Abstract
Before the use of hypothermia as a treatment for comatose post-cardiac arrest patients, several prognostic variables were widely accepted as reliable and valid for the prediction of poor outcome. Recent studies using hypothermia have reported on patients with recovery of consciousness in spite of absent or extensor motor responses after 3 days, absent bilateral cortical N20 responses after 24 hours, serum neuron-specific enolase levels greater than 33 μg/L, and early myoclonus status epilepticus. Hypothermia and its associated use of sedative and paralytic agents may delay neurologic recovery and affect the optimal timing of prognostic variables. Recent developments in brain imaging may provide additional objective prognostic information and deserve further study. Pending the results of future validation studies in patients treated with hypothermia, we recommend that irreversible management decisions not be made based on a single prognostic parameter, and, if there is uncertainty, these decisions be delayed for several days to allow for repeated testing.
Keywords: Cardiac arrest; Coma; Prognostication; Hypothermia; Neurological examination; Myoclonus status epilepticus; Electroencephalography; Neuron-specific enolase, S100B protein; Somatosensory evoked potentials; Brain magnetic resonance imaging
Introduction
Cardiovascular disease is a leading cause of death, accounting for approximately one third of all deaths annually in the United States [1]. About 295,000 people in the United States have an out-of-hospital cardiac arrest (OHCA) each year and only 10% to 20% of those affected survive [1]. Approximately 80% of patients with successful restoration of spontaneous circulation after cardiopulmonary resuscitation (CPR) remain in a comatose state [2], and approximately one third of these regain consciousness [3]. However, neurological sequelae are common in these survivors and vary from minor to severe cognitive and motor impairments [4].
Accurate prediction of neurological outcome in comatose cardiac arrest patients is paramount not only to assist families with decision making regarding withdrawal of life support versus continued supportive care, but also to avoid the prolonged use of limited health care resources when there is no chance of meaningful recovery. It is estimated that the costs of CPR are $406,605 per life saved. Because only a minority of patients survives to an independent lifestyle, many patients are taken off life support early even if they do not meet specific criteria for poor outcome. Thus, early accurate prediction of neurological outcome in these patients may not only save resources, but also lives, and is therefore a very important health care issue.
Before the use of therapeutic hypothermia [5, 6], the prognostication of comatose patients after cardiac arrest predominantly relied on the bedside neurological examination in conjunction with electrophysiological testing. The 2006 American Academy of Neurology (AAN) practice parameter for prediction of outcome in comatose survivors after CPR provides useful prognostication guidelines [7]; however, these guidelines were based on studies performed before the use of hypothermia and the authors rightfully urge caution in the interpretation of various prognosticators in patients who have been treated with hypothermia.
Survival from OHCA depends on a strong chain of survival [8], involving rapid emergency medical services assistance, effective CPR, expedited transportation to a medical center, and effective post-resuscitation care. As therapeutic hypothermia has been proven to be effective in improving both survival and neurological outcome [5, 6], it should be considered standard treatment for comatose post-cardiac arrest survivors [9, 10]. There are large interhospital variations in survival after successful resuscitation from OHCA [11]. A recent policy statement from the American Heart Association outlines the rationale for regional systems of care for OHCA [12]. In the near future, post-cardiac arrest survivors will be directed to “cardiac arrest centers” that offer therapeutic hypothermia. In this review, the current knowledge of prognostic variables for patients in coma after cardiac arrest will be discussed.
Neurological Examination
For decades the bedside neurologic examination has been advocated as the “the gold standard” for outcome prediction of comatose post-cardiac arrest survivors [13]. The authors of the 2006 AAN practice parameter concluded that patients with absent pupillary or corneal reflexes, or absent or extensor motor responses after 3 days after the cardiac arrest, or the presence of myoclonus status epilepticus (SE) within the first day are highly likely to have a poor outcome [7]. Poor outcome was defined as death or persisting unconsciousness after 1 month or unconsciousness or severe disability requiring constant nursing care after 6 months [7]. Although the specificity for poor outcome of these variables was found to be high, they have limited sensitivity: many patients who do not meet any of these criteria fare poorly as well.
Brainstem Reflexes
Absence of one or more brainstem reflexes in the first few hours after return of spontaneous circulation cannot be regarded as being specific for poor outcome, because some patients who lack these reflexes early on may in fact regain consciousness in the ensuing days. Conversely, patients who do not regain their brainstem reflexes in the absence of sedating medications after 72 hours are unlikely to regain consciousness; however, the sensitivity for poor outcome of absent brainstem reflexes at this time point is low. Levy et al. [13] reported a 12% sensitivity for prediction of poor outcome (defined as persistent vegetative state or death) at the 72 hours’ time point for absent pupillary responses and 16% for absence of corneal reflexes. None of the patients with absent pupillary reflexes or corneal reflexes after 3 days recovered to good functional outcome or moderate disability at 1 year. Although Levy et al.'s [13] landmark paper published in 1985 greatly enhanced our ability to predict outcome after hypoxic-ischemic brain injury caused by cardiac arrest, its results should be applied with caution in the present time because critical care of comatose patients has evolved considerably since then. Additionally, only 71% of Levy et al.'s [13] cohort had cardiac arrest as the cause of their hypoxic-ischemic brain injury and none of the patients received treatment with hypothermia.
