Abstract
Objective
The current study examined the impact of risperidone and divalproex on affective and working memory circuitry in patients with pediatric bipolar disorder (PBD).
Method
This was a six-week double blind randomized trial of risperidone plus placebo vs. divalproex plus placebo for patients with mania (n=21; 13.6±2.5 years). The fMRI outcomes were measured using a block design affective N-back task with angry, happy and neutral face stimuli at baseline and at 6-week follow-up. Matched healthy controls (HC; n=15, 14.5±2.8 years) were also scanned twice.
Results
Post hoc analyses on the significant interaction in a 3×2×2 ANOVA which included patient groups and HC revealed that the risperidone group showed greater activation after treatment in response to the angry face condition in the left subgenual anterior cingulate cortex (ACC) and striatum relative to the divalproex group. The divalproex group showed greater activation relative to the risperidone group in left inferior frontal gyrus and right middle temporal gyrus. Over the treatment course, the risperidone group showed greater change in activation in the left ventral striatum than the divalproex group, and the divalproex group showed greater activation change in left inferior frontal gyrus and right middle temporal gyrus than the risperidone group. Furthermore, each patient group showed increased activation relative to HC in fronto-striato-temporal regions over time. The happy face condition was potentially less emotionally challenging in this study and did not elicit notable findings.
Conclusion
When patients performed a working memory task under emotional duress inherent in the paradigm, divalproex enhanced activation in a fronto-temporal circuit while risperidone increased activation in the dopamine (D2) receptor-rich ventral striatum.
Keywords: risperidone, divalproex, pediatric bipolar disorder, fMRI
INTRODUCTION
There is accumulating evidence for the efficacy and effectiveness of divalproex sodium (divalproex)1,2,3 and risperidone4, 5, 6 in pediatric bipolar disorder (PBD). Risperidone has FDA approval for use in pediatric mania, while evidence for divalproex remains more equivocal.3, 7 Findings from open trials in pediatric mania that showed that divalproex is useful.2, 3, 8, 9 are in contrast to those from the double-blind placebo-controlled trial of divalproex extended release showing no benefit relative to placebo.10 A mechanistic understanding of how these medications impact brain function may offer further insight into how these two medications yield differential results. Functional neuroimaging studies using appropriate challenge paradigms represent one promising strategy for investigating how brain mechanisms could explain differential therapeutic benefit across different classes of medications. Importantly, understanding how treatments impact the critical interface of affective and cognitive brain circuitries can shed light on how affect regulation is influenced by cognitive operations and vice versa.11 This is especially valuable given the intricate connections between the two underlying neural operations.12 Such close connectivity between the cognitive and affective brain circuitry explains the combined affective and working memory impairment in PBD.11, 13, 14 Hence, the current study of pediatric mania embraces the emerging trend15, 16, 17, 18 of using task-elicited regional brain activation of a cognitive task (e.g. working memory) under emotional challenge as the primary outcome measure in a pharmacological study.
Working memory has been shown to be impaired in our three year longitudinal study of PBD,19 but pharmacotherapy with lamotrigine was shown to improve working memory in this population.20 In healthy controls (HC), working memory function is supported by fronto-striatal-parietal circuitry.21, 22, 23 In fact, in a sample that included mixed group of medicated and unmedicated PBD patients, Chang et al24 showed increased activity in PBD patients relative to HC while performing a visuospatial working memory task in several prefrontal regions, including the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and anterior cingulate cortex (ACC) . Using an N-back working memory task performed under negative emotional challenge with unmedicated PBD patients, Passarotti et al25 showed that affective regions and cognitive prefrontal regions are less engaged in PBD, suggesting alteration in the brain circuitry at the interface of affective and cognitive functions. Using another task that probed such an interface where colored emotional words were matched with colored dots, lamotrigine monotherapy led to an increase in ventromedial prefrontal cortex (VMPFC) activation in PBD patients relative to HC.16 Also, decreased activation was noted in the DLPFC in patients, illustrating the additional role of this cognitive region. Furthermore, we and others have documented alterations in fronto-temporal circuitry during facial emotion processing in PBD.26, 27, 28, 29, 30 These studies further informed us that emotional faces could be an effective probe of affective circuitry function in PBD patients.
Therefore, we compared the effects on brain function of antipsychotic (risperidone) and anti-epileptic (divalproex) medications, using a double-blind randomized controlled trial (DBRCT) in pediatric mania, with a block design affective N-back working memory task to probe the interface of cognitive and affective circuitries. Basic neurochemical research implicates fronto-temporal mechanisms of action with divalproex due to its action in the second messenger system31 and ventral fronto-striatal systems with risperidone due to its known serotonin-dopamine antagonistic activity in these regions. Serotonin rich subgenual ACC16, 32, 33, 34 and dopamine (D2) receptor-rich ventral striatum35, 36 have been implicated in bipolar disorder and could be predictably involved in the action of risperidone, a known serotonin-dopamine antagonist.
METHOD
Design
This was a six-week out-patient DBRCT of risperidone plus placebo (that resembled divalproex capsule) vs. divalproex plus placebo (that resembled risperidone tablet) for manic and mixed episodes of bipolar disorder. This study was approved by the University of Illinois at Chicago's Institutional Review Board. Written informed consent was obtained after the study was completely described to the participants. Parents and adolescents older than 16 years gave written permission and children younger than 16 years gave assent to participate. The clinical trials registry number is NCT00176202.
Sample
Inclusion criteria were a DSM-IV diagnosis of mixed or manic bipolar disorder; 12 to 18 years old; and medication free or currently clinically unstable on medication, justifying termination of the ineffective regimen. With consent, all subjects receiving psychotropic medications were washed out and free of any medication for a week prior to baseline scanning, and for 4 weeks in the case of fluoxetine or aripiprazole. Prior exposure to SGAs and anti-epileptic medications was acceptable. Exclusion criteria included: active substance abuse; serious medical problems; autism and non-affective psychotic disorders. Additionally, HC scoring ≥12 on YMRS or ≥28 on CDRS-R were excluded. Using these criteria, we recruited 44 subjects into the study. After excluding subjects whose fMRI data were unusable due to motion artifacts (HC: n=1; risperidone group: n=4; divalproex group: n=3), the final sample included in the analyses included 15 HC and 21 patients randomized to either risperidone (n=10) or divalproex (n=11). No subjects dropped out of the study. Sample characteristics are summarized in Table 1. See Figure S1, available online, for the CONSORT Diagram.
Table 1.
