Abstract
Bone marrow-derived cells (BMDCs) contribute to postnatal vascular growth by differentiating into endothelial cells or secreting angiogenic factors. However, the extent of their endothelial differentiation highly varies according to the angiogenic models used. Wound healing is an intricate process in which the skin repairs itself after injury. As a process also observed in cancer progression, neoangiogenesis into wound tissues is profoundly involved in this healing process, suggesting the contribution of BMDCs. However, the extent of the differentiation of BMDCs to endothelial cells in wound healing is unclear. In this study, using the green fluorescent protein-bone marrow chim-eric experiment and high resolution confocal microscopy at a single cell level, we observed no endothelial differentiation of BMDCs in 2 acute wound healing models (dorsal excisional wound and ear punch) and a chronic wound healing model (decubitus ulcer). Instead, a major proportion of BMDCs were macrophages. Indeed, colony-stimulating factor 1 (CSF-1) inhibition depleted approximately 80% of the BMDCs at the wound healing site. CSF-1–mutant (CSF-1op/op) mice showed significantly reduced neoangiogenesis into the wound site, supporting the substantial role of BMDCs as macrophages. Our data show that the proangiogenic effects of macrophages, but not the endothelial differentiation, are the major contribution of BMDCs in wound healing.
Introduction
Vasculogenesis describes the process of de novo blood vessel formation from vascular precursor cells. Formation of the first major vessels, such as the dorsal aorta or the cardinal veins, is well known to occur through embryonic vasculogenesis.1 However, the involvement of vasculogenesis in postnatal vessel growth is largely unclear. The concept that bone marrow-derived cells (BMDCs) contribute to postnatal neoangiogenesis via endothelial differentiation was first proposed by Asahara et al.2 They named a cell population of BMDCs with the capacity to differentiate into endothelial cells as endothelial progenitor cells (EPCs). Since then, endothelial differentiation from BMDCs has been widely accepted as a substantial mechanism for vascular repair in ischemic tissues and for neoangiogenesis required for tumor growth and metastasis.3,4 However, there has been apparent controversy surrounding the temporal and quantitative aspects of endothelial differentiation from BMDCs.5–7 The lack of a unifying specific marker to define EPCs complicates the situation.8 One possible explanation for this controversy is that the majority of BMDCs recruited to the tumor vessels may be incorporated only at early phases of tumor vascular growth but not at later stages.9 BMDCs may not have a long-term endothelial fate but rather may find roles as vascular supportive cells.10 Otherwise, the highly variable extent of endothelial differentiation from BMDCs may reflect differences in genetic background or the nature of the angiogenic stimulus.4 On the other hand, recent reports showed that direct contribution of endothelial cells may not be a major mechanism in BMDC-mediated enhancement of angiogenesis. Instead, they reported that paracrine factors of BMDCs appear to play a central role.7,11,12
Cutaneous wound healing is an intricate process in which the skin repairs itself after injury. BMDCs participate in this process as various cell types, such as inflammatory cells, fibrocytes, and mesenchymal stem cells,13 and not only release cytokines, promoting wound repair, but also differentiate into keratinocytes and dermal myofibroblasts.14,15 Neoangiogenesis into healing tissues is profoundly involved in this process. Therefore, the roles of BMDCs in tumor angiogenesis described earlier in the “Introduction” have implications with respect to wound healing. Currently, it is generally understood that circulating EPCs contribute to a proportion of endothelial cells in wound healing tissues.13,16 In accordance with this, transplantation of cultured or isolated EPCs accelerates wound closure and promotes neoangiogenesis into wound tissues, suggesting that exogenous EPC transplantation could be useful in dermal wound repair and skin regeneration.11,17 However, there has been neither high resolution confocal analysis at a single-cell level showing the participation of BMDCs in newly forming endothelial tubes nor a quantitative analysis for the extent of BMDC endothelial differentiation. Therefore, there is no definite confirmation that BMDCs differentiate into mature endothelial cells in the process of wound healing.
Macrophages are white blood cells present in many tissues, produced by the differentiation of monocytes in a colony stimulating factor-1 (CSF-1)–dependent manner.18 Their role is to phagocytose cellular debris and pathogens and to stimulate lymphocytes and other immune cells to respond to the pathogen. Moreover, recent accumulating evidence shows that macrophages contribute to angiogenesis both in physiologic and pathologic settings.19–22 De Palma et al reported that a major contribution of BMDCs in tumor angiogenesis is the proangiogenic effects of macrophages.23,24
In this study, we thoroughly examined endothelial differentiation of BMDCs in the process of wound healing through high-resolution confocal microscopy. Most of the BMDCs were shown to be CSF-1–dependent macrophages. Moreover, CSF-1–mutant (CSF-1op/op) mice (op = osteopetrotic), which have a greatly reduced number of macrophages, showed significantly delayed wound healing and reduced neoangiogenesis into the wound healing area, supporting the substantial effects of BMDCs as macrophages in wound healing.
Methods
Mice
C57BL/6 mice (SLC), CSF-1op/op mice (The Jackson Laboratory), and CAG-EGFP transgenic mice (The Jackson Laboratory) were used. In some control experiments, we used Flk-1+/EGFP mice25 and LysM-Cre mice26 crossed with Flox-CAT-EGFP mice27 (LysM-Cre/Flox-CAT-EGFP). All animal experiments were approved by Keio University and were performed in accordance with the Guidelines of Keio University for Animal and Recombinant DNA experiments.