The Brain Resuscitation Clinical Trials I Study Group found a sensitivity of 19% and a false-positive rate (1-specificity) of 0% (95% CI, 0–25) for absent pupillary responses to light at 72 hours in a study of 262 patients [14]. A more recent study conducted by Zandbergen et al. [15] including 407 patients found a sensitivity for prediction of poor outcome of absent pupillary reflexes of 22% and absent corneal responses at 72 hours of 28%, with a false-positive rate of 0%.
In a small retrospective study with 37 comatose post-cardiac arrest patients treated with hypothermia, none of six patients without pupillary reflexes and none without corneal reflexes at 72 hours after the arrest recovered awareness [16]; however, a recent prospective study of 111 comatose patients after cardiac arrest treated with induced hypothermia found a false-positive rate of the absence of one or more brainstem reflexes between 36 and 72 hours of 4% (95% CI, 1–15) for poor outcome, suggesting that hypothermia may affect the validity of the brainstem examination [17••]. One caveat of this study is that patients were examined “after passive rewarming and off sedation.” Because details on the use of sedative medications (type, dose, and duration of use prior to the examination) are not reported, the potential of a lingering effect of sedative agents on the neurological examination cannot be excluded. A recent prospective study including 53 comatose post-cardiac arrest patients treated with hypothermia showed that sedative agents were used within 12 hours or less prior to the 72-hour examination in 83% of patients and that absent corneal reflexes were not 100% specific for poor outcome at this time point [18].
In summary, the absence of one or more brainstem reflexes at 72 hours used to be an accurate indicator of poor outcome in comatose post-cardiac arrest survivors; however, the validity of this finding is in question in patients treated with hypothermia and requires further study.
Motor Response
Reports on the prognostic accuracy of the best motor response to noxious stimuli in post-cardiac arrest patients have shown less consistent results. In general, the prognostic accuracy of an impaired motor response is greater when it is assessed after 72 hours than after 24 or 48 hours after the arrest [7, 14, 15]. In Levy et al.'s [13] study, none of the 69 patients who had flexor posturing, extensor posturing, or no motor response (M-score ≤ 3) to noxious stimuli 72 hours after the arrest recovered to a good neurological outcome.
A recent meta-analysis including 25 (prospective and retrospective) studies revealed that at day 2 and 3 after the arrest the motor score (M-score ≤ 3) predicts poor outcome with similar accuracy as the somatosensory evoked potentials (SSEPs) [19]; however, as the authors point out, these findings cannot necessarily be applied to patients treated with hypothermia.
A recent study of 37 patients treated with therapeutic hypothermia describes that 2 of 14 patients with no motor response at day 3 regained awareness [16]. This suggests that early abnormal or absent motor responses do not always predict poor outcome after hypothermia. A prospective study of 72 post-cardiac arrest patients treated with hypothermia assessed the predictive value of the motor response the first day after discontinuation of sedation [20]. The investigators found that an M-score greater than 3 on the first day after sedation was stopped predicted favorable outcome (cerebral performance category [CPC] 1 and 2), with 100% specificity and 43% sensitivity [20]; however, the results of their study also demonstrate that an M-score of 1 up to 4 days after discontinuation of sedation did not invariably predict poor outcome in each patient. A Glasgow coma scale score of 4 at day 4 after sedation was discontinued predicted poor outcome, with a specificity of 95% and a sensitivity of 47%.
Another prospective study of 111 comatose cardiac arrest survivors treated with hypothermia found a false-positive mortality prediction of 24% for an M-score ≤ 2 assessed between 36 and 72 hours in predicting poor outcome [17••]. The authors conclude that in patients treated with hypothermia, the best motor response to painful stimuli is a less reliable outcome predictor than in studies performed before the era of hypothermia summarized in the 2006 AAN practice parameter [17••]. Whether the use of sedation is a confounder in these studies remains uncertain.
In summary, the absence of a motor response to pain even up to 4 days after the arrest cannot any longer be used as a single predictor of poor outcome in patients who have been treated with hypothermia. Further studies are required to validate the prognostic accuracy of this clinical examination finding in this context.
Myoclonus Status Epilepticus
Myoclonus SE has been defined as spontaneous, repetitive, unrelenting, generalized multifocal myoclonus affecting the face, trunk, and extremities in comatose patients [7]. Myoclonus SE typically presents in the first day after the arrest. Many patients have associated evidence of SE by electroencephalography (EEG), but this is not a requirement for the diagnosis. Prior to the introduction of hypothermia, myoclonus SE was invariably associated with a poor prognosis in patients with coma caused by a primary cardiac arrest [21]. In rare instances good outcomes have been described in patients with myoclonus SE after a cardiac arrest secondary to respiratory failure.