Demographic Variables and Clinical Characteristics for the Three Groups.
| HC (n=15) | Risperidone Group (n=10) | Divalproex Group (n=11) | Analysis | |
|---|---|---|---|---|
| Mean (SD/%) | Mean (SD/%) | Mean (SD/%) | (F), p value | |
| Variables | ||||
| Age (years) | 14.5 (2.8) | 12.4 (1.6) | 13.1 (2.6) | (2.14), p=.13 |
| WASI- FSIQ | 107.3 (11.8) | 98.70 (5.6) | 97.90 (14.6) | (2.86), p=.06 |
| SESa | 2.7 (1.2) | 2.8 (0.7) | 1.9 (1.1) | (2.85), p=.07 |
| YMRS (pre, post) | (26.07), p=.00001 | |||
| Baseline YMRS | .93 (1.5) | 27.2 (6.4) | 25.3 (6.6) | Risp vs HC: p=.00001 Dvpx vs HC: p=.00001 Risp vs Dvpx: p=.37 |
| Follow-up YMRS | 1.1 (1.9) | 10.0 (6.4)b | 14.0 (9.3)b | Risp vs HC: p=.0009 Dvpx vs HC: p=.00005 Risp vs Dvpx: p=.14 |
| CDRS (pre, post) | (1.84) p=.18 | |||
| Baseline CDRS-R | 19.2 (2.24) | 44.8 (14.1) | 45.8 (13.6) | Risp vs HC: p=.000001 Dvpx vs HC: p=.000001 Risp vs Dvpx: p=.84 |
| Follow-up CDRS-R | 19.1 (1.5) | 33.1 (14.2) b | 38.9 (14.9) | Risp vs HC: p=.003 Dvpx vs HC: p=.00006 Risp vs Dvpx: p=.23 |
| Variables | N (%) | N (%) | N (%) | Fisher's Exact P (two-tailed) |
| Sex | p>.66 | |||
| Male | 9 (60) | 5 (50) | 7 (64) | |
| Female | 6 (40) | 5 (50) | 4 (36) | |
| Handedness | p>.17 | |||
| Right | 15 (100%) | 9 (90%) | 9 (82%) | |
| Left | 0 (0%) | 1(10%) | 2 (18%) | |
| Race | p>.67 | |||
| Caucasian | 9 (60) | 5 (50) | 8 (73) | |
| Other | 6 (40) | 5 (50) | 4 (27) | |
| Episode | ||||
| Manic | - | 7 (70) | 8 (72.7) | |
| Mixed | - | 3(30) | 3 (27.3) | |
| Comorbidity | (N) | (N) | (N) | |
| ADHD | - | 3 | 4 | |
| Psychosis | - | 1 | - | |
| GAD | - | - | 1 |
Note: CDRS-R = Child Depression Rating Scale-Revised; Dvpx = Divalproex sodium; GAD=Generalized Anxiety Disorder; HC = Healthy Control; Risp = Risperidone; WASI-FSIQ = Wechsler Abbreviated Scale of Intelligence Full Scale Intelligent Quotient (Matrix Reasoning and Vocabulary Subtests); YMRS = Young Mania Rating Scale.
Mean revised Hollingshead socio-economic status (SES)
follow-up scores differ significantly from baseline scores within each patient groups, relative to HC at both baseline and post-treatment, and change during the trial period relative to HC (p<.001).
Assessment and efficacy measures
Each child and their parent or legal guardian were interviewed using the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS37) supplemented by the episode characterization of bipolar disorder from the KSADS - Present and Lifetime version.38 Diagnostic interviews were completed by doctoral level clinicians with established inter-rater reliability. The primary clinical efficacy measure was the Young Mania Rating Scale (YMRS39). The Child Depression Rating Scale-Revised was also administered (CDRS-R40). Three masters-level independent clinical raters with established reliability administered these clinical outcome measures on a weekly basis.
Study dosing of risperidone and divalproex
The mean (standard deviation, SD) risperidone dose at endpoint was 1.45 (±0.35) mg/day in nonresponders and 1.35 (±0.46) mg/day in responders (defined as improvement >=50% on the YMRS scores). The mean (SD) divalproex dose at endpoint was 866.67 (±214.52) mg/day in non-responders and 852.14 (±275.13) mg/day in responders. For the risperidone group there were 6 responders (60%) and 4 non-responders (40%), whereas for the divalproex group there were 4 responders (36%) and 7 non-responders (64%). The mean serum valproic level at end point was 98 μg/mL, and 95% of patients achieved a therapeutic serum valproic level of > 75 μg/mL by the 5th day. No titration of medications was allowed after day 7. One subject in the divalproex group received lorazepam as a rescue medication at a dose of 2 mg for severe agitation during the first week of the trial.
fMRI Session: Affective 2-back Task with Emotional Faces
Participants underwent an fMRI scanning session at baseline and at completion of treatment. In each session, they were administered a 2-back working memory task with emotional faces, for 7 min. The paradigm involved blocks of two runs i.e., angry and neutral, and happy and neutral. The first run consisted of blocks of angry and neutral faces and the second run consisted of happy and neutral faces. On each trial, an emotional face stimulus (i.e., angry, happy or neutral) was presented for 3 sec. and subjects responded by pressing a response key if they saw the same face that was presented two trials earlier. In this task, a 2-back match always involved a match both in face identity and emotion. There were 160 Gur faces41 with either neutral, angry or happy face emotions balanced by gender, race and affect. Each condition (angry or happy vs. neutral face) consisted of four 30 sec. trial blocks (each with ten trials), with blocks of 10 angry, happy or neutral faces presented in a pseudorandom sequence (Figure 1). A 20-sec. fixation mark in-between blocks allowed for emotional arousal to return to baseline and for rest periods during testing.
Figure 1.
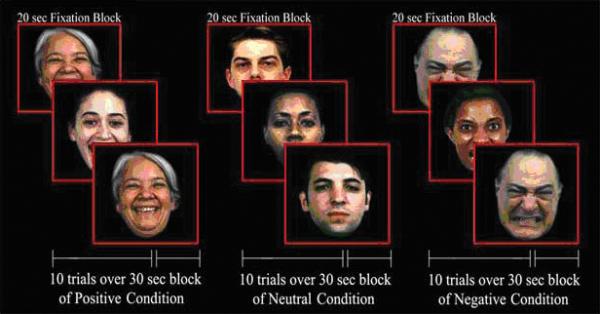
2-Back Working Memory Task with Emotional Faces
MRI Protocol
Gradient-echo planar functional imaging and structural acquisitions were performed with a 3.0 Tesla whole body scanner (Signa, General Electric Medical System, Milwaukee, WI). We acquired twenty-five slices in the axial plane (TE=25ms; flip angle=90°; field of view=20×20cm2; acquisition matrix=64×64; TR=2.5s; slice thickness=5 mm with 1mm gap). Anatomical images were also acquired in the axial plane (three-dimensional spoiled gradient recalled [SPGR], 1.5mm thick contiguous axial slices) and were later co-registered with the functional data.