GFP-bone marrow chimeric experiment
Reconstitution of mice with green fluorescent protein positive (GFP+) bone marrow cells was performed as described elsewhere. Two-month-old recipient C57BL/6 mice were lethally irradiated with a total dose of 9 Gy, and 300 μL of cell suspension of the mononuclear cells (5 × 106) harvested from CAG-EGFP transgenic mice was administered via tail vein injection. Three months after transplantation, FACS analysis revealed that the recipient cells were replaced by donor-derived GFP+ cells at a rate of more than 90% in the peripheral blood (data not shown). Thereafter, we applied wound healing models to the GFP-BM chimeric mice.
Dorsal excisional wound model
A full-thickness excisional wound was made using a sterile biopsy punch with a diameter of 4 mm (Keisei Medical Industrial) on the dorsal region as previously described.28 The wounds were left open, and the animals were housed in individual cages. Dorsal wounds were macroscopically monitored by taking digital photographs at indicated time points. The wound areas (percentage of wound area to initial wound area) were calculated from the photographs using ImageJ software Version 1.44 (www.rsb.info.nih.gov/ij; n = 7 per group). Approximately 50 mg/kg (body weight) of Ki2022729 dissolved in 200 μL of methylcellulose; 200 μL of methylcellulose only; 50 mg/kg (body weight) of AFS98,30 a rat monoclonal antimurine CSFR-1 antibody (IgG2a), dissolved in 200 μL of phosphate-buffered saline (PBS); or an isotype-matched irrelevant rat IgG dissolved in 200 μL of PBS was intraperitoneally injected daily from 2 days before wounding to 7 days after wounding.
Ear punch wound model
A 1.5-mm hole was made in the center of the ears using a metal ear punch (Natsume) as described elsewhere.31
Decubitus ulcer model
Development of decubitus ulcers was performed as previously described.32 Briefly, mice were anesthetized, bilateral skin covering hind limbs was cut down, and 5-mm length of bilateral sciatic nerve was removed. Mice were housed free in less straw-bedded cages.
Histologic and morphometric analysis
Removed wound tissues were fixed by 4% paraformaldehyde in PBS overnight. Then, tissues were saturated in 30% sucrose for 24 hours and embedded in cryofreezing medium. Cryosections (10-μm thickness) were prepared and stained with hematoxylin and eosin or were subjected to immunohistochemical analysis. The primary monoclonal antibodies included hamster anti-CD31 (2H8; Chemicon International), rat anti-CD31 (MEC 13.3; BD Biosciences PharMingen), α-smooth muscle actin (1A4; fluorescein isothiocyanate or Cy3-conjugated; Sigma-Aldrich), and F4/80 (CI:A3–1; Serotec). The primary polyclonal antibodies included anti-GFP (Invitrogen; Alexa488-conjugated), collagen IV (Cosmo Bio), and keratin5 (Covance). After several washes in PBS, sections were incubated for 1 hour at room temperature with the following secondary antibodies: AlexaFluor-488 fluorescence-conjugated IgGs (Invitrogen) or Cy3/Cy5/DyLight549/DyeLight649-conjugated IgGs (Jackson ImmunoResearch Laboratories). In preparation for nuclear staining, specimens were treated with 4,6-diamidino-2-phenylindole (Invitrogen). For the 5-bromo-2′-deoxyuridine (BrdU) incorporation assay, 100 μg of BrdU (BD Biosciences PharMingen) per gram of body weight dissolved in sterile PBS was injected intraperitoneally 2 hours before death. Prepared sections were stained using a BrdU immunohistochemistry system (Calbiochem).
Whole-mount immunostaining
Preparation of whole-mount samples was performed as previously described.19 Removed wound tissues were fixed in 4% paraformaldehyde in PBS overnight and stored in methanol at −20°C. For immunohistochemistry, samples were stained in primary antibodies diluted in 0.1% Triton X at 4°C for 2 days. Staining with secondary antibodies in 0.1% Triton X was performed overnight. Thereafter, samples were postfixed in 4% paraformaldehyde in PBS overnight. Then, the samples were mounted using a Prolong Antifade Kit (Invitrogen).
Flow cytometric analysis of dissociated cells in wound tissues
Wound tissues were minced with a scalpel and incubated for 30 minutes at 37°C in Dulbecco minimum essential medium containing 1% collagenase D (from Clostridium histolyticum) and 1 unit/mL dispase. Dissociated single cells were preincubated with Fc block (BD Biosciences) to avoid nonspecific binding of antibodies and then incubated with intended antibodies. Stained samples were analyzed by SORP FACSAria (BD Biosciences) with FlowJo software Version 7.6.3 (TreeStar) or CELLQuest Pro software Version 5.1 (BD Biosciences). Debris and dead cells were excluded by forward and side scatter and a negative gate for propidium iodide staining. The primary antibodies included anti-vascular endothelial-cadherin, anti-CD45, anti-F4/80, anti-CD11b, anti-CD86, anti-CD48 (eBioscience), anti-Mrc1/macrophage mannose receptor (R&D Systems), and anti-LYVE-1 (RELIATech).
