Abstract
Background
The pathophysiology of the presumed perihematomal edema (PPE) immediately surrounding an acute intracererbral hemorrhage is poorly understood and its composition may influence clinical outcome.
Method
Twenty three patients from the Diagnostic Accuracy of MRI in Spontaneous intracerebral Hemorrhage (DASH) study were prospectively enrolled and studied with MRI. Perfusion-weighted imaging (PWI), diffusion weighted imaging (DWI) and FLAIR sequences were coregistered. TMax and apparent diffusion coefficient (ADC) values in the PPE ROIs were compared with contralateral mirror and remote ipsilateral hemispheric ROIs.
Results
Compared to mirror and ipsilateral hemispheric ROIs, TMax delay and ADC were consistently increased in the PPE. Two thirds of the patients also exhibited patchy regions of restricted diffusion in the PPE.
Conclusion
The MRI profile of the PPE in acute ICH exhibits delayed perfusion and increased diffusivity mixed with areas of reduced diffusion.
Introduction
Perihematomal edema occurs within the first few days after intracerebral hemorrhage (ICH) onset.1 Experimental studies suggest that the mechanism of perihematomal edema formation is multifactorial and may be of cellular (cytotoxic) and/or vasogenic origin.2
Multimodal MRI can help characterize presumed perihematomal edema (PPE).3 Vasogenic edema increases the apparent diffusion coefficient (ADC) and cytotoxic edema decreases ADC on diffusion weighted imaging (DWI). Perfusion weighted imaging (PWI) can identify critically hypoperfused regions.4 Previous MRI studies consistently demonstrated hypoperfusion in the PPE, but studies of DWI have reported conflicting results.5-8 One study found decreased ADC in the PPE while three others concluded that ADC was globally increased.5-7, 9
We investigated the MRI profile of PPE of 20 consecutive patients in the Diagnostic Accuracy of MRI in Spontaneous intra-cerebral Hemorrhage (DASH) study.
Methods
Patients
The DASH study is a prospective NIH funded study, which aims to assess the feasibility and diagnostic yield of routine brain MRI in spontaneous (non-traumatic) ICH. Inclusion criteria for this sub-study of 23 consecutive patients were: supratentorial ICH, no brain surgery prior to MRI, MRI within 3 days after symptom onset including DWI, PWI using multiecho multishot parallel imaging10 and FLAIR. Three patients with technically inadequate PWI were excluded.
MRI Protocol
The brain MRI protocol has been previously described.10
Coregistration and ROI Generation
MRIs were analyzed using Medical Image Processing Analysis and Visualization (MIPAV) software.11
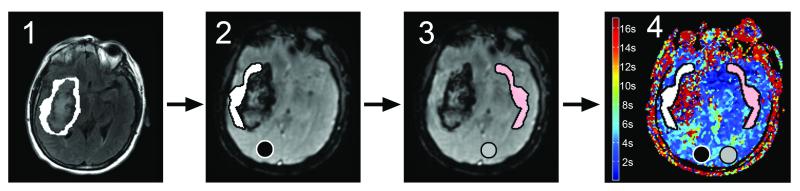
The coregistration process and PPE definition are illustrated in Figure 1.
Figure 1.
ROI Generation: 1- FLAIR was coregistered to T2* PWI (first TE). PPE was outlined on FLAIR and then transposed to T2* PWI. 2- All the hyperintense signal caused by blood on T2* was removed from the PPE ROI to avoid contamination of the PWI maps. Ipsilateral hemisphere ROI was drawn in the spared hemisphere 3- Both ROIs were flipped on the opposite hemisphere 4- ROIs were transposed on PWI maps to measure Mean Tmax value. (Same process was performed with ADC map).
Relative ADC (rADC) was defined as the mean ADC value of the ipsilateral ROI divided by the mean ADC value of its mirror ROI.
Restricted diffusion lesions were qualitatively identified by a hyperintense signal on B1000 and a corresponding hypointense signal on ADC map within the PPE.
Statistical Analysis
Wilcoxon signed-rank or Friedman tests and Mann-Whitney U tests were used to compare related and non-related volumes, TMax and ADC values. The Spearman Rank test was used to estimate the correlation. Fisher’s exact and McNemar’s tests were used to compare independent and related proportions. All tests were two-tailed and statistical significance was defined at α<0.05. Descriptive data are presented as median (IQR) values except when specified otherwise.
Results
Baseline Characteristics
Baseline characteristics of the 20 included patients are presented in Table 1.
Table 1.