Image Processing and Data Analysis
We used FIASCO software (Functional Imaging Analysis Software - Computational Olio42) to implement 3D motion estimation and motion correction and to remove slow signal drift. Individual volumes were excluded from analyses if, relative to median head position, head displacement was greater than 1.5mm or head rotation was greater than 0.5 degrees. The groups did not differ in terms of the number of volumes retained after discarding volumes with motion artifact. For each subject, voxel-wise effect size (r) maps were obtained by contrasting activation for affective versus neutral face emotion blocks separately for baseline and follow-up. A Fisher z transform was applied to normalize the effect size maps (zr)43. Subjects’ zr-maps and SPGR anatomical images were warped into Talairach space using automated procedures in AFNI (Analysis of Functional NeuroImages44). We re-sampled each individual's normalized functional map (3.125 × 3.125 × 6 mm grid) to an isotropic 3 × 3× 3 mm grid to provide a voxel dimension similar to that of the in-plane resolution of the acquired data.
Based on our hypotheses, we conducted a whole-brain voxel-wise 3 × 2 × 2 ANOVA in AFNI that compared the three groups (risperidone, divalproex, HC) for each condition (angry vs. neutral faces, happy vs. neutral faces) and testing time (baseline, follow-up) to examine differences in brain activation at baseline and follow-up in risperidone or divalproex groups relative to each other and to HC, the reference group for healthy brain activation. Restricting our analyses to clusters that were significant in the 3-way interaction, we carried out pair-wise comparisons to further deconstruct the significant interaction and clarify the directionality of the within-group and between-group differences at baseline and follow-up. To correct for a total of 21 comparisons, we applied Bonferroni correction resulting in an adjusted probability for significance of p=0.002. Then, using this adjusted probability value, we carried out AlphaSim whole brain Monte Carlo simulations45 to correct for voxel-wise multiple comparisons in the fMRI analyses. To do so, we identified significant voxel clusters by adopting a contiguity threshold (uncorrected p=.002; minimum volume threshold= 108 mm3) that ensured a corrected p<0.05. We also performed Spearman correlation analyses to further explore the relationship between changes in behavioral performance (median reaction time [RT], accuracy) as well as clinical measures (YMRS, CDRS-R) with changes in fMRI brain activation in anatomical ROIs for which we had a priori predictions (i.e., amygdala, VLPFC, medial prefrontal cortex (MPFC), DLPFC, subgenual and dorsal ACC) and showed significant interaction effects in the ANOVA. Anatomical ROIs were defined in standard Talairach space using AFNI tools. Spearman correlation analyses were used due to small samples and limited power. The ROIs in AFNI format, as well as the rationale for anatomical ROI definition, are available at our Pediatric BRAIN Center website: http://www.psych.uic.edu/brain-center/publications_supplement.html.
RESULTS
Demographic and Clinical Data
Table 1 summarizes clinical and demographic data for the three groups with both patient groups showing significant improvement in manic and depressive symptoms. Separate ANOVAs for each demographic measure revealed that the three groups did not differ significantly for age, estimated IQ, or SES (p>.05). Similarly, no significant group differences were found for handedness, gender, or racial composition using Fisher's p tests (two-tailed). Moreover, we conducted two separate 3 × 2 ANOVAs, one for YMRS and one for CDRS-R scores, with group (risperidone group, divalproex group, HC) as between-subject factor and testing time (baseline, follow-up) as a within-subject factor (Table 1).
Behavioral Results
We carried out separate repeated measures ANOVAs for median RT and accuracy data, with group (risperidone group, divalproex group, HC) as a between-subjects factor, and testing time (baseline, follow-up) and face emotion condition (angry, happy, neutral) as within-subjects factors. Median RT and mean accuracy for each group are reported in Table 2. We traditionally use median RT instead of mean RT since it considerably reduces the high RT variability that is often present in psychiatric population.
Table 2. Behavioral Results.
Median Reaction Time and Accuracy for the Risperidone group, the Divalproex group and Healthy Controls (HC).
| Risperidone Group (n=10) | Divalproex Group (n=11) | Healthy Controls (n=15) | |
|---|---|---|---|
| Response Time (in ms) | Median RT (SD) | Median RT (SD) | Median RT (SD) |
| Baseline | |||
| Happy Condition | 920 (112) | 919 (108) | 719 (131) |
| Angry Condition | 920 (111) | 1196 (266) | 826 (133) |
| Neutral Condition | 855 (222) | 1155 (267) | 708 (146) |
| Follow-up | |||
| Happy Condition | 927 (223) | 1077 (233) | 897 (173) |
| Angry Condition | 1005 (145) | 1256 (186) | 941 (207) |
| Neutral Condition | 868 (89) | 1166 (204) | 828 (71) |
| Accuracy (% correct) | % (SD) | % (SD) | % (SD) |
| Baseline | |||
| Happy Condition | 97 (0.04) | 95 (0.04) | 96 (0.07) |
| Angry Condition | 97 (0.03) | 96 (0.03) | 97 (0.05) |
| Neutral Condition | 96 (0.03) | 96 (0.03) | 96 (0.06) |
| Follow-up | |||
| Happy Condition | 98 (0.01) | 83 (0.13) | 99 (.01) |
| Angry Condition | 98 (0.02) | 91 (0.05) | 87 (.09) |
| Neutral Condition | 97 (0.02) | 82 (0.12) | 93 (.08) |
Note: ms=milliseconds; RT=reaction time; SD=standard deviation
Median Reaction Time
A main effect of testing time [F(1,33)=6.16, p<.02] revealed that median RT was overall slower at follow-up than baseline, but this effect did not differ across groups. Moreover, there was a significant interaction of group by face emotion condition [F(4,66)=7.01, p<.00009]. For angry faces, the divalproex group had slower responses than the risperidone group [F(1,33)=18.30, p<.0002] and HC [F(1,33)=37.44, p<.000001], who did not differ from each other (p<.17). The same pattern of results occurred for neutral faces, for which the divalproex group had slower responses than the risperidone group [F(1,33)=23.90, p<.00003] and HC [F(1,33)=49.89, p<.000001], who did not differ from each other (p<.11). For happy faces both the divalproex group [F(1,33)=15.74, p<.0004] and the risperidone group [F(1,33)=5.47, p<.03] had slower responses than HC, but did not differ from each other (p<.16).