Quantitative reverse-transcribed PCR analysis
Total RNA was prepared from wound tissues or from isolated cells by FACS, and reverse transcription was performed using Superscript II (Invitrogen). Quantitative polymerase chain reaction (PCR) assays were performed on an ABI 7500 Fast Real-Time PCR Systems using TaqMan Fast Universal PCR master mixture (Applied Biosystems) and TaqMan Gene Expression Assay Mix of csf-1 (Mm00432688_ml), csfr-1 (Mm00432689_ml), nos2 (Mm00440502_ml), il6 (Mm00446190_ml), mmp2 (Mm00439498_ml), mmp9 (Mm00442991_ml), mmp13 (Mm00439491_ml), vegfa (Mm00437304_m1), angpt1 (Mm00456503_ml), and cxcl12 (Mm00445552_ml). A mouse β-actin (Mm00607939_sl) assay mix served as an endogenous control. Data were analyzed by 7500 Fast System SDS Software, Version 1.3.1. Each experiment was performed with 4 replicates from each sample, and the results were averaged.
Western blotting
Western blot analysis was performed as described elsewhere.19 The first antibodies used were anti–vascular endothelial growth factor (VEGF), anti–matrix metalloproteinase-2 (MMP-2), or anti–MMP-9 (Santa Cruz Biotechnology). The amount of total protein was examined by reblotting with anti–β-actin (Sigma-Aldrich).
Confocal microscopy and image acquisition
Fluorescent images (Figures 1B-K, 2A-Y, 3D-J,M-S, 5D-E,G-H, 6C-N,Q-R, and 7B-C) were obtained using a confocal laser scanning microscope (FV1000-D; Olympus) at room temperature. Scanning was performed in sequential laser emission mode to avoid scanning at other wavelengths. All of the images for quantification (Figures 1E-K, 2A-Y, 3E-J, N-S, 6Q-R, and 7B-C) were digitally recorded with high magnification objective lenses (40×/1.3 NA oil objective) to minimize their pseudo-positive detection of the neighboring layers, while some images not for quantification (Figures 1B-D, 3D, M, 5D-E, G-H, and 6E-N) were scanned with low magnification lenses (10×/0.4 NA). FV10-ASW Viewer 3.0 software (Olympus) was used to process the images (brightness and contrast), and export them into jpg format. For constructing merged images for quadruple immunohistochemistry (Figures 2A-Y and 3F-J, O-S), triple-colored images were overlaid with DAPI using Adobe Photoshop CS2. Quantification of cells of interest was performed in 500 μm × 500 μm fields of view per sample in each scanned image. Scion image software Version 4.0.3.2 (Scion Corporation) was used for quantification of capillary density. For image acquisition of samples stained with H&E in bright field view (Figures 2C, L and 6C, D), an inverted microscope (CKX41; Olympus) equipped with an objective lens (10×/0.4 NA) and a digital camera (DP20; Olympus) were used. Acquired images were analyzed with the use of image-filing software (cellSens; Olympus). Macroscopic views (Figures 1B-D, 2B, K, and 6A, O, P) were captured by a digital camera, FinePix Z70 (Fujifilm) in a closeup mode, and were exported into jpg format.
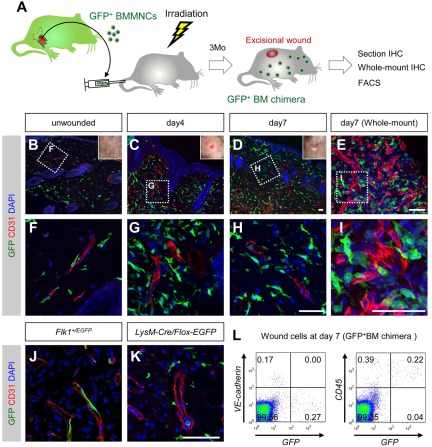
Figure 1.
BMDCs do not differentiate into endothelial cells in dorsal excisional wounds. (A) The procedure for the dorsal excisional wound model combined with the GFP-bone marrow chimeric experiment. (B-H) Sectional immunohistochemistry for indicated antibodies. Grids in panels B through D indicate the fields in panels F through H, respectively. None of the CD31+ vascular endothelium is stained for GFP. (E,I) Whole-mount immunohistochemistry for indicated antibodies. A grid in panel E indicates the field in panel I. (J-K) Sectional immunohistochemistry for the tissues 7 days after wounding in Flk-1+/EGFP mice (J) or LysM-Cre/Flox-CAT-EGFP mice (K). GFP was detected only in endothelial cells of Flk-1+/EGFP mice and myeloid cells of LysM-Cre/Flox-CAT-EGFP mice. (L) FACS analysis for dissociated cells in wound tissues (day 7) of mice reconstituted with GFP+ bone marrow cells. None of GFP+ cells is positive for vascular endothelial-cadherin, and most GFP+ cells are positive for CD45. Bars represent 50 μm.
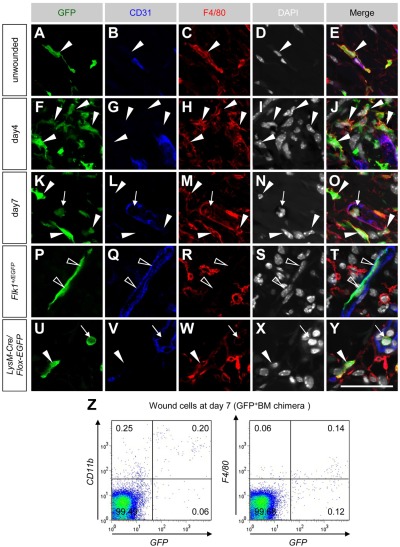
Figure 2.