Baseline Characteristics.
| Age, Mean (SD), years | 61± (17) |
| NIH Stroke Scale | 12 (5-20) |
| Glasgow Coma Scale | 14 (7-15) |
| Topography deep/lobar | 11/9 |
| MRI FLAIR Volume, cc | 23 (12-58) |
| MRI T2* Volume, cc | 27 (21-81) |
| Time from symptom onset to MRI, Hours |
26 (12-64) |
| Edema Volume, cc | 22 (10-33) |
| Edema ROI Volume, cc | 14 (8-26) |
| Hemisphere Volume, cc | 34 (30-42) |
| ICH Cause | Primary: Hypertension (11), Possible Cerebral Amyloid Angiopathy (CAA) (3), Unknown (1) Secondary: Vascular malformation (3), Cerebral venous thrombosis (1), hemorrhagic transformation of an infarction (1). |
Perfusion Imaging
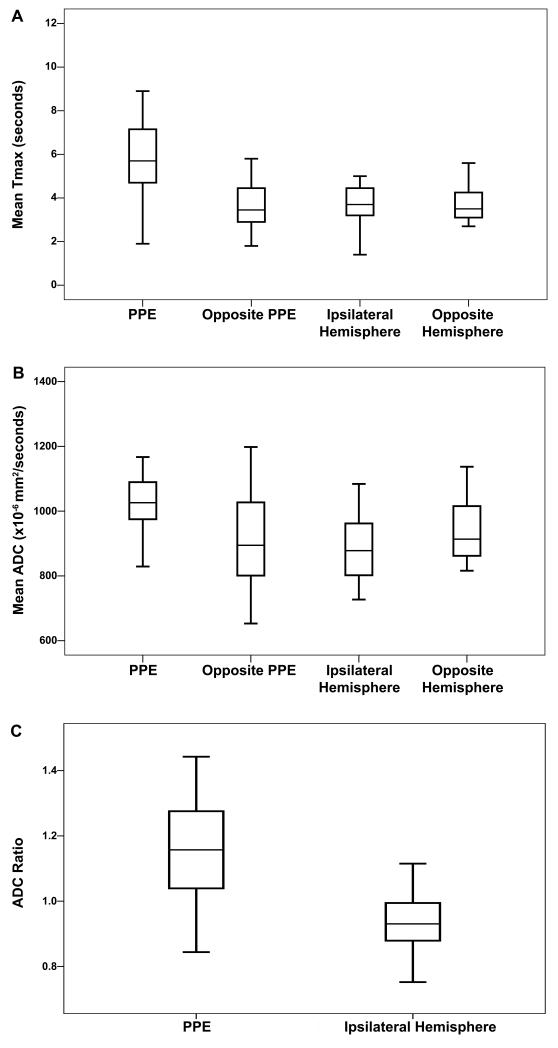
In regions of PPE the TMax delay of 5.7 seconds (4.7-7.2) was higher compared to the corresponding contralateral mirror ROI, 3.5 seconds (2.9-4.5), p<0.001, and ipsilateral hemisphere ROI: 3.7 seconds (3.2-4.5), p<0.001 (Figure 2a). PPE TMax delay was higher in patients scanned within 24 hours after symptom onset (n=10) p=0.017. Among the 7 patients with a mean TMax delay above 6 seconds, ICH volumes on FLAIR were larger 49 cc (19-60) compared with the remaining patients 14cc (8-18), p=0.046, however, edema volumes were not different.
Figure 2.
Mean Tmax value (a); Mean Absolute ADC (b) and rADC (c) in the PPE, its corresponding opposite ROI, in the ipsilateral hemisphere and its corresponding ROI.
Diffusion Imaging
The mean ADC measured in the PPE, 1026 (972-1099) ×10−6 mm2/second, was higher than the mirror ROI: 895 (801-1033) ×10−6 mm2/second, p=0.004, and the ipsilateral hemisphere ROI: 878 (802-962) ×10−6 mm2/second, p<0.001(Figure 2b). In the PPE, rADC, 1.16 (1.04-1.28), was higher compared to the rADC measured in the contralateral hemisphere ROI: 0.93 (0.88-0.99), p=0.003. There was no relation between the absolute or rADC in the PPE and ICH volume, edema volume, MRI delay or corresponding TMax delay. Fourteen patients (70%) exhibited restricted diffusion lesions within the PPE with a mean ADC value of 814 (744-957) ×10−6 mm2/second. These patients had larger ICH volumes measured on FLAIR, 28cc. (15.4-74.6) vs. 11.3cc (8.7-13.9), p=0.03, but there was no relation with the corresponding TMax delay, edema volume or time from symptom onset to MRI.
MRI profile according to ICH cause
Patients with both primary and secondary causes of ICH exhibited a delayed TMax and increased ADC in the PPE.
Among the 14 patients with restricted diffusion lesions in the PPE, ICH causes were hypertension (10), CAA (1), vascular malformation (2) and hemorrhagic transformation of brain infarction (1).