Accuracy
The main effect of group [F(2,33)=8.80, p<.001] was significant. Planned comparisons revealed that across both testing times the divalproex group exhibited lower accuracy compared to both the risperidone group [F(1,33)=17.03, p<.0002] and HC [F(1,33)=7.92, p<.008], who did not differ from each other (p=.10). No other significant results were found.
fMRI Results
A significant group by face emotion by testing time interaction was found [F(2,33)=4.18, p<0.03]. A list of the clusters that were significant based on the ANOVA interactions and the relative between- and within-group differences in activation at baseline and follow-up are reported in Tables 3-5. See Supplement 1, available online, for additional results.
Table 3.
Between-Group Comparisons for Angry or Happy vs. Neutral Faces at Baseline or Follow-up.
| Talairach Coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values | |
|---|---|---|---|---|---|
| BASELINE | |||||
| Angry vs. Neutral | |||||
| Risperidone Group > HC | none | none | none | none | none |
| HC> Risperidone Group | 45 20 -3 | R inferior frontal gyrus | 47 | 108 | 5.10 |
| -0 16 -12 | L subgenual ACC | 25 | 108 | 4.65 | |
| -37 17 5 | L insula | 13 | 162 | 6.24 | |
| -49 -67 8 | L middle temporal gyrus | 19 | 297 | 4.91 | |
| Divalproex Group > HC | none | none | none | none | none |
| HC> Divalproex Group | 47 17 2 | R inferior frontal gyrus | 47/45 | 135 | 5.77 |
| 20 14 -1 | R putamen | 486 | 5.34 | ||
| -19 5 -1 | L putamen | 108 | 4.91 | ||
| 8 5 -13 | R subgenual ACC | 25 | 108 | 6.13 | |
| Risperidone Group > Divalproex Group | none | none | none | none | none |
| Divalproex Group> Risperidone Group | none | none | none | none | none |
| Happy vs. Neutral | |||||
| Risperidone Group > HC | none | none | none | none | none |
| HC> Risperidone Group | none | none | none | none | none |
| Divalproex Group > HC | none | none | none | none | none |
| HC> Divalproex Group | none | none | none | none | none |
| Risperidone Group > Divalproex Group | none | none | none | none | none |
| Divalproex Group > Risperidone Group | none | none | none | none | none |
| FOLLOW-UP | |||||
| Angry vs. Neutral | |||||
| Risperidone Group > HC | 56 -46 -19 | R inferior temporal gyrus | 37 | 108 | 4.10 |
| -6 8 -14 | L subgenual ACC | 25 | 108 | 3.99 | |
| -11 16 6 | L caudate | 108 | 2.74 | ||
| HC> Risperidone Group | none | none | none | none | none |
| Divalproex Group > HC | -36 35 -14 | L VLPFC | 47/11 | 108 | 2.15 |
| 37 50 -1 | R VLPFC | 45/46 | 270 | 3.05 | |
| 20 41 29 | R middle frontal gyrus | 9 | 108 | 3.71 | |
| 65 -31 -7 | R middle temporal gyrus | 21 | 189 | 3.44 | |
| HC> Divalproex Group | none | none | none | none | none |
| Risperidone Group > Divalproex Group | -2 -3 -8 | L subgenual ACC | 25 | 108 | 2.98 |
| -22 -5 -3 | L ventral striatum | 108 | 3.34 | ||
| Divalproex Group> Risperidone Group | -40 32 -7 | L inferior frontal gyrus | 47/11 | 108 | 2.82 |
| 63 -34 -7 | R middle temporal gyrus | 21 | 189 | 2.93 | |
| Happy vs. Neutral | |||||
| Risperidone Group > HC | 44 26 -13 | R inferior frontal gyrus | 47 | 216 | 4.77 |
| -49 -19 2 | L superior temporal gyrus | 22 | 297 | 5.21 | |
| HC> Risperidone Group | none | none | none | none | none |
| Divalproex Group > HC | none | none | none | none | none |
| HC> Divalproex Group | none | none | none | none | none |
| Risperidone Group > Divalproex Group | -25 -88 5 | L middle occipital gyrus | 18 | 324 | 4.36 |
| Divalproex Group> Risperidone Group | 62 -40 -4 | R middle temporal gyrus | 21 | 108 | 4.17 |
Note: Talairach coordinates and t values for clusters for which there is a significant three-way interaction (corrected p<0.05, with cluster contiguity threshold) in the whole-brain Analysis of Variance (ANOVA). ACC= anterior cingulate cortex; BA = Brodmann Area; HC=healthy control; L=left; R=right; VLPFC=ventrolateral prefrontal cortex.
Table 5.
Within Group Differences at Follow-up vs. Baseline.
| Talairach Coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values | |
|---|---|---|---|---|---|
| HC | |||||
| Angry vs. Neutral | |||||
| Follow-up > Baseline | none | none | none | none | none |
| Baseline > Follow-up | 26, 11, 2 | R putamen | 135 | 3.73 | |
| 5 -16 32 | R midcingulate gyrus | 24 | 135 | 3.67 | |
| Happy vs Neutral | |||||
| Follow-up > Baseline | 62, -43, 29 | R inferior parietal gyrus | 40 | 135 | 4.58 |
| Baseline > Follow-up | none | none | none | none | none |
| Risperidone Group | |||||
| Angry vs. Neutral | |||||
| Follow-up > Baseline | 11, -7, 29 | R midcingulate gyrus | 24 | 162 | 4.03 |
| 26, -13, -4 | R ventral striatum | 108 | 3.15 | ||
| -12, -4, -2 | L ventral striatum | 108 | 4.74 | ||
| Baseline > Follow-up | none | none | none | none | none |
| Happy vs. Neutral | |||||
| Follow-up > Baseline | none | none | none | none | none |
| Baseline > Follow-up | none | none | none | none | none |
| Divalproex Group | |||||
| Angry vs. Neutral | |||||
| Follow-up > Baseline | 20, 65, 5 | R medial frontal gyrus | 10 | 243 | 3.87 |
| 53, -34, -4 | R middle temporal gyrus | 21 | 108 | 4.24 | |
| Baseline > Follow-up | none | none | none | none | none |
| Happy vs. Neutral | |||||
| Follow-up > Baseline | -46, 20, 14 | L inferior frontal gyrus | 45 | 108 | 3.24 |
| Baseline > Follow-up | none | none | none | none | none |
Note: Talairach coordinates and t values for clusters for which there is a significant three-way interaction (corrected p<0.05, with cluster contiguity threshold) in the whole-brain Analysis of Variance (ANOVA). BA = Brodmann Area; HC=healthy control; L=left; R=right.