A major proportion of BMDCs recruited into wound tissues are macrophages in dorsal excisional wounds. (A-O) Sectional immunohistochemistry for indicated antibodies in unwounded and wounded tissues of mice reconstituted with GFP+ bone marrow cells. Most GFP+ cells located perivascularly (closed arrowheads) or in the vascular lumen (arrows) expressed F4/80. (P-Y) Sectional immunohistochemistry for the tissues 7 days after wounding in Flk-1+/EGFP mice (P-T) or LysM-Cre/Flox-CAT-EGFP mice (U-Y). GFP was detected only in endothelial cells of Flk-1+/EGFP mice (open arrowheads) and F4/80+ monocytes (arrows)/macrophages (closed arrowheads) of LysM-Cre/Flox-CAT-EGFP mice. (Z) FACS analysis for dissociated cells in wound tissues at day 7 indicated that approximately 70% of GFP+ cells are positive for CD11b, but F4/80 expression in FACS was weak and highly variable. Bar represents 50 μm.
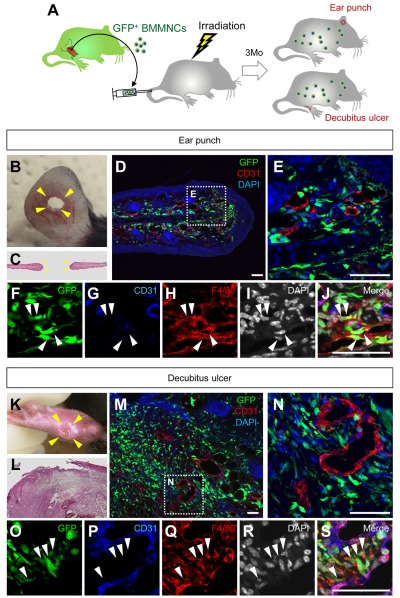
Figure 3.
BMDCs do not differentiate into endothelial cells in ear punch wounds and decubitus ulcers. (A) The procedure for the ear punch wound model or the decubitus ulcer model combined with the GFP-bone marrow chimeric experiment. (B-S) Macroscopic views (hematoxylin and eosin staining) or sectional immunohistochemistry for indicated antibodies. Grids in panels D and M indicate the fields in panels F-J and panels O-S, respectively. GFP+ cells that accumulated in the granulation tissues are mostly F4/80+ macrophages (arrowheads). Bars represent 50 μm.
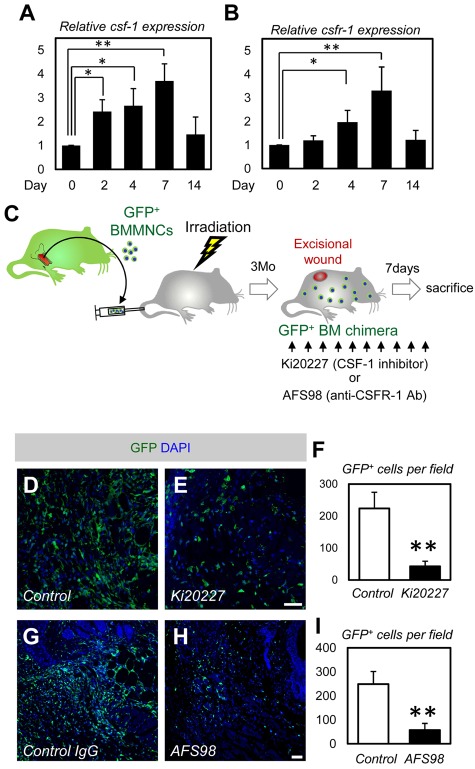
Figure 5.
CSF-1 inhibition mostly depletes BMDCs in wound healing. (A-B) Quantitative PCR analysis for csf-1 and csfr-1 (n = 4). (C) The procedure for CSF-1 inhibition combined with the dorsal excisional wound model and the GFP-BM chimeric experiment. (D-E) Immunohistochemistry for dorsal skin wounds of PBS- or Ki20227-injected GFP-BM chimeric mice. Ki20227 treatment depleted a large number of GFP+ BMDCs in the wound site. (F) Quantification of the GFP+ cells (n = 3). (G-H) Immunohistochemistry for dorsal skin wounds of control IgGs- or AFS98-injected GFP-BM chimeric mice. AFS98 treatment depleted a large number of GFP+ BMDCs in the wound site. (I) Quantification of the GFP+ cells (n = 3). Bars represent 100 μm. *P < .05, **P < .01.
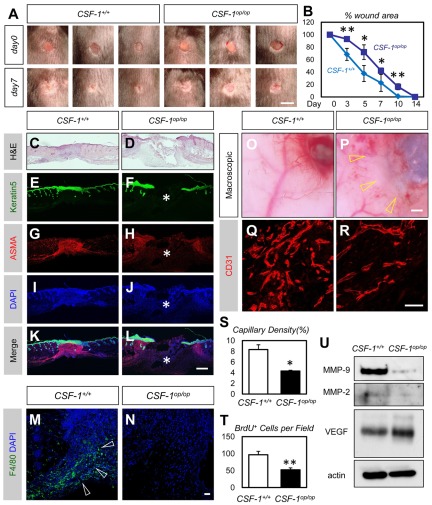
Figure 6.
