specific role of SPCs in migration. Fate-mapping revealed that SPCs stay in the leading zone of the primordium during the whole course of the migration. Interestingly, photoconversion of Kaede in cells in the leading primordium after the fusion (Nechiporuk and Raible, 2008) labelled neuromasts that are more anteriorly located than those that we find receive a contribution from SPCs. Thus, cells derived from the SPCs seem to remain a distinct population of leading zone cells that are deposited to form the posterior-most neuromasts.
Later roles of Fgf signalling
Fgf signalling is required for several important aspects of lateral line morphogenesis, neuromast formation and the correct migration of the primordium (Lecaudey et al., 2008; Nechiporuk and Raible, 2008; Aman and Piotrowski, 2008). Previous studies have shown that blocking Fgf signalling disrupts the normal polarity of the primordium, including the restricted expression of chemokine receptors that is required for migration. It has thus been proposed that the primary role of Fgf signalling is in patterning within the primordium, which in turn is required for correct migration. Our findings suggest a model in which Fgfs also have a more direct role in coordinating cell migration. If the role of Fgfs in chemoattraction we have uncovered continues at later stages, Fgf ligands produced by the cells in the leading zone might act to attract Fgfr1-expressing cells in the trailing part. Consistent with this, blocking the Fgf pathway leads to elongated morphology of the primordium and uncoordinated migration (Lecaudey et al., 2008). Aman and Piotrowski (Aman and Piotrowski, 2008) reported normal coordinated primordium migration in transgenic hsp70:dkk1 embryos, in which both Wnt and Fgf signalling are impaired. However, in such a situation, the loss of Fgf signalling might be compensated for by other events downstream of Wnt inactivation. Alternatively, the chemoattractive role of Fgfs might be transient, and downregulated after fusion of SPCs with the main primordium, when the expression patterns of some Fgf pathway components change. Distinguishing between these alternatives will require the ability to uncouple the multiple downstream effects of Fgf signalling in the migrating primordium.
In summary, our work reveals a novel role for the Fgf pathway in chemoattraction-mediated assembly of a migrating cell group in the posterior lateral line. It will be interesting to uncover whether similar chemotactic mechanisms could initiate or maintain a compact state of other migratory cell groups during normal development or cancer invasion.
Supplementary Material
Acknowledgments
We thank Elke Ober for help with the laser ablation experiments and the staff of the NIMR fish facility for their work.
Footnotes
Funding
This study was funded by the Medical Research Council [U117532048]. M.A.B. was supported by Fellowships from the Fondation Fyssen (France) and the European Molecular Biology Organization (EMBO). Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.080275/-/DC1
References
- Adamska M., Léger S., Brand M., Hadrys T., Braun T., Bober E. (2000). Inner ear and lateral line expression of a zebrafish Nkx5-1 gene and its downregulation in the ears of FGF8 mutant, ace. Mech. Dev. 97, 161–165 [DOI] [PubMed] [Google Scholar]
- Aman A., Piotrowski T. (2008). Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev. Cell 15, 749–761 [DOI] [PubMed] [Google Scholar]
- Boldajipour B., Mahabaleshwar H., Kardash E., Reichman-Fried M., Blaser H., Minina S., Wilson D., Xu Q., Raz E. (2008). Control of chemokine-guided cell migration by ligand sequestration. Cell 132, 463–473 [DOI] [PubMed] [Google Scholar]
- Cinquin O., Crittenden S. L., Morgan D. E., Kimble J. (2010). Progression from a stem cell-like state to early differentiation in the C. elegans germ line. Proc. Natl. Acad. Sci. USA 107, 2048–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambly-Chaudiere C., Cubedo N., Ghysen A. (2007). Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev. Biol. 7, 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N. B., Sapede D., Saint-Etienne L., Thisse C., Thisse B., Dambly-Chaudiere C., Rosa F. M., Ghysen A. (2002). Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc. Natl. Acad. Sci. USA 99, 16297–16302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey K., Amaya E. (2010). FGF signalling: diverse roles during early vertebrate embryogenesis. Development 137, 3731–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D., Weijer C. J. (2006). Chemotactic cell movement during Dictyostelium development and gastrulation. Curr. Opin. Genet. Dev. 16, 367–373 [DOI] [PubMed] [Google Scholar]