The prevalence of myoclonus SE following cardiac arrest varies between studies. In the prospective cohort study by Zandbergen et al. [15] with 407 patients, status myoclonus was observed in 4% of patients at 24 hours after the arrest and all of them had a poor outcome. However, it should be noted that only 2% of patients included in this study received hypothermia.
A retrospective study with 107 patients (of whom 59% were treated with hypothermia) investigated the relationship between post-anoxic SE (including myoclonic SE and nonconvulsive SE) and neurological outcome [22]. Post-anoxic SE was defined by EEG characteristics only. Patients underwent EEG recording at least once in the first few days after the arrest. EEG evidence of post-anoxic SE was present in one third of patients and an independent and strong predictor for poor outcome [22]; however, three patients with post-anoxic SE, all treated with hypothermia, regained consciousness and one was living independently at 1 year.
A prospective study of the same group with 111 cardiac arrest patients treated with hypothermia described myoclonus in 33% of patients [17••]. Two of these survived to 3 to 6 months. In a separate report more details are described on three patients treated with hypothermia who regained consciousness despite early myoclonus SE by clinical criteria with an EEG correlate [23•]. These patients had preserved brainstem reflexes, normal cortical N20 responses by SSEPs, and reactive EEGs. All three patients received treatment for the SE and improved to a CPC of 1, 2, and 3 at 6 months [23•]. Conversely, a small retrospective study with 37 patients treated with hypothermia after cardiac arrest described that none of eight patients with myoclonus SE recovered awareness (false-positive rate of 0%; 95% CI, 0–40) [16].
In summary, the previous concept that myoclonus SE early after a circulatory arrest invariably predicts poor outcome does not appear to hold true in patients treated with hypothermia. Intact brainstem reflexes, preserved cortical responses by SSEP, and a reactive EEG may identify a subset of these patients who have a chance of regaining consciousness. Furthermore, early treatment of SE may favorably affect outcome and needs further study. Both hypothermia and the accompanying use of sedating and paralyzing drugs can masquerade clinical evidence of epileptiform activity. Therefore, pending larger studies, routine EEG monitoring during hypothermia and pharmacologic treatment of epileptiform activity should be considered.
Electroencephalography
Electroencephalography has been studied extensively in comatose post-cardiac arrest patients prior to the use of hypothermia [24]. In general, early recovery of cortical activity and EEG reactivity correlate with good outcome, whereas clinical evidence of seizures and EEG evidence of generalized epileptiform activity correlate with poor outcome [25]. Prior to the introduction of hypothermia, several EEG patterns have been strongly associated with poor outcome, most notably generalized suppression (< 20 μV), burst-suppression pattern with generalized epileptiform activity, and generalized periodic complexes on a flat background; however, none of these EEG patterns predicts poor outcome with certainty [7].
Scollo-Lavizzari and Bassetti [26] conducted a meta-analysis of 408 comatose cardiac arrest patients who had EEG recordings 6 hours or more after resuscitation. The authors found meaningful neurological recovery in 79% of patients with dominant normal alpha activity and 0% in patients with alpha coma, low-voltage delta activity, periodic-generalized phenomena with low-voltage background activity, and a very flat or isoelectric EEG (< 20 μV). Forty-three percent of patients with predominant theta-delta activity had a good outcome.
Continuous or serial EEG monitoring is likely to provide more accurate prognostic information than a single EEG recording. In a series of 231 patients who underwent sequential EEG monitoring starting immediately after resuscitation, 74% of patients with and 30% of patients without electrocortical activity on the initial EEG regained consciousness [27]. Incomplete EEG recovery on sequential EEG recordings was associated with poor outcome.
Electroencephalographic monitoring is cheap and easy to perform at the bedside, but an important disadvantage is its susceptibility to sedative drugs, metabolic derangements, and hypothermia. Rundgren et al. [28] assessed the prognostic value of amplitude-integrated EEG (aEEG) patterns in 95 post-cardiac arrest patients treated with hypothermia. Thirty-one patients had continuous aEEG patterns at the beginning of the recording and 62 after return to normothermia. The presence of a continuous aEEG pattern strongly correlated with neurologic recovery: 29 of 31 (90%) patients with a continuous aEEG pattern at the beginning of the recording and 54 of 62 (87%) patients with a continuous aEEG pattern after return to normothermia regained consciousness, supporting the view that aEEG may have prognostic value in the setting of hypothermia.
In summary, EEG is noninvasive and easy to use even in unstable patients, but its widespread application as a prognostic test for comatose post-cardiac arrest patients has been hampered by the lack of a universally accepted classification system and its susceptibility to drugs and metabolic disorders. Recent advances in quantitative EEG analysis and continuous bedside recordings may overcome some of these limitations [29, 30]. Preliminary studies in post-cardiac arrest patients treated with hypothermia suggest a potential use of continuous EEG recording as a prognostic test in this setting, but further validation studies are needed.