Decreased neovascularization in the wound area of CSF-1op/op mice. (A) Representative macroscopic views of full-thickness excisional wounds in the back skin of CSF-1+/+ or CSF-1op/op mice. Note delayed wound closure in CSF-1op/op mice. (B) Percentage of wound closure of dorsal excisional wounds (n = 6). (C-L) Hematoxylin and eosin staining (C-D) or immunohistochemistry of Keratin5 (green), α-smooth muscle actin (red), and 4,6-diamidino-2-phenylindole (blue) (E-L) in the healing edges at 7 days after wounding. Note delayed epidermal closure (E-F) and dermal contraction (G-H) in CSF-1op/op mice (asterisks). (M-N) Immunohistochemistry of F4/80 (green) and 4,6-diamidino-2-phenylindole (blue) in the healing edges at 7 days after wounding. Macrophages are abundantly recruited in the healing edge of CSF-1+/+ (arrowheads) but not in that of CSF-1op/op mice. (O-P) Representative macroscopic views of the ventral sides of the dorsal excisional wounds in CSF-1+/+ or CSF-1op/op mice (7 days after wounding). Note decreased vessels in the wound area of CSF-1op/op mice. (Q-R) Images for sectional immunohistochemistry of CD31. Neovascularization is decreased in the wound area of CSF-1op/op mice compared with that of CSF-1+/+ mice. (S-T) Quantification in the capillary density or the number of BrdU+ cells in the healing edges 7 days after wounding (n = 6). (U) Representative Western blotting for the wound tissues at 7 days after wounding. Although VEGF expression was not altered, MMP-9 and MMP-2 were down-regulated in CSF-1op/op mice. Bars represent 5 mm in A; 500 μm in C-L,O-P; and 50 μm in M-N,Q-R. *P < .05. **P < .01.
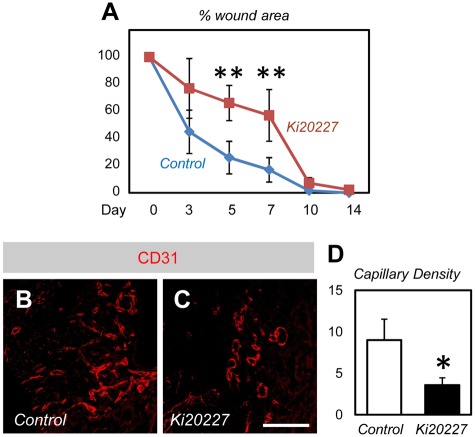
Figure 7.
Delayed wound healing in Ki20227-treated mice. (A) Percentage wound closure of dorsal excisional wounds (n = 6). (B-C) Immunohistochemistry of CD31 in the healing edges at 7 days after wounding. Neovascularization is decreased in the wound area of Ki20227-treated mice compared with that of vehicle-treated mice. (D) Quantification in the capillary density in the healing edges 7 days after wounding (n = 6). Bar represents 50 μm. *P < .05. **P < .01.
Statistical analysis
All results are expressed as the mean ± SD. The comparison between the averaged variables of 2 groups was evaluated using the 2-tailed Student t test. P values of < .05 were considered statistically significant.
Results
BMDCs do not differentiate into endothelial cells in dorsal excisional wounds
The initial step in our study was to examine the distribution of BMDCs in one of the most popular wound healing models, the dorsal excisional wound model28 (Figure 1A). Immunohistochemical analysis of mice reconstituted with GFP+ bone marrow cells revealed that GFP+ cells are abundant at the wound healing edge compared with that in the unwounded tissue (Figure 1B-D). High resolution confocal images showed that none of the vascular endothelium is stained for GFP (Figure 1F-H) (> 50 fields of view in each period), suggesting that BMDCs do not differentiate into endothelial cells in the healing process for dorsal excisional wounds. Although most GFP+ cells located perivascularly in unwounded tissues (Figure 1F), numerous GFP+ cells were detected in the area distant from the vessels in the wound healing area (Figure 1G-H). For control experiments to discriminate between vascular and perivascular localization of GFP+ cells, we used Flk-1+/EGFP mice25 and LysM-Cre/Flox-CAT-EGFP mice.26,27 In accordance with the nature of these mice, GFP was detected only in endothelial cells of Flk-1+/EGFP mice (Figure 1J), and myeloid cells of LysM-Cre/Flox-CAT-EGFP mice (Figure 1K). Next, we used whole-mount tissue immunohistochemistry, a technique suitable for confocal microscopy, to discriminate the cellular localization between endothelial lumen and perivascular space.7,19 Using this methodology, we found all GFP+ cells near the vessels are located perivascularly (> 50 fields of view; Figure 1E,I). FACS analysis for dissociated cells in wound tissues at day 7 indicated that none of the GFP+ cells was positive for the endothelial marker, vascular endothelial-cadherin (Figure 1L). Instead, approximately 85% of GFP+ cells were positive for the pan-hematopoietic marker, CD45 (Figure 1L). These data suggest that BMDCs recruited into wound tissues do not differentiate into endothelial cells but are mostly hematopoietic cells.
A major proportion of BMDCs recruited into wound tissues are macrophages
Previous studies have implicated that most BMDCs recruited into tumors are monocyte-lineage cells.7,12,23 Therefore, we examined expression of a macrophage marker F4/80 in unwounded and wounded tissues of mice reconstituted with GFP+ bone marrow cells. Most GFP+ cells expressed F4/80 (Figure 2A-O), suggesting that the major contribution of BMDCs in the dorsal excisional wound model is participation as macrophages. In control experiments, GFP+ F4/80+ macrophages were only detected in LysM-Cre/Flox-CAT-EGFP mice but not in Flk-1+/EGFP mice (Figure 2P-Y). FACS analysis for dissociated cells in wound tissues at day 7 indicated that approximately 70% of GFP+ cells were positive for CD11b (Figure 2Z), although F4/80 expression in FACS analysis was relatively weak (Figure 2Z) as indicated in a previous study.33 Taken together, a major proportion of BMDCs recruited into dorsal excisional wounds are macrophages.