I. Between Group Differences at Baseline for Angry and Happy Face Emotion Condition: Table 3
At baseline, the two patient groups did not differ significantly for either face emotion condition, confirming that our randomization was effective. For the angry face condition, the risperidone group exhibited lower activation relative to HC at baseline in the right VLPFC, left insula, subgenual ACC, and middle temporal gyrus, but showed no regions of greater activation. Relative to HC, the divalproex group exhibited lower activation at baseline in the right VLPFC, subgenual ACC, and bilateral putamen, but no regions of greater activation. There were no significant group differences for the happy face condition at baseline. Baseline brain activation for both angry and happy face conditions in YMRS responders vs non-responders is also reported in Tables S1 and S2, available online.
II. Between Group Differences at Follow-up for Angry and Happy Face Emotion Condition: Table 3
For the angry face condition at follow-up, the risperidone group exhibited greater activation relative to the divalproex group, in the left ventral striatum and subgenual ACC and lower activation in the left VLPFC and right middle temporal gyrus. At follow-up, the risperidone group exhibited increased activation relative to HC in the right VLPFC, left subgenual ACC and caudate, and no decreased activation. Relative to HC, the divalproex group showed increased activation in right DLPFC, middle temporal gyrus, and bilateral VLPFC, and no decreased activation. For the happy face condition at follow-up, the risperidone group exhibited greater activation than the divalproex group in left middle occipital gyrus and lower activation in right middle temporal gyrus. Relative to HC, the risperidone group exhibited greater activation for the happy face condition at follow-up in right VLPFC and left superior temporal gyrus and no decreased activation, while the divalproex group did not show significant differences compared to HC.
III. Between Group Differences at Follow-up vs. Baseline for Angry and Happy Face Emotion Condition: Table 4
Table 4.
Between-Group Comparisons at Follow-up vs. Baseline: A) for Angry or Happy vs. Neutral Faces; B) Collapsed over Face Emotion Condition.
| Talairach Coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values | |
|---|---|---|---|---|---|
| Angry vs. Neutral | |||||
| Risperidone Group > HC | -4, 32, 14 | L dorsal ACC | 32 | 162 | 3.55 |
| -7, 17, -13 | L subgenual ACC | 25 | 108 | 3.67 | |
| 21, -6, -2 | R ventral striatum | 189 | 3.44 | ||
| -7, 11, -1 | L ventral striatum | 135 | 2.77 | ||
| 2, -19, 29 | R posterior cingulate gyrus | 23 | 405 | 4.21 | |
| 53, -25, -4 | R middle temporal gyrus | 21 | 189 | 4.30 | |
| HC> Risperidone Group | none | none | none | none | none |
| Happy vs. Neutral | |||||
| Risperidone Group > HC | none | none | none | none | none |
| HC > Risperidone Group | none | none | none | none | none |
| Angry vs. Neutral | |||||
| Divalproex Group > HC | 14, 62, -1 | R medial frontal gyrus | 10 | 162 | 3.73 |
| -40, 35, -4 | L inferior frontal gyrus | 47,11 | 135 | 3.51 | |
| 6, 20, -5 | L subgenual ACC | 108 | 2.71 | ||
| 8, 14, 14 | R dorsal caudate | 162 | 4.19 | ||
| -7, 17, 14 | L dorsal caudate | 108 | 2.73 | ||
| 20, 11, -4 | R putamen | 405 | 3.67 | ||
| 15, -5, -6 | R ventral striatum | 297 | 4.02 | ||
| 57, -34, -7 | R middle temporal gyrus | 21 | 216 | 3.53 | |
| HC > Divalproex Group | none | none | none | none | none |
| Happy vs. Neutral | |||||
| Divalproex Group > HC | none | none | none | none | none |
| HC > Divalproex Group | none | none | none | none | none |
| Angry vs. Neutral | |||||
| Risperidone Group >Divalproex Group | -17, -8, -2 | L ventral striatum | 108 | 2.32 | |
| Divalproex Group > Risperidone Group | -40, 35, -5 | L inferior frontal gyrus | 47 | 108 | 2.71 |
| 62, -33, -5 | R middle temporal gyrus | 21 | 162 | 2.23 | |
| Happy vs. Neutral | |||||
| 14, -34, 65 | R postcentral gyrus | 3 | 162 | 4.01 | |
| Risperidone Group > Divalproex Group | |||||
| Divalproex Group > Risperidone Group | none | none | none | none | none |
Note: Talairach coordinates and t values for clusters for which there is a significant three-way interaction (corrected p<0.05, with cluster contiguity threshold) in the whole-brain Analysis of Variance (ANOVA). ACC= anterior cingulate cortex; BA = Brodmann Area; HC=healthy control; L=left; R=right.
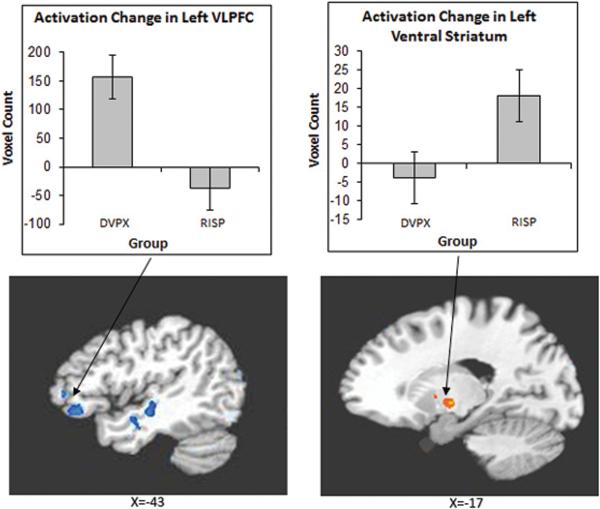
For the angry face condition, the risperidone group showed a greater increase in activation from baseline to follow-up than the divalproex group in left ventral striatum and decreased activation in left VLPFC and right middle temporal gyrus. Figure 2 illustrates differential activation between the two patient groups.
Figure 2.
Group by Time interaction in the left Ventrolateral Prefrontal Cortex (VLPFC) and left ventral striatum in the divalproex and risperidone groups for the angry face condition. Note: DVPX=Divalproex; RISP=Risperidone; VLPFC=ventrolateral prefrontal Cortex.
Relative to HC, the risperidone group exhibited increased activation from baseline to follow-up in left dorsal and subgenual ACC, bilateral ventral striatum, right posterior cingulate, and middle temporal gyrus, and no regions of decreased activation.
The divalproex group exhibited increased activation relative to HC from baseline to follow-up in right medial frontal gyrus, ventral striatum, putamen, and middle temporal gyrus, bilateral caudate, left VLPFC and subgenual ACC, and no decreased activation.