Somatosensory Evoked Potentials
SSEPs are elicited by electric stimulation of the median nerve and test the integrity of the afferent sensory pathways including activation of the primary cortical somatosensory area (N20 peak). Based on a meta-analysis of eight studies, the 2006 AAN practice parameter reports an excellent predictive value for poor outcome of bilateral absence of N20 responses with an overall false-positive rate of 0.7% (95% CI, 0.1–3.7) and a pooled sensitivity of 46% [7]. In the largest of the eight studies bilateral absence of cortical N20 responses consistently predicted poor outcome in the 383 patients who were tested at least once between 24 and 72 hours after the arrest with a false-positive rate of 0% (95% CI, 0–17) [15]. Treating physicians were blinded to the 24- and 48-hour SSEP results, but not to the 72-hour ones, and the percentage of patients with a poor outcome in the study was high at 87%, factors that may have overestimated the prognostic accuracy of bilateral absent N20 peaks for poor outcome.
Data on the prognostic accuracy of SSEPs in post-cardiac arrest patients treated with hypothermia are limited. A pilot study reported accurate poor outcome prediction by bilateral absence of recordable N20 peaks during hypothermia with 26% sensitivity [31]. All 13 patients with absent SSEPs during hypothermia had a poor outcome. Another study described poor outcome in all 33 patients with absent SSEPs after hypothermia, but treating physicians were not blinded to the SSEP results [17••]. In contrast, a recent study reported recovery of consciousness in two post-cardiac arrest patients treated with hypothermia with absent or minimally detectable cortical N20 responses on day 3 after the arrest. Both patients regained normal cognitive function [32•].
In summary, SSEPs are very useful as a prognostic test in comatose post-cardiac arrest patients because of the high specificity for poor outcome, ease of use, low cost, and the minimal influence of drugs and metabolic derangements on the test result. A limitation is the relative low sensitivity for poor outcome. Because the large majority of studies supporting the robust prognostic accuracy of SSEPs were conducted before the use of hypothermia, further investigation of their prognostic accuracy is pivotal in prospective studies with patients treated with hypothermia and with investigators blinded to the test result (avoiding a self-fulfilling prophecy).
Biochemical Markers of Cerebral Injury
Biochemical markers of central nervous system injury are generally easily obtainable and may be an objective measure of brain damage, unaffected by sedatives or clinician interpretation [33–36]. Neuron-specific enolase (NSE) is an isomer of enolase, a cytoplasmic glycolytic neuronal enzyme present in neurons and neuroendocrine cells. Astroglial S100 protein is a glial cellular calcium-binding protein. Increased levels of serum NSE and S100 protein have been detected in patients with different causes of central nervous system injury [35]. Both markers also have been investigated in several pilot studies of comatose post-cardiac arrest patients yielding substantial differences in false-positive rates and sensitivities for poor outcome [15, 37].
In the prospective study by Zandbergen et al. [15], serum NSE levels were determined in 231 comatose post-cardiac arrest patients at 24, 48, and 72 hours after the arrest [7]. An NSE level greater than 33 μg/L at any of the three time points was detected in 60% of patients and predicted poor outcome with a false-positive rate of 0% (95% CI, 0–3). The false-positive rate of serum S100 levels was 5% at a cut-off level of 0.7 μg/L [7, 15]. Based on the results of this study, the 2006 AAN practice parameter for prediction of outcome in comatose survivors after CPR states that “Serum NSE levels > 33 μg/L at days 1 to 3 post CPR accurately predict poor outcome (recommendation level B).” However, the large majority of patients in Zandbergen et al.'s [15] study were not treated with hypothermia.
Although it is evident that serum levels of NSE and S100 protein are often increased after cardiac arrest, the optimal timing of sampling and the best cut-off levels for prognostication of poor outcome remain topics for debate. Comatose post-cardiac arrest patients have been reported to have regained consciousness in spite of serum NSE levels of 85 μg/L within the first 3 days [18, 38, 39], and cut-off values as high as 80 μg/L have been proposed to accurately predict poor outcome, with 100% specificity and 63% sensitivity [39]. It also remains uncertain whether serum NSE or S100 protein is more accurate for prognostication. In the study by Zandbergen et al. [15], NSE performed better than S100 protein in the first 3 days. In contrast, a multicenter prospective study performed in Japan with 80 comatose post-cardiac arrest patients (56% treated with hypothermia) found an S100 protein cut-off value of 0.05 μg/L at 24 hours to be 100% specific and 100% sensitive for poor outcome, whereas NSE cut-off levels of 39.8 μg/L at 24 hours achieved a specificity of 100% and a sensitivity of 72% [40]. The authors concluded that S100 protein is superior to NSE for early prediction of neurological outcome following cardiac arrest.
Few studies have evaluated serum NSE and S100 protein levels after cardiac arrest with induced hypothermia. An elegant study of 68 patients randomized to hypothermia and normothermia demonstrated that the cut-off values for NSE and S100 serum levels between 24 and 48 hours predictive of poor outcome (with > 95% specificity) were higher in the hypothermia group (31.2 μg/L and 0.49 μg/L) than in the normothermia group (13.3 μg/L and 0.23 μg/L) [41]. In an unblinded study with slightly over a 100 patients treated with hypothermia and sampled at 2, 24, 48, and 72 hours after the arrest, an NSE value of greater than 28 μg/L at 48 hours (100% specificity and 67% sensitivity) was a superior predictor for poor outcome than an S100 protein level greater than 0.51 μg/L at 24 hours (96% specificity and 62% sensitivity) [42]. Another unblinded study of 90 post-cardiac arrest patients treated with hypothermia demonstrated that an NSE level greater than greater than 33 μg/L at 48 hours predicted poor outcome with 100% specificity and 43% sensitivity [43].