BMDCs do not differentiate into endothelial cells in ear punch wounds
We used another acute wound healing model, the ear punch wound model, in which vessel growth is known to profoundly affect the epidermal closure31,34 (Figure 3A-B). Four days after wounding, GFP+ BMDCs were abundant at wound healing edges compared with the area distant from the wounds (Figure 3C-D). Similar to that of the dorsal excisional wounds, none of the vascular endothelium was stained for GFP, suggesting that BMDCs do not differentiate into endothelial cells in the ear punch wound model (Figure 3D-E). Instead, most GFP+ cells in the wound healing edges coexpressed F4/80 (Figure 3F-J), suggesting that the major contribution of BMDCs in the ear punch wound model is participation as macrophages.
BMDCs do not differentiate into endothelial cells in decubitus ulcers
So far, most experimental and molecular approaches to clarify the mechanism underlying human wound healing have been limited to the findings in animal models of acute wound healing. However, in clinics, a major problem in wound management is the refractory nature of chronic wounds, such as diabetic foot ulcers and decubitus ulcers.35 Moreover, it is possible that BMDCs participate in the continuous remodeling of vessels as endothelial cells in chronic wounds. Therefore, we tested a mouse decubitus ulcer model.32 In this model, GFP+ BMDCs were massively accumulated throughout the granulation tissues in the ulcer (Figure 3K-M). However, similar to the 2 models of acute wound healing, none of the vascular endothelium was stained for GFP, suggesting that BMDCs do not differentiate into endothelial cells in the decubitus ulcer model (Figure 3N). Instead, most GFP+ cells in the wound healing edges coexpressed F4/80 (Figure 3O-S). These data suggest that BMDCs do not differentiate into endothelial cells in chronic or acute wounds.
Macrophages recruited into the wound tissues possess “M2 polarized” properties and express abundant MMPs
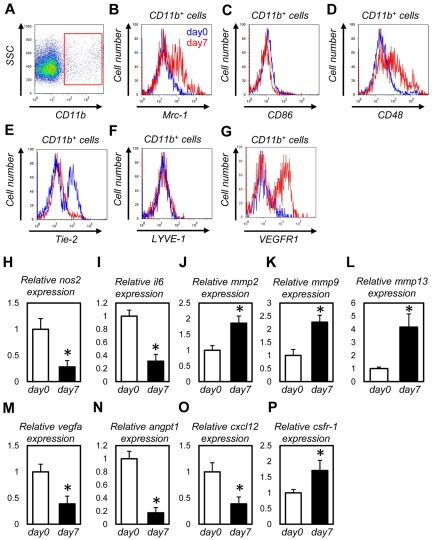
Macrophages are a heterogeneous population of cells that play diverse roles in pathologic situations, such as inflammation and cancer.21 One classification of macrophages measures “macrophage polarization” and groups them into the activated (M1) or the alternatively activated (M2) type.36 We examined the subtypes and gene expression profiles of macrophages in wound tissues. Using FACS, we isolated CD11b+ monocytes/macrophages from unwounded tissues or dorsal excisional wounds at day 7 (Figure 4A) and compared their expression levels of cell surface markers and the mRNA levels of paracrine factors. Macrophages in wound tissues showed higher expression of an M2 marker, Mrc1 (macrophage mannose receptor)36 (Figure 4B), and lower expression of a M1 marker, CD8636 (Figure 4C). Lower expression of nos2 and interleukin6 (il6) and higher expression of csfr-1 also represents the M2 phenotypes36 in wound macrophages (Figure 4I-J,P). Although VEGFR1 and CD4833 were higher, Tie223 was lower in wound macrophages than those in unwounded tissues (Figure 4D-E,G). Wound macrophages showed higher expression of genes for angiogenesis-related MMPs, such as MMP-2, MMP-9, and MMP-13 (Figure 4J-L), which are also known to be provided by macrophages.19,28,37 The expression of vegfa, angpt-1,34 and cxcl-1212 is lower in wound macrophages than those in unwounded tissues (Figure 4M-O). As the mRNAs harvested from whole wound tissues showed higher expression of vegfa, angpt-1, and cxcl-12 than those from unwounded tissues (data not shown), it is conceivable that cells other than macrophages (eg, epidermal cells and fibroblasts) are the major sources of these angiogenic factors. Moreover, CD11b− cells showed far more abundant expression of vegfa, angpt-1, and cxcl-12 than CD11b+ cells (data not shown). Macrophages might contribute to wound healing via matrix remodeling rather than secreting angiogenic factors in a paracrine manner.
Figure 4.
Macrophages recruited into the wound tissues possess M2-polarized properties and express abundant MMPs. (A-H) FACS analysis for CD11b+ cells from unwounded tissues (blue lines) or dorsal excisional wounds at day 7 (red lines). Macrophages in wound tissues showed higher expression of Mrc1 (B), CD48 (D), and VEGFR1 (G) and lower expression of CD86 (C) and Tie-2 (E). (I-Q) Quantitative PCR analysis for CD11b+ cells from unwounded tissues (day 0) or dorsal excisional wounds at day 7 (n = 4). Macrophages in wound tissues showed higher expression of mmp2 (J), mmp9 (K), mmp13 (L), and csfr-1 (P) and lower expression of nos2 (H), il6 (I), vegfa (M), angpt1 (N), and cxcl-12 (O). *P < .05.