For the happy face condition, the risperidone group showed increased activation relative to the divalproex group from baseline to follow-up in the right postcentral gyrus and no regions of decreased activation. There were no significant differences between the risperidone group and HC. Relative to HC, with treatment, the divalproex group exhibited no increased or decreased activation. Between group comparisons at follow-up relative to baseline, collapsed over face emotion condition, can be found in Table S3, available online.
IV. Within Group Differences at Follow-up vs. Baseline for Angry and Happy Face Emotion Condition: Table 5
For the angry face condition, the risperidone group showed greater activation at follow-up, relative to baseline, in right midcingulate gyrus and bilateral ventral striatum, and no decreased activation. There were no significant differences due to treatment in this group for the happy face condition. For the angry condition, the divalproex group showed increased activation at follow-up, relative to baseline, in right medial frontal gyrus and middle temporal gyrus and no decreased activation. For the happy face condition, the divalproex group showed greater left VLPFC, and no decreased activation. For the angry face condition, HC exhibited no increased activation and decreased activation in right putamen and mid-cingulate gyrus, whereas for the happy face condition HC showed increased activation in right inferior parietal gyrus and no decreased activation
Neural Correlates of Changes in Clinical Measures scores from Baseline to Follow-up
For each condition, and each of our patient groups, exploratory Spearman's correlation analyses (two-tailed) were performed between changes in YMRS scores from baseline to follow-up, and changes in BOLD signal activation from baseline to follow-up in specific ROIs (i.e. amygdala, VLPFC, dorsal ACC, subgenual ACC) based on the significant clusters from the whole-brain ANOVA interaction. We also conducted these correlations for the two patient groups combined. For these exploratory multiple comparisons, we report results that were significant at an uncorrected p<.05. For the risperidone group, the improvement in manic symptoms was correlated with decreased activity in bilateral amygdala (left: r=.98, p=.001; right: r=.88, p=.02) and increased activity in VLPFC (left: r=.70, p=.04; right: r=.85, p=.004); whereas in the divalproex group, significant correlation was found with the decreased activity in the left amygdala (r=.64, p=.05). Across all patients, the change in YMRS scores was positively correlated with changes in the left amygdala (r=.74, p=.001), and right VLPFC (r=.61, p=.005) (Table S4, available online). No significant correlations were found between changes in CDRS-R scores and activity changes in any of the ROIs. Because of the exploratory nature and uncorrected p values of these analyses, these results are preliminary until replicated in future.
DISCUSSION
Our study compared the neural network engagement in PBD patients with an SGA vs. an anti-epileptic during a working memory task with emotional face stimuli. In line with our hypothesis, when risperidone and divalproex were directly compared over the course of treatment, there was significantly greater engagement during the angry face condition of the ventral striatum with risperidone and the VLPFC and middle temporal gyrus with divalproex. After treatment, the risperidone group also showed greater activation relative to the divalproex group in the subgenual ACC, along with the ventral striatum. Additionally, after treatment and consistent with the increase over the course of treatment, the divalproex group showed greater activation relative to the risperidone group in VLPFC and middle temporal gyrus. Furthermore, during the angry face condition, each patient group showed increased activation over time relative to HC in the VLPFC, ACC,11, 46, 47, 48 ventral striatum49, 50 and middle temporal lobe51, 52 all of which may be critical for mood stabilization in patients, irrespective of the type of medication. Across all comparisons, the happy face condition did not elicit notable findings to the same extent as the angry face condition, probably by the virtue of it being a less effective emotional probe to elicit BOLD signal responses. With regards to the behavioral results, along with reduced accuracy in the divalproex group relative to the other two groups across testing times, RT was also uniformly slower in the divalproex group in both conditions. This may be, in part, due to the psychomotor slowing known to be caused by divalproex.53
Differential Response between Risperidone and Divalproex
While both risperidone and divalproex led to reduction in manic symptoms, and the BOLD signal changes relative to HC were also similar in each patient group, on direct comparison there were mechanistic differences between these two medications. The SGA, risperidone, relative to divalproex, resulted in greater increase in bilateral ventral striatum activity while attending to angry stimuli during the working memory task. Healthy adult human studies have demonstrated a strong correlation between the plasma concentration of risperidone and D2 receptor occupancy in ventral striatum.35 Dynamic molecular imaging has also shown dopamine release in the striatum during negative emotion processing in human volunteers.54 A reduction in serotonin in the frontal cortex and striatum in rats impaired mood and working memory that, in turn, were shown to be reversed with risperidone36, underscoring our current findings in PBD. Furthermore, risperidone is known to be specifically effective in reducing aggression55 and the current study illustrated how angry faces served as a probe better than happy faces. A careful future evaluation of the change in participants’ aggressive symptoms while attending to angry emotional faces will elucidate the role of risperidone in specifically reducing participants’ aggression. While there was no significantly greater increase in subgenual ACC activation over time with risperidone relative to divalproex, there was greater activation in this region after the treatment. This is a replication of our previous finding where we were able to demonstrate increased subgenual activity with risperidone in pediatric mania while performing a color matching task, which is also a cognitive-affective interface task.56 The subgenual ACC has been shown to be underactive in untreated patients with bipolar disorder while performing an attentional task57 and appears to be a key region concerned with modulating autonomic responses and neurotransmission in animals.34 Risperidone may improve this region's ability to gain emotional control through serotonin-dopamine antagonism. Consistent with our findings, stimulation of the subgenual ACC ameliorated symptoms in adult depression33 and perfusion was normalized with a serotonin reuptake inhibitor in the same region.32, 58
After treatment, patients randomized to divalproex showed greater fronto-temporal activity than those who were randomized to risperidone. In the angry face condition, the divalproex group showed an increase in activity post-treatment relative to HC in the VLPFC, an area known for involvement in selection and stimulus comparison tasks,59 such as sorting and remembering faces in the current N-back task. Consistent with our current findings, treatment with another antiepileptic drug, i.e. lamotrigine, led to increased VLPFC activity during an affective color matching task probing the affective and cognitive interface.16 A similar increase in bilateral middle temporal lobe activity was noted with lamotrigine using a similar affective N-back task in PBD.60 The mesial or the middle temporal gyrus is known for the dual function of working memory and facial emotion processing.61, 62 In fact, divalproex was shown to normalize myo-inositol and phosphomonoester concentrations in fronto-temporal regions via phosphorinositol second messenger system (PI-cycle), given its role in bipolar patients treated with this medication.31 Furthermore, the medial part of the middle temporal gyrus is implicated in human lesion studies causing epilepsy, which can be treated by divalproex.51, 52
In the happy face condition, there were no notable group differences noted over the course of treatment, but some occurred post-treatment. The risperidone group showed greater activity in the occipital cortex which may reflect greater effort in visually scanning the faces63 to hold them in short- term memory, while the divalproex group showed greater activity in middle temporal gyrus known to support working memory and facial emotion processing.61, 62
Fronto-Striato-Temporal Circuitry Emerged in Response to Mood Stabilization to Serve the Dual Function of Working Memory and Affect Modulation in PBD
Previous findings from fMRI treatment studies of PBD with lamotrigine,16, 60 and risperidone and divalproex11 are in line with those of the current study showing enhanced activity in the higher cortical VLPFC and DLPFC, ventral striatum, and pre and subgenual ACC alongside clinical improvement in mania with both medications, in all patients, relative to HC. The cognitive studies of working memory in healthy adults21, 64, 65 and PBD post-treatment16 have demonstrated enhanced engagement of VLPFC and ACC similar to our current findings, underscoring the dual function of VLPFC's affective and cognitive control. Similarly, the temporal lobe51, 52 and the ventral striatum49, 50 known to be engaged in modulating affect, working memory, and attention appeared to be more engaged with treatment in the current study, illustrated with the angry face condition. In addition, treatment resulted in the enhanced engagement of affect control regions - i.e. the VLPFC and the subgenual ACC - similar to our previous studies in PBD.16 Also, the posterior cingulate cortex, known to be engaged during attentional control,16, 66, 67 was deployed in supporting the working memory processing. Although not apparent in the whole brain analysis, the ROI analysis revealed a decrease in amygdala activity and an increase in VLPFC activity related to the reduction in YMRS score in all patients. These findings indicate a relationship between mood stabilization with SGA and divalproex and improvements in these areas are consistent with those of previous studies68, 69 proposing top-down control of the VLPFC over the amygdala.