In summary, the main serum markers of cerebral injury that have been investigated in comatose post-cardiac arrest patients for prognostication purposes are serum NSE and S100 protein. Because NSE is also present in platelets and red blood cells, hemolysis increases serum values and may result in false-positive test results. Thus, NSE levels derived from hemolyzed blood samples should not be used for prognostication purposes. Many studies used retrospective study designs, included small patient numbers, did not use rigorous blinding, and were performed before the use of hypothermia. Variable cut-off points for poor outcome have been identified across studies, which may be due to differences in laboratory assays and processing, different time points of data acquisition, and variations in definitions of poor outcome. Recent data suggest that serum cut-off levels predictive of outcome differ between patients treated with hypothermia and those not treated with hypothermia. There are currently insufficient data on the predictive accuracy of NSE and S100 protein in post-cardiac arrest patients treated with hypothermia to advise their routine use in clinical practice; however, the results of these biomarker values may, in conjunction with other test results, indicate an increased likelihood of the presence of irreversible brain injury and help guide clinical decision making. Further study of their clinical utility in properly designed studies is needed.
Neuroimaging
Cranial CT and brain MRI have become essential tools in the diagnosis, management, and prognostication of patients with acute brain injuries and have also been studied as an adjunct in post-cardiac arrest prognostication. An unenhanced brain CT scan may demonstrate loss of distinction between gray and white matter, the severity of which (quantified by the ratio in Hounsfield units of gray matter to white matter) was found to be predictive of death in a study of 25 comatose post-cardiac arrest patients imaged within 48 hours after the event [44]. Inamasu et al. [45] studied the CT scans of 75 comatose post-cardiac arrest patients immediately after resuscitation and found that both loss of gray and white matter distinction and the presence of sulcal effacement were correlated with poor outcome. Both studies were retrospective, unblinded, and performed in patients who were not treated with hypothermia.
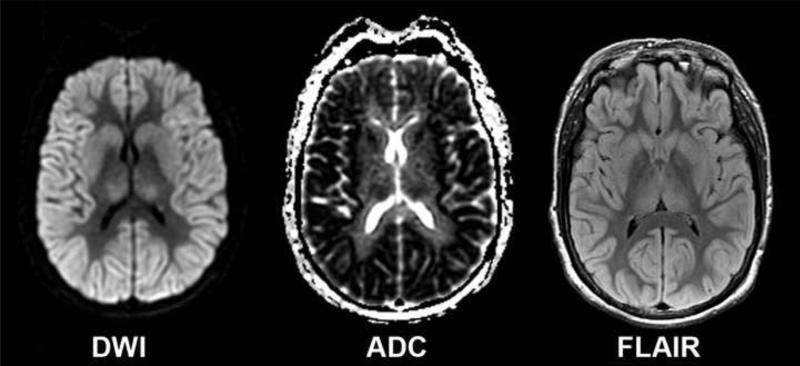
Brain MRI studies in comatose post-cardiac arrest patients suggest that widespread cortical abnormalities on diffusion-weighted imaging (DWI) or fluid-attenuated inversion recovery (FLAIR) are associated with poor outcome [46, 47]. A retrospective study of 80 patients (14 patients treated with hypothermia) showed that whole-brain apparent diffusion coefficients (ADCs) were lower in poor outcome than in good outcome patients [48]. In a prospective quantitative brain MRI study of comatose post-cardiac arrest survivors, 86% of patients were able to undergo brain MRI [49•]. The study included 51 patients (61% treated with hypothermia) with 62 MRIs and showed that patients with more than 10% of brain volume below 650 × 106 mm2/sec on the ADC map did not regain consciousness. The same group showed that brain DWI MRI changes in comatose post-cardiac arrest patients are region- and time-dependent. DWI changes are most apparent between 2 and 5 days after the arrest, and cortical structures, in particular the occipital and temporal lobes, and the putamen exhibit the most profound ADC reductions in poor outcome patients [49•, 50]. Figure 1 shows an example of a post-cardiac arrest patient with severe brain MRI changes who died 5 days after the arrest. Berek et al. [51] evaluated the use of magnetic resonance spectroscopy (MRS) as a prognostic indicator in 30 post-cardiac arrest patients. The presence of a lactate peak on MRS correlated with death or severe disability at 1 month [51].
Figure 1.