Discussion
Our results demonstrate a consistent MRI profile in the region of PPE surrounding acute ICH: significant perfusion delay and facilitated diffusion admixed with patchy areas of restricted diffusion.
Perihematomal hypoperfusion was inversely related to time from symptom onset and associated with large ICH volumes as previously shown.5-8 One third of our patients exhibited TMax>6 seconds delay which has been associated with critical hypoperfusion in brain infarction.4
ADC maps suggest that PPE consists of areas with cytotoxic and vasogenic edema. Interestingly, the presence of cytotoxic edema was found in the majority of patients with a primary ICH cause. It was associated with hematoma size, but not with the severity of hypoperfusion. These findings suggest that cytotoxic edema in the PPE might be related to ICH mass effect or the accumulation of cytotoxic factors such as thrombin or iron, rather than hypoperfusion.2
Our study has several limitations. First, MR imaging was performed 1 to 3 days after ICH onset, i.e. after the majority of ICH expansion has occurred. Imaging performed at an earlier time point might yield different results. Second, we chose TMax as the perfusion parameter to assess hypoperfusion. Another option would have been to use mean transit time (MTT). TMax maps provide better signal-to-noise ratio compared to the MTT maps and recent data suggest that TMax may better represent critical tissue hypoperfusion than MTT.4 Finally, our results derived from a small sample size need confirmation in a larger study and do not allow for analyzing correlations between MRI profile and functional outcome.
Acknowledgements and Funding
Source of Funding: The funding for this study was provided by the National Institutes of Health (NIH) grants RO1 NS034866, Principal Investigator, Christine A.C. Wijman. The NIH played no role in the design and the conduct of the study; collection, management, analysis, and interpretation of the data; or preparation or approval of the manuscript.
Footnotes
The authors have nothing to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gebel JM, Jr., Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–2641. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 3.Carhuapoma JR, Hanley DF, Banerjee M, Beauchamp NJ. Brain edema after human cerebral hemorrhage: A magnetic resonance imaging volumetric analysis. J Neurosurg Anesthesiol. 2003;15:230–233. doi: 10.1097/00008506-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Olivot JM, Mlynash M, Zaharchuk G, Straka M, Bammer R, Schwartz N, Lansberg MG, Moseley ME, Albers GW. Perfusion mri (tmax and mtt) correlation with xenon ct cerebral blood flow in stroke patients. Neurology. 2009;72:1140–1145. doi: 10.1212/01.wnl.0000345372.49233.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidwell CS, Saver JL, Mattiello J, Warach S, Liebeskind DS, Starkman S, Vespa PM, Villablanca JP, Martin NA, Frazee J, Alger JR. Diffusion-perfusion mr evaluation of perihematomal injury in hyperacute intracerebral hemorrhage. Neurology. 2001;57:1611–1617. doi: 10.1212/wnl.57.9.1611. [DOI] [PubMed] [Google Scholar]
- 6.Schellinger PD, Fiebach JB, Hoffmann K, Becker K, Orakcioglu B, Kollmar R, Juttler E, Schramm P, Schwab S, Sartor K, Hacke W. Stroke mri in intracerebral hemorrhage: Is there a perihemorrhagic penumbra. Stroke. 2003;34:1674–1679. doi: 10.1161/01.STR.0000076010.10696.55. [DOI] [PubMed] [Google Scholar]
- 7.Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke. 2004;35:1879–1885. doi: 10.1161/01.STR.0000131807.54742.1a. [DOI] [PubMed] [Google Scholar]
- 8.Pascual AM, Lopez-Mut JV, Benlloch V, Chamarro R, Soler J, Lainez MJ. Perfusion-weighted magnetic resonance imaging in acute intracerebral hemorrhage at baseline and during the 1st and 2nd week: A longitudinal study. Cerebrovasc Dis. 2007;23:6–13. doi: 10.1159/000095752. [DOI] [PubMed] [Google Scholar]
- 9.Carhuapoma JR, Barker PB, Hanley DF, Wang P, Beauchamp NJ. Human brain hemorrhage: Quantification of perihematoma edema by use of diffusion-weighted mr imaging. AJNR Am J Neuroradiol. 2002;23:1322–1326. [PMC free article] [PubMed] [Google Scholar]
- 10.Newbould RD, Skare ST, Jochimsen TH, Alley MT, Moseley ME, Albers GW, Bammer R. Perfusion mapping with multiecho multishot parallel imaging epi. Magn Reson Med. 2007;58:70–81. doi: 10.1002/mrm.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAuliffe M, Lalonde F, McGarry D, Gandler W, Csaky K, Trus B. Medical image processing, analysis & visualization in clinical research. IEEE Computer Based Medical Sciences (CBMS) 2001:381–386. [Google Scholar]