These findings must be interpreted in light of a few limitations. The sample size is small, but yields interpretable findings given the conserved power with fMRI techniques involving the acquisition of several hundreds of repeated measures of voxel-wise activity within the brain for each single subject. Unlike behavioral studies, fMRI studies require fewer subjects to have enough power to characterize group differences in brain function.70 Furthermore, the use of fMRI tasks containing many trials, for which the BOLD signal in each voxel can be averaged across, coupled with the use of stringent statistical procedures that apply voxel-wise probability corrections (e.g. AlphaSim cluster thresholding), considerably increases the study power to detect reliable group difference effects. Finally, the results reported in this study are in line with those from previous studies that employed similar tasks with findings that fall within the parameters of a priori hypothesis,14,16 which further confirms that the results were not driven by the sample size in this study. However, the ROI- based fronto-limbic changes must be interpreted with caution given the uncorrected p value until they can be replicated in a larger sample. The block design did not allow us to differentiate the pattern of brain activity for correct and incorrect trials, especially given that the patients who received divalproex showed lower accuracy throughout the study. However, given that the study compared the two medications and the divalproex group showed reduced accuracy both pre-and post- treatment, the incorrect trials are likely not responsible for drug effects, though future studies are needed to replicate these findings. Additionally, a block design offered greater statistical power and signal stability relative to an event-related design. Future studies will also need to further explore whether the beneficial effects of medications in normalizing cognitive circuitry activity is secondary to mood stabilization, or whether medications directly and differentially improve working memory function and will determine the relationship between brain activation and clinical response over the course of treatment. The strengths of the study include the use of a DBRCT design, the comparison of two classes of medications, the use of IQ-, age-, and gender-matched patient and HC groups, the utilization of a task addressing the critical interface of cognitive and emotional processes closer to real-life application, and the contribution of new mechanistic knowledge of how individual medications influence brain function.
Supplementary Material
Acknowledgments
This research was funded by National Institute of Health grants 1 K23 RR018638-01 and MO1-RR-13987, and Berger Colbeth Endowed Funds.
Dr. Pavuluri receives grant or research support from the National Institutes of Health (NIH), the National Institute of Mental Health, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the American Foundation for Suicide Prevention, and the Marshall Reynolds Foundation. She is the recipient of the Berger-Colbeth Term Chair in Child Psychiatry and serves on the speakers’ bureau of Bristol-Myers Squibb (Abilify). Dr. Passarotti receives funding from a NARSAD Young Investigator Award. Dr. Sweeney receives funding from NIH, GlaxoSmithKline, Johnson and Johnson, AstraZeneca, and Eli Lilly and Co.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article is discussed in an editorial by Dr. Daniel P. Dickstein on page xxx.
Supplemental material cited in this article is available online.
Disclosure: Dr. Wegbreit and Ms. Fitzgerald report no biomedical financial interests or potential conflicts of interest.
Clinical trial registration information -- Risperidone and Divalproex Sodium With MRI Assessment in Pediatric Bipolar; http://www.clinicaltrials.gov; NCT00176202.
Reference List
- 1.Findling RL, McNamara NK, Stansbrey R, et al. Combination lithium and divalproex sodium in pediatric bipolar symptom re-stabilization. J Am Acad Child Adolesc Psychiatry. 2006;45:142–148. doi: 10.1097/01.chi.0000189135.05060.8a. [DOI] [PubMed] [Google Scholar]
- 2.Pavuluri MN, Henry DB, Carbray JA, Naylor MW, Janicak PG. Divalproex sodium for pediatric mixed mania: a 6-month prospective trial. Bipolar Disord. 2005;7:266–273. doi: 10.1111/j.1399-5618.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 3.Wagner KD, Weller EB, Carlson GA, et al. An open-label trial of divalproex in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2002;41:1224–1230. doi: 10.1097/00004583-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Chang KD. The use of atypical antipsychotics in pediatric bipolar disorder. J Clin Psychiatry. 2008;69(Suppl 4):4–8. 4-8. [PubMed] [Google Scholar]
- 5.Biederman J, Mick E, Hammerness P, et al. Open-label, 8-week trial of olanzapine and risperidone for the treatment of bipolar disorder in preschool-age children. Biol Psychiatry. 2005;58:589–594. doi: 10.1016/j.biopsych.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Haas M, DelBello MP, Pandina G, et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2009;11:687–700. doi: 10.1111/j.1399-5618.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 7.Pavuluri MN, Henry DB, Carbray JA, Sampson G, Naylor MW, Janicak PG. Open-label prospective trial of risperidone in combination with lithium or divalproex sodium in pediatric mania. J Affect Disord. 2004;82:S103–S111. doi: 10.1016/j.jad.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Deltito JA, Levitan J, Damore J, Hajal F, Zambenedetti M. Naturalistic experience with the use of divalproex sodium on an in-patient unit for adolescent psychiatric patients. Acta Psychiatr Scand. 1998;97:236–240. doi: 10.1111/j.1600-0447.1998.tb09994.x. [DOI] [PubMed] [Google Scholar]
- 9.Papatheodorou G, Kutcher SP, Katic M, Szalai JP. The efficacy and safety of divalproex sodium in the treatment of acute mania in adolescents and young adults: an open clinical trial. J Clin Psychopharmacol. 1995;15:110–116. doi: 10.1097/00004714-199504000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Segal S. A double-blind, placebo-controlled trial to evaluate the safety and efficacy of depakote ER for the treatment of mania associated with bipolar disorder in children and adolescents [online] 2009 Available from URL: http://www.clinicalstudyresults.org/drugdetails/?.company_id=1&sort=c.company_name&page=1&drug_id=1561.