Brain MRI showing the diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC), and fluid-attenuated inversion recovery (FLAIR) sequences of a 50 year-old woman who had been resuscitated for 18 minutes for a ventricular fibrillation cardiac arrest. At 72 hours after the event she had no motor response to pain, intact brainstem reflexes, a peak NSE value greater than 130 μg/L, and bilaterally absent N20 responses by somatosensory evoked potential. The MRI was obtained 81 hours after the arrest and shows widespread areas of reduced diffusion and increased T2 signal involving the entire cortex, the basal ganglia, and the thalamus. Five days after the arrest she died after supportive care was withdrawn.
In summary, brain CT and MRI hold promise as useful prognostic adjuncts in patients with hypoxic-ischemia brain injury caused by cardiac arrest. Imaging studies have the advantage to be relatively objective and unaffected by sedative drugs and metabolic derangements. From a practical point of view, it is easier to obtain a brain CT in these patients with potential hemodynamic instability and the presence of metallic hardware or pacemakers. Conversely, MRI provides more detailed information and may have greater prognostic accuracy. Both CT and MRI require further validation studies in large prospective patient cohorts treated with hypothermia.
Conclusions
Before the routine use of hypothermia as a treatment for patients in coma after cardiac arrest, several prognostic variables were widely accepted as reliable and valid for the prediction of poor neurological outcome. Recent studies in patients who have been treated with hypothermia call the validity of some of these variables into question, and have described patients who regained consciousness, sometimes with good neurological outcome, in spite of absent or extensor motor responses after 3 days, absent bilateral cortical N20 responses by SSEP after 24 hours, serum NSE levels greater than 33 μg/L within 3 days, and early myoclonus SE. Treatment with hypothermia and its associated use of sedative and paralytic agents may alter the natural history of neurologic recovery and the optimal timing for assessment of these parameters for prognostication. Therefore, there is an urgent need for additional large prospective validation trials for each of these prognostic variables. Recent developments in brain imaging provide new, objective and potentially more sensitive markers of early global ischemic brain injury and deserve further study.
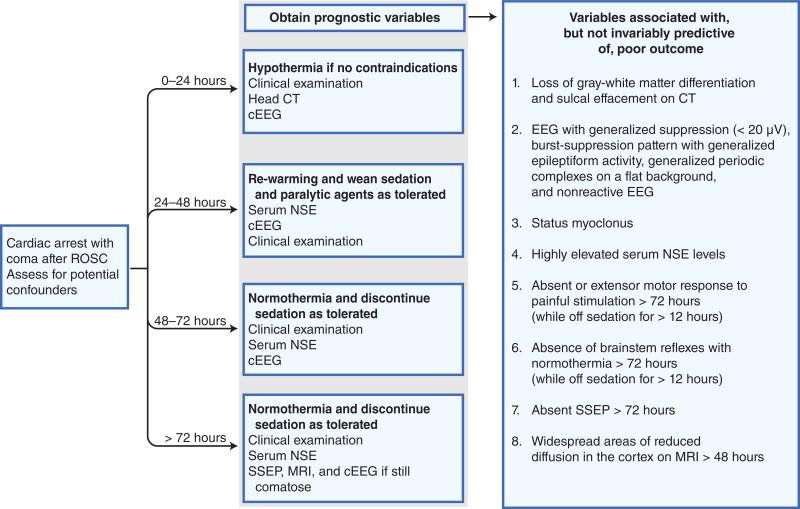
Pending the results of future validation studies in patients treated with hypothermia, caution should be exhibited in making irreversible management decisions based on the results of a single prognostic parameter. If there is uncertainty, it is reasonable to delay such decisions for several days allowing for repeated testing. A proposed algorithm for prognostication of outcome for comatose post-cardiac arrest patients treated with hypothermia is shown in Figure 2. Eventually, a quantitative prognostic outcome model incorporating multiple variables will likely prove to be most powerful in accurate prognostication and in guiding decision making in regard to continuation or withdrawal of life support in these patients.
Figure 2.
Algorithm for prognostication of outcome for comatose post-cardiac arrest patients treated with hypothermia. cEEG—continuous electroencephalography; EEG—electroencephalography; NSE—neuron-specific enolase; ROSC—return of spontaneous circulation; SSEP—somatosensory evoked potentials.
Acknowledgment
S. Persoon has received the following grants: The Netherlands Heart Foundation (2003B263) and the Foundation “De Drie Lichten” (41/09). C.A.C. Wijman has received the following grants: NIH grants (R01HL089116 and 2R01NS034866) and AHA National Scientist Development award (0430275N).
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Edgar A. Samaniego, Stanford Neurocritical Care Program, Stanford Stroke Center, Department of Neurology and Neurological Sciences, Stanford University, USA
Suzanne Persoon, Stanford Neurocritical Care Program, Stanford Stroke Center, Department of Neurology and Neurological Sciences, Stanford University, USA.