- Feng Y., Xu Q. (2010). Pivotal role of hmx2 and hmx3 in zebrafish inner ear and lateral line development. Dev. Biol. 339, 507–518 [DOI] [PubMed] [Google Scholar]
- Freyer L., Aggarwal V., Morrow B. E. (2011). Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development 138, 5403–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Gilmour D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quevedo R., Lee Y., Poss K. D., Wilkinson D. G. (2010). Neuronal regulation of the spatial patterning of neurogenesis. Dev. Cell 18, 136–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety S. S., Wilkinson D. G. (2010). Morpholino artifacts provide pitfalls and reveal a novel role for pro-apoptotic genes in hindbrain boundary development. Dev. Biol. 350, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudiere C. (2007). The lateral line microcosmos. Genes Dev. 21, 2118–2130 [DOI] [PubMed] [Google Scholar]
- Haas P., Gilmour D. (2006). Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev. Cell 10, 673–680 [DOI] [PubMed] [Google Scholar]
- Hatta K., Tsujii H., Omura T. (2006). Cell tracking using a photoconvertible fluorescent protein. Nat. Protoc. 2, 960–967 [DOI] [PubMed] [Google Scholar]
- Kadam S., Ghosh S., Stathopoulos A. (2012). Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands. Development 139, 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Kozlowski D. J., Whitfield T. T., Hukriede N. A., Lam W. K., Weinberg E. S. (2005). The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev. Biol. 277, 27–41 [DOI] [PubMed] [Google Scholar]
- Lecaudey V., Cakan-Akdogan G., Norton W. H., Gilmour D. (2008). Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 135, 2695–2705 [DOI] [PubMed] [Google Scholar]
- Lee Y., Grill S., Sanchez A., Murphy-Ryan M., Poss K. D. (2005). Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173–5183 [DOI] [PubMed] [Google Scholar]
- Li Q., Shirabe K., Kuwada J. Y. (2004). Chemokine signaling regulates sensory cell migration in zebrafish. Dev. Biol. 269, 123–136 [DOI] [PubMed] [Google Scholar]
- McGraw H. F., Drerup C. M., Culbertson M. D., Linbo T., Raible D. W., Nechiporuk A. V. (2011). Lef1 is required for progenitor cell identity in the zebrafish lateral line primordium. Development 138, 3921–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk A., Raible D. W. (2008). FGF-dependent mechanosensory organ patterning in zebrafish. Science 320, 1774–1777 [DOI] [PubMed] [Google Scholar]
- Robu M. E., Larson J. D., Nasevicius A., Beiraghi S., Brenner C., Farber S. A., Ekker S. C. (2007). p53 activation by knockdown technologies. PLoS Genet. 3, e78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin A. F., Nuñez V. A., Sapède D., Tassin V., Dambly-Chaudière C., Ghysen A. (2010). Origin and early development of the posterior lateral line system of zebrafish. J. Neurosci. 30, 8234–8244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Scholl A. M., Kuhn E. B., Stadt H. A., Decker J. R., Pegram K., Hutson M. R., Kirby M. L. (2011). FGF8 signaling is chemotactic for cardiac neural crest cells. Dev. Biol. 354, 18–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. (2010). Making senses: development of vertebrate cranial placodes. Int. Rev. Cell Mol. Biol. 283, 129–234 [DOI] [PubMed] [Google Scholar]
- Sun X., Cheng G., Hao M., Zheng J., Zhou X., Zhang J., Taichman R. S., Pienta K. J., Wang J. (2010). CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 29, 709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D., Samakovlis C., Krasnow M. A. (1996). branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87, 1091–1101 [DOI] [PubMed] [Google Scholar]
- Tiveron M. C., Cremer H. (2008). CXCL12/CXCR4 signalling in neuronal cell migration. Curr. Opin. Neurobiol. 18, 237–244 [DOI] [PubMed] [Google Scholar]
- Uv A., Cantera R., Samakovlis C. (2003). Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 13, 301–309 [DOI] [PubMed] [Google Scholar]
- Valdivia L. E., Young R. M., Hawkins T. A., Stickney H. L., Cavodeassi F., Schwarz Q., Pullin L. M., Villegas R., Moro E., Argenton F., et al. (2011). Lef1-dependent Wnt/β-catenin signalling drives the proliferative engine that maintains tissue homeostasis during lateral line development. Development 138, 3931–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin G., Haas P., Gilmour D. (2007). The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr. Biol. 17, 1026–1031 [DOI] [PubMed] [Google Scholar]
- Xu Q., Wilkinson D. G. (1998). In situ hybridization of mRNA with hapten labelled probes. In In Situ Hybridization: A Practical Approach, 2nd edition (ed. Wilkinson D. G.), pp. 87–106 Oxford: Oxford University Press; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.