- 11.Pavuluri MN, O'connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 13.Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI Study of the Neural Correlates of Incidental versus Directed Emotion Processing in Pediatric Bipolar Disorder. J Am Acad Child Adolesc Psychiatry. 2009 doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang K, Karchemskiy A, Kelley R, et al. Effect of divalproex on brain morphometry, chemistry, and function in youth at high-risk for bipolar disorder: a pilot study. J Child Adolesc Psychopharmacol. 2009;19:51–59. doi: 10.1089/cap.2008.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavuluri MN, Passarotti AM, Parnes SA, Fitzgerald JM, Sweeney JA. A pharmacological functional magnetic resonance imaging study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20:395–406. doi: 10.1089/cap.2009.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry. 2010;71:1526–1534. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavuluri MN, West A, Hill SK, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youths. J Am Acad Child Adolesc Psychiatry. 2009;48:299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavuluri MN, Passarotti AM, Mohammed T, Carbray JA, Sweeney JA. Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disord. 2010;12:213–220. doi: 10.1111/j.1399-5618.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen AM, Stern CE, Look RB, Tracey J, Rosen BR. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci USA. 1998;95:7721–7726. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 25.Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 27.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci. 2004;1021:376–83. doi: 10.1196/annals.1308.048. 376-383. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg HP, Krystal JH, Bansal R, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Silverstone PH, Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with both lithium and sodium valproate may normalize phosphoinositol cycle activity in bipolar patients. Hum Psychopharmacol. 2002;17:321–327. doi: 10.1002/hup.420. [DOI] [PubMed] [Google Scholar]
- 32.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 33.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodaka F, Ito H, Takano H, et al. Effect of risperidone on high-affinity state of dopamine D2 receptors: a PET study with agonist ligand [11C](R)-2-CH3O-N-n-propylnorapomorphine. Int J Neuropsychopharmacol. 2010:1–7. doi: 10.1017/S1461145710001148. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins TA, Elliott JJ, Ardis TC, et al. Tryptophan depletion impairs object-recognition memory in the rat: reversal by risperidone. Behav Brain Res. 2010;208:479–483. doi: 10.1016/j.bbr.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 40.Poznanski EO, Mokros HB. Children's Depression Rating Scale, Revised (CDRS-R) Western Psychological Services; Los Angeles, CA: 1995. [Google Scholar]
- 41.Gur RE, McGrath C, Chan RM, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 42.Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Physica-Verlag; Heidelberg: 1996. pp. 39–49. [Google Scholar]
- 43.Rosenthal R. Meta-analytic procedures for social research. Sage; Newbury Park, CA: 1991. [Google Scholar]
- 44.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 45.Ward BD. Simultaneous inference for fMRI data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 2000. [Google Scholar]
- 46.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 47.Konishi S, Kawazu M, Uchida I, Kikyo H, Asakura I, Miyashita Y. Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex. 1999;9:745–753. doi: 10.1093/cercor/9.7.745. [DOI] [PubMed] [Google Scholar]
- 48.Rubia K, Russell T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 49.Rosen HR, Rich BA. Neurocognitive correlates of emotional stimulus processing in pediatric bipolar disorder: a review. Postgrad Med. 2010;122:94–104. doi: 10.3810/pgm.2010.07.2177. [DOI] [PubMed] [Google Scholar]
- 50.Provost JS, Petrides M, Monchi O. Dissociating the role of the caudate nucleus and dorsolateral prefrontal cortex in the monitoring of events within human working memory. Eur J Neurosci. 2010;32:873–880. doi: 10.1111/j.1460-9568.2010.07333.x. [DOI] [PubMed] [Google Scholar]
- 51.Barcia JA, Gallego JM. Intraventricular and intracerebral delivery of anti-epileptic drugs in the kindling model. Neurotherapeutics. 2009;6:337–343. doi: 10.1016/j.nurt.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartzkroin PA, Haglund MM. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia. 1986;27:523–533. doi: 10.1111/j.1528-1157.1986.tb03578.x. [DOI] [PubMed] [Google Scholar]
- 53.Pavuluri MN. Effects of early intervention on the course of bipolar disorder: theories and realities. Curr Psychiatry Rep. 2010;12:490–498. doi: 10.1007/s11920-010-0155-1. [DOI] [PubMed] [Google Scholar]
- 54.Badgaiyan RD. Dopamine is released in the striatum during human emotional processing. Neuroreport. 2010;21:1172–1176. doi: 10.1097/WNR.0b013e3283410955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klampfl K, Taurines R, Preuss A, et al. Serum concentrations, therapeutic response and side effects in children and adolescents with impulsive-aggressive symptoms during risperidone therapy. Pharmacopsychiatry. 2010;43:58–65. doi: 10.1055/s-0029-1239540. [DOI] [PubMed] [Google Scholar]
- 56.Pavuluri MN, Passarotti AM, Lu LH, Carbray JA, Sweeney JA. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder: fMRI outcomes. Psychiatry Res. 2011;193:28–37. doi: 10.1016/j.pscychresns.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 58.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 59.Petrides M. Frontal lobes and behaviour. Curr Opin Neurobiol. 1994;4:207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 60.Passarotti AM, Sweeney JA, Pavuluri MN. Lamotrigine treatment improves prefrontal activity in pediatric bipolar disorder.. Poster session presented at the annual ANCP Conference; Miami, FL. 2010. [Google Scholar]
- 61.Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev. 2010;117:210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48:2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Soto D, Greene CM, Chaudhary A, Rotshtein P. Competition in Working Memory Reduces Frontal Guidance of Visual Selection [Epub ahead of print]. Cereb Cortex. 2011 Jul 20; doi: 10.1093/cercor/bhr190. [DOI] [PubMed] [Google Scholar]
- 64.Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- 65.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 66.Bledowski C, Rahm B, Rowe JB. What “works” in working memory? Separate systems for selection and updating of critical information. J Neurosci. 2009;29:13735–13741. doi: 10.1523/JNEUROSCI.2547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.