Christine A.C. Wijman, Department of Neurology, University Medical Center Utrecht, The Netherlands
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879. doi: 10.1001/jama.291.7.870. [DOI] [PubMed] [Google Scholar]
- 3.Zandbergen EG, de Haan RJ, Reitsma JB, Hijdra A. Survival and recovery of consciousness in anoxic-ischemic coma after cardiopulmonary resuscitation. Intensive Care Med. 2003;29:1911–1915. doi: 10.1007/s00134-003-1951-4. [DOI] [PubMed] [Google Scholar]
- 4.Lim C, Alexander MP, LaFleche G, et al. The neurological and cognitive sequelae of cardiac arrest. Neurology. 2004;63:1774–1778. doi: 10.1212/01.wnl.0000144189.83077.8e. [DOI] [PubMed] [Google Scholar]
- 5.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 6.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 7.Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 8.Nolan J, Soar J, Eikeland H. The chain of survival. Resuscitation. 2006;71:270–271. doi: 10.1016/j.resuscitation.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 10.Nolan JP, Soar J. Postresuscitation care: entering a new era. Curr Opin Crit Care. 2010;16:216–222. doi: 10.1097/MCC.0b013e3283383dca. [DOI] [PubMed] [Google Scholar]
- 11.Carr BG, Kahn JM, Merchant RM, et al. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80:30–34. doi: 10.1016/j.resuscitation.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation. 121:709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 13.Levy DE, Caronna JJ, Singer BH, et al. Predicting outcome from hypoxic-ischemic coma. JAMA. 1985;253:1420–1426. [PubMed] [Google Scholar]
- 14.Edgren E, Hedstrand U, Kelsey S, et al. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet. 1994;343:1055–1059. doi: 10.1016/s0140-6736(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 15.Zandbergen EG, Hijdra A, Koelman JH, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66:62–68. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 16.Al Thenayan E, Savard M, Sharpe M, et al. Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest. Neurology. 2008;71:1535–1537. doi: 10.1212/01.wnl.0000334205.81148.31. [DOI] [PubMed] [Google Scholar]
- 17••.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–307. doi: 10.1002/ana.21984. [In this study the prognostic value of several variables was studied in 111 post-comatose survivors of cardiac arrest treated with hypothermia. Status myoclonus, absent motor response to pain, and incomplete brainstem reflexes falsely predicted poor outcome in a small subset of patients. Based on their observations, the authors conclude that hypothermia may alter outcome prediction after cardiac arrest.] [DOI] [PubMed] [Google Scholar]
- 18.Samaniego EA, Mlynash M, Caulfield AF, et al. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2010 Aug 3; doi: 10.1007/s12028-010-9412-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YC, Phan TG, Jolley DJ, et al. Accuracy of clinical signs, SEP, and EEG in predicting outcome of hypoxic coma: a meta-analysis. Neurology. 2010;74:572–580. doi: 10.1212/WNL.0b013e3181cff761. [DOI] [PubMed] [Google Scholar]
- 20.Schefold JC, Storm C, Kruger A, et al. The Glasgow coma score is a predictor of good outcome in cardiac arrest patients treated with therapeutic hypothermia. Resuscitation. 2009;80:658–661. doi: 10.1016/j.resuscitation.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Celesia GG, Grigg MM, Ross E. Generalized status myoclonicus in acute anoxic and toxic-metabolic encephalopathies. Arch Neurol. 1988;45:781–784. doi: 10.1001/archneur.1988.00520310099023. [DOI] [PubMed] [Google Scholar]
- 22.Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2007;69:255–260. doi: 10.1212/01.wnl.0000265819.36639.e0. [DOI] [PubMed] [Google Scholar]
- 23•.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72:744–749. doi: 10.1212/01.wnl.0000343006.60851.62. [This study reports details on three patients treated with hypothermia who regained consciousness despite early post-anoxic myoclonus SE by clinical criteria with an EEG correlate.] [DOI] [PubMed] [Google Scholar]
- 24.Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473–485. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Snyder BD, Hauser WA, Loewenson RB, et al. Neurologic prognosis after cardiopulmonary arrest: III. Seizure activity. Neurology. 1980;30:1292–1297. doi: 10.1212/wnl.30.12.1292. [DOI] [PubMed] [Google Scholar]
- 26.Scollo-Lavizzari G, Bassetti C. Prognostic value of EEG in post-anoxic coma after cardiac arrest. Eur Neurol. 1987;26:161–170. doi: 10.1159/000116329. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen EO, Holm S. The natural course of neurological recovery following cardiopulmonary resuscitation. Resuscitation. 1998;36:111–122. doi: 10.1016/s0300-9572(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 28.Rundgren M, Westhall E, Cronberg T, et al. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. 2010;38:1838–1844. doi: 10.1097/CCM.0b013e3181eaa1e7. [DOI] [PubMed] [Google Scholar]
- 29.Wennervirta JE, Ermes MJ, Tiainen SM, et al. Hypothermia-treated cardiac arrest patients with good neurological outcome differ early in quantitative variables of EEG suppression and epileptiform activity. Crit Care Med. 2009;37:2427–2435. doi: 10.1097/CCM.0b013e3181a0ff84. [DOI] [PubMed] [Google Scholar]