CSF-1 inhibition mostly depletes BMDCs in wound healing
All of the results obtained in the GFP+BM chimeric experiments indicated that the major contribution of BMDCs in wound healing is participation as macrophages. CSF-138 is a cytokine required for the differentiation of monocyte-lineage cells, including macrophages.18 The expression of CSF-1 and the receptor CSFR-1 is up-regulated under conditions of increased macrophage recruitment, such as inflammation and cancer.39 Therefore, we examined the expression of CSF-1 and CSFR-1 in the healing process of dorsal excisional wounds. Quantitative PCR analysis showed that the expression of CSF-1 and CSFR-1 increased as early as 2 days after wounding and peaked on day 7 (Figure 5A-B). Thereafter, the expression of CSF-1 and CSFR-1 decreased on day 14 to the expression level before wounding. These data suggest that the CSF-1/CSFR-1 signaling contributes to macrophage recruitment during wound healing as also observed in cancer progression. If the major contribution of BMDCs in wound healing is truly participation as macrophages, blocking CSF-1 signaling should mostly deplete BMDCs in wound tissues. To test this, we injected CSF-1 inhibitor (Ki20227)29 or anti-CSFR1 neutralizing antibodies (AFS98) 30 into mice reconstituted with GFP+ bone marrow cells and applied the dorsal excisional wound model (Figure 5C). Ki20227 or AFS98 administration depleted approximately 80% of the GFP+ BMDCs in the wound healing site (Figure 5D-I). For the GFP+ cells that remained after treatment with Ki20227 or AFS98, we still detected no GFP+ endothelial cells. These remaining GFP+ cells could be bone marrow-derived fibrocytes or mesenchymal stem cells.
Decreased neovascularization in the wound area of CSF-1op/op mice
These results led us to examine the substantial effects of BMDCs as macrophages in wound healing. For this purpose, we used CSF-1–mutant (CSF-1op/op) mice and evaluated the role of CSF-1 signaling in the dorsal excisional wound model. Macroscopically, wound closure was apparently delayed in CSF-1op/op mice on day 7 (Figure 6A). When we quantified the wound areas on days 0, 3, 5, 7, and 14, CSF-1op/op mice showed a significantly larger raw surface from day 3 to day 10 than that for CSF-1+/+ mice (Figure 6B). Histologically, hematoxylin and eosin staining and keratin5 staining showed delayed epidermal closure in CSF-1op/op mice (Figure 6C-F). Immunohistochemical analysis for α-smooth muscle actin showed delayed dermal contraction by myofibroblasts13 in CSF-1op/op mice (Figure 6G-L). Immunohistochemically, CSF-1op/op mice showed a greatly reduced level of F4/80+ macrophages in the healing edges 7 days after wounding compared with that of CSF-1+/+ mice (Figure 6M-N). Macroscopic observations and immunohistochemical analysis for CD31 indicated significantly decreased blood vessel density at the wound edge in CSF-1op/op mice compared with that of CSF-1+/+ mice (Figure 6O-S). Because of the decreased blood supply, proliferation of cells in the granulation tissues of CSF-1op/op mice was significantly decreased (Figure 6T). Although VEGF expression was not altered, MMP-9 and MMP-2 were down-regulated in CSF-1op/op mice (Figure 6U) in agreement with the expression profiles of wound macrophages (Figure 4J-M).
Pharmacologic inhibition of CSF-1 decreased neovascularization in the wound area
Next, we examined whether pharmacologic CSF-1 inhibition by Ki20227 treatment also delayed wound healing. We injected Ki20227 into mice in the dorsal excisional wound model. Ki20227-treated mice showed a significantly larger raw surface from day 3 to day 7 than that for vehicle-treated mice (Figure 7A). Immunohistochemical analysis for CD31 indicated significantly decreased blood vessel density at the wound edge in Ki20227-treated mice compared with that of vehicle-treated mice (Figure 7B-D).
Discussion
In this study, we showed that BMDCs do not participate as bona fide endothelial cells in both acute and chronic wound healing models. Instead, most of the recruited BMDCs were macrophages, confirmed by the fact that blocking CSF-1 signaling depleted most BMDCs recruited into the wound tissues. The substantial contribution of BMDCs as macrophages was demonstrated by significantly delayed wound healing and reduced neoangiogenesis into the wound healing area in CSF-1op/op mice or mice treated with CSF-1 inhibitors.
A previous study showed that only approximately 50% of BMDCs are positive for CD45 in the excisional wound healing model.15 Considering that CD45 is ubiquitously expressed in the hematopoietic lineage cells, including macrophages, this percentage seems low compared with our FACS analysis (Figure 1L) and our data showing that CSF-1 inhibition depletes approximately 80% of the BMDCs. This difference may be the result of the timing of wounding; Ishii et al15 performed the excisional wound healing model at 4 weeks after bone marrow transplantation, whereas we performed the model 3 months after bone marrow transplantation. It is plausible that the number of BM-derived CD45− nonhematopoietic cells may decrease in this time lag. Another explanation is that macrophage depletion may also decrease the level of other kinds of BMDCs, such as fibrocytes or mesenchymal stem cells, by affecting macrophage-derived paracrine factors. Actually, cell proliferation in granulation tissues decreased in CSF-1op/op mice (Figure 6T). The expression of CD45 in fibrocytes decreases on differentiation or maturation of the cells, and this makes it difficult to recognize them by the CD45 marker.13
Although our data indicated that the endothelial differentiation of BMDCs in wound healing is unlikely, certain conditions have shown a significant contribution of BMDCs as endothelial cells. It appears that BMDCs participate in the vasculature of certain aggressive cancer models but not in low-grade cancer models, wound healing models, or physiologic development. There are some reports that demonstrated a fairly high contribution of BMDCs as endothelial cells. In humans, more than 25% of the endothelial cells are derived from bone marrow in heart transplant patients.40 Moreover, 12% of the endothelial cells are derived from bone marrow in certain lymphogenic tumors.41
Using CSF-1op/op mice, we found that CSF-1–dependent macrophages substantially contributed to neoangiogenesis in wound tissues. Regarding the molecular mechanism for macrophage contribution to wound angiogenesis, we found that the expression of MMP-2 and MMP-9, but not VEGF, is altered in CSF-1op/op mice. Although it is extremely difficult to determine which factor is critical for that mechanism, it is expected that matrix remodeling through proteases may be the central role of macrophages as observed in tumor angiogenesis.19 A recent study clearly demonstrated that macrophages promote endothelial tip cell fusion downstream of VEGF-mediated tip cell induction in the developing stage.20 Although visualization of tip cells in wound healing edges is technically difficult, impaired tip cell fusion may underlie the reduced neoangiogenesis into wound tissues in CSF-1op/op mice.
