Abstract
Nineteen Aedes aegypti larvae were collected in rural Antigua, West Indies, from an 18-liter plastic bucket. The location was in a rural area at the northern end of Antigua bordering the coast of Dickenson Bay and approximately 50 m south of Halcyon Cove Beach (17°09′42.54″N, 61°50′44.50″W; elevation 16 m). Atypical morphology was noted in larvae and 3 reared adult females. Fourth instars showed a reduction in length of the lateral hair on the saddle (seta 1–X) with measurements ranging from 0.36 to 0.57 the length of the saddle. Two atypical female specimens displayed an abundance of dull white to gold scales that blanketed the abdomen. A 3rd specimen bore fine, golden scales on the mesonotum and bronze scales on the vertices of the head. These adult specimens demonstrated morphological characteristics that closely parallel described mutations, although the genetic basis for these characters was not confirmed. The remaining adults in the collection were morphologically typical. Adults and larvae were compared to field populations from Florida, Bahamas, and Antigua, as well as to the Rockefeller strain maintained at Rutgers University.
Keywords: Aedes aegypti, variants, Antigua, morphology, mutants
One of the earliest accounts of Aedes aegypti (L.) from the island nation of Antigua was recorded in 1910 by Theobald (Kumm 1931), although the location and number of specimens were not specified. Subsequent collections were well documented in the 1965 Collection Records of the Project Mosquitoes of Middle America, also known as the MOMA project (Belkin and Heinemann 1976). In addition to Antigua, collections recorded include those from nearby islands, including the Leeward Islands of Anguilla, Barbuda, Montserrat, Nevis, and St. Kitts. Thirteen larval collections of Ae. aegypti were reported from Antigua: 8 were from artificial containers, 4 from tree holes, and 1 from the bromeliad Billbergia pyramidalis (Sims). Additionally, 1 blooded female was collected. Other species associated with these collections were Culex quinquefasciatus Say on 5 occasions and Ochlerotatus taeniorhynchus (Wied.) (=Aedes taeniorhynchus (Wied.), see Reinert 2000). The collection associated with the saltwater species Oc. taeniorhynchus was taken from a “small tree hole (in “Laballie” tree) at edge of domestic area near sea; at 1.2 m above ground; water clear; bottom with dead plant matter; partial shade.” Salinity was not noted. Collection localities were from the areas of Saints John (west-central), Phillip (southeastern), Paul (southern), and Mary (western). No collections were recorded from the northern end of the island during the MOMA project.
On the island of Anguilla, approximately 175 km northwest of Antigua, a sizable population of feral Ae. aegypti was found in numerous eroded rock hole depressions formed in the limestone substrate (Parker et al. 1983). This population was noticeably different from the domestic population found on the island, and although considered as Ae. aegypti sensu stricto, the adults tended to be darker especially on the 5th hindtarsomere. Subsequent biochemical genetic comparisons showed that the 2 populations were significantly differentiated and did not constitute a single panmictic unit (Wallis and Tabachnick 1990).
First, 2nd, and 3rd instars were collected October 2009 from a white, 18-liter plastic bucket located in a rural area adjacent to a resort property. The bucket was partially filled with a hardened substance resembling plaster and was topped with leaf litter; the water was very turbid. The area was canopied with mature trees and appeared to be a small construction dump site (17°09′42.54″N, 61°50′44.50″W; elevation 16 m). The bucket apparently had been undisturbed for a lengthy period despite its close proximity to human habitation.
The larvae were identified as a mixture of Ae. aegypti and Cx. quinquefasciatus. In the Ae. aegypti larvae, no apparent morphological or behavioral traits were observed that set this collection apart from a typical collection. Four 4th instars were preserved in alcohol, and the remainders were reared at room temperature on a diet of pulverized tropical fish food. Twelve viable adults emerged and were fed a honey–water mixture; 3 additional females of typical form died on the water surface.
Morphological comparisons were made with field populations from Key Largo, FL (Monroe County); Bradenton, FL (Manatee County); Nassau, Bahamas; and several locations in Antigua: Fort James, Golden Grove Mill, and Parour Road in Saint John, and Bethesda in Saint Paul. Additionally, comparisons were made to colony specimens of the Rockefeller (ROCK) strain maintained at the Rutgers University.
Mosquitoes of North America (Carpenter and LaCasse 1955) was used as a reference for the morphological descriptions. Anatomical terms were those referenced in Harbach and Knight (1980) and Darsie and Ward (2005). Digital images were taken with a trinocular mounted Moticam 2000® 2.0-megapixel camera (Motic Group Co., LTD, Hong Kong, China), and measurements of saddle morphologies were aided by Motic Images Plus 2.0© software (Motic Group Co., LTD).
Four 4th instars were examined. No abnormal pigmentation was observed at the time of collection and the integument color at the time of preservation was dark gray. The length of setae 1–X on the preserved specimens ranged from 0.36 to 0.57 of the length of the saddle, not “about as long as the saddle” as described by Carpenter and LaCasse (1955). Pupae were dark-colored and showed normal behavior.
Fifteen adults were examined (4♂, 11♀). Three specimens were considered to be morphological variants. Two females showed abdominal scale color that was dingy white to gold; some scales appeared colorless. This scale coloration extended to the visible sterna (I–VII-S). Specimen 1 showed the most extreme degree of white and gold coloration (Fig. 1A). The basal silver-white lateral patches or spots of typical specimens remained intact and not dulled or discolored (Fig. 1B). The abdominal scale color of specimen 2 was similar to specimen 1, although somewhat fewer pale scales were present (Fig. 1C). Specimen 3 exhibited narrow, gold scales on the mesonotum that obscured portions of the lyre markings, particularly the centrad mesonotal white lines. Bronze scales completely covered the head vertices (Fig. 1D).
Fig. 1.