- 30.Koenig MA, Kaplan PW, Thakor NV. Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurol Clin. 2006;24:89–106. doi: 10.1016/j.ncl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Bouwes A, Binnekade JM, Zandstra DF, et al. Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation. Neurology. 2009;73:1457–1461. doi: 10.1212/WNL.0b013e3181bf98f4. [DOI] [PubMed] [Google Scholar]
- 32•.Leithner C, Ploner CJ, Hasper D, Storm C. Does hypothermia influence the predictive value of bilateral absent N20 after cardiac arrest? Neurology. 2010;74:965–969. doi: 10.1212/WNL.0b013e3181d5a631. [This study reports recovery of consciousness in two post-cardiac arrest patients treated with hypothermia with absent or minimally detectable cortical N20 responses by SSEPs on day 3 after the arrest.] [DOI] [PubMed] [Google Scholar]
- 33.Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis. 2005;20:213–219. doi: 10.1159/000087701. [DOI] [PubMed] [Google Scholar]
- 34.Pahlman S, Esscher T, Bergvall P, Odelstad L. Purification and characterization of human neuron-specific enolase: radioimmunoassay development. Tumour Biol. 1984;5:127–139. [PubMed] [Google Scholar]
- 35.Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 36.Pleines UE, Morganti-Kossmann MC, Rancan M, et al. S-100 beta reflects the extent of injury and outcome, whereas neuronal specific enolase is a better indicator of neuroinflammation in patients with severe traumatic brain injury. J Neurotrauma. 2001;18:491–498. doi: 10.1089/089771501300227297. [DOI] [PubMed] [Google Scholar]
- 37.Zandbergen EG, de Haan RJ, Hijdra A. Systematic review of prediction of poor outcome in anoxic-ischaemic coma with biochemical markers of brain damage. Intensive Care Med. 2001;27:1661–1667. doi: 10.1007/s001340101076. [DOI] [PubMed] [Google Scholar]
- 38.Zingler VC, Krumm B, Bertsch T, et al. Early prediction of neurological outcome after cardiopulmonary resuscitation: a multimodal approach combining neurobiochemical and electrophysiological investigations may provide high prognostic certainty in patients after cardiac arrest. Eur Neurol. 2003;49:79–84. doi: 10.1159/000068503. [DOI] [PubMed] [Google Scholar]
- 39.Reisinger J, Hollinger K, Lang W, et al. Prediction of neurological outcome after cardiopulmonary resuscitation by serial determination of serum neuron-specific enolase. Eur Heart J. 2007;28:52–58. doi: 10.1093/eurheartj/ehl316. [DOI] [PubMed] [Google Scholar]
- 40.Shinozaki K, Oda S, Sadahiro T, et al. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation. 2009;80:870–875. doi: 10.1016/j.resuscitation.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34:2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 42.Rundgren M, Karlsson T, Nielsen N, et al. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80:784–789. doi: 10.1016/j.resuscitation.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Oksanen T, Tiainen M, Skrifvars MB, et al. Predictive power of serum NSE and OHCA score regarding 6-month neurologic outcome after out-of-hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation. 2009;80:165–170. doi: 10.1016/j.resuscitation.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Torbey MT, Selim M, Knorr J, et al. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke. 2000;31:2163–2167. doi: 10.1161/01.str.31.9.2163. [DOI] [PubMed] [Google Scholar]
- 45.Inamasu J, Miyatake S, Suzuki M, et al. Early CT signs in out-of-hospital cardiac arrest survivors: temporal profile and prognostic significance. Resuscitation. 2010;81:534–538. doi: 10.1016/j.resuscitation.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Wijdicks EF, Campeau NG, Miller GM. MR imaging in comatose survivors of cardiac resuscitation. AJNR Am J Neuroradiol. 2001;22:1561–1565. [PMC free article] [PubMed] [Google Scholar]
- 47.Jarnum H, Knutsson L, Rundgren M, et al. Diffusion and perfusion MRI of the brain in comatose patients treated with mild hypothermia after cardiac arrest: a prospective observational study. Resuscitation. 2009;80:425–430. doi: 10.1016/j.resuscitation.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Wu O, Sorensen AG, Benner T, et al. Comatose patients with cardiac arrest: predicting clinical outcome with diffusion-weighted MR imaging. Radiology. 2009;252:173–181. doi: 10.1148/radiol.2521081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Wijman CA, Mlynash M, Caulfield AF, et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65:394–402. doi: 10.1002/ana.21632. [This prospective study of 83 post-cardiac arrest patients shows that brain MRI is feasible in these patients and that quantitative DWI holds promise as a prognostic adjunct. DWI may not only identify those who die or cannot regain consciousness, but it may also provide information on the likelihood of long-term neurological impairment among survivors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mlynash M, Campbell DM, Leproust EM, et al. Temporal and spatial profile of brain diffusion-weighted MRI after cardiac arrest. Stroke. 2010;41:1665–1672. doi: 10.1161/STROKEAHA.110.582452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berek K, Lechleitner P, Luef G, et al. Early determination of neurological outcome after prehospital cardiopulmonary resuscitation. Stroke. 1995;26:543–549. doi: 10.1161/01.str.26.4.543. [DOI] [PubMed] [Google Scholar]