Finally, our data show that a proangiogenic effect of CSF-1–dependent macrophages, but not the endothelial differentiation, is the major contribution of BMDCs in the wound healing process. Blockade of CSF-1 signaling is one of the most promising strategies against various cancers and may potentially substitute VEGF blockade as an antiangiogenic therapy.19,21 Currently, several drugs that inhibit CSF-1 signaling are in early clinical trials.21 Clinical implications drawn from our study indicate that CSF-1 inhibition may impair wound healing as a possible side effect.
Acknowledgments
The authors thank Masatsugu Ema (University of Tsukuba) for providing the Flk-1+/EGFP mice.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Specially Promoted Research) and by the Keio Kanrinmaru Project.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.O. and A.N.-I. performed experiments and analyzed data; K.K. assisted in manuscript preparation; T.S. designed experiments, interpreted results, and assisted in manuscript preparation; and Y.K. designed experiments, interpreted results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshiaki Kubota, Center for Integrated Medical Research, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: ykubo33@a3.keio.jp.
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6(11):835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS, Benezra R, Lyden DC, et al. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci U S A. 2008;105(34):E54. doi: 10.1073/pnas.0804876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purhonen S, Palm J, Rossi D, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105(18):6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan DJ, Ciarrocchi A, Mellick AS, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21(12):1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009;16(4):269–273. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- 11.Suh W, Kim KL, Kim JM, et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23(10):1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 12.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28(5):905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 15.Ishii G, Sangai T, Sugiyama K, et al. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells. 2005;23(5):699–706. doi: 10.1634/stemcells.2004-0183. [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 17.Barcelos LS, Duplaa C, Kränkel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104(9):1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecchini MG, Dominguez MG, Mocci S, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120(6):1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 19.Kubota Y, Takubo K, Shimizu T, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206(5):1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobov IB, Rao S, Carroll TJ, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437(7057):417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9(6):789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 24.Ruhrberg C, De Palma M. A double agent in cancer: deciphering macrophage roles in human tumors. Nat Med. 2010;16(8):861–862. doi: 10.1038/nm0810-861. [DOI] [PubMed] [Google Scholar]
- 25.Ema M, Yokomizo T, Wakamatsu A, Terunuma T, Yamamoto M, Takahashi S. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, CD31, Tie2, endoglin, and CD34 in the mouse embryo. Blood. 2006;108(13):4018–4024. doi: 10.1182/blood-2006-03-012872. [DOI] [PubMed] [Google Scholar]
- 26.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto S, Niwa H, Tashiro F, et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 2000;470(3):263–268. doi: 10.1016/s0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- 28.Hattori N, Mochizuki S, Kishi K, et al. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009;175(2):533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno H, Kubo K, Murooka H, et al. A c-fms tyrosine kinase inhibitor, Ki20227, suppresses osteoclast differentiation and osteolytic bone destruction in a bone metastasis model. Mol Cancer Ther. 2006;5(11):2634–2643. doi: 10.1158/1535-7163.MCT-05-0313. [DOI] [PubMed] [Google Scholar]
- 30.Sudo T, Nishikawa S, Ogawa M, et al. Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene. 1995;11(12):2469–2476. [PubMed] [Google Scholar]
- 31.Oike Y, Yasunaga K, Ito Y, et al. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc Natl Acad Sci U S A. 2003;100(16):9494–9499. doi: 10.1073/pnas.1531901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonul B, Soylemezoglu T, Yanicoglu L, Guvendik G. Effects of epidermal growth factor on serum zinc and plasma prostaglandin E2 levels of mice with pressure sores. Prostaglandins. 1993;45(2):153–157. doi: 10.1016/0090-6980(93)90030-b. [DOI] [PubMed] [Google Scholar]
- 33.Pucci F, Venneri MA, Biziato D, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114(4):901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 34.Cho CH, Sung HK, Kim KT, et al. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci U S A. 2006;103(13):4946–4951. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366(9488):1725–1735. doi: 10.1016/S0140-6736(05)67699-4. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherr CJ, Rettenmier CW, Sacca R, Roussel MF, Look AT, Stanley ER. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- 39.Douglass TG, Driggers L, Zhang JG, et al. Macrophage colony stimulating factor: not just for macrophages anymore! A gateway into complex biologies. Int Immunopharmacol. 2008;8(10):1354–1376. doi: 10.1016/j.intimp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112(19):2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 41.Peters BA, Diaz LA, Polyak K, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11(3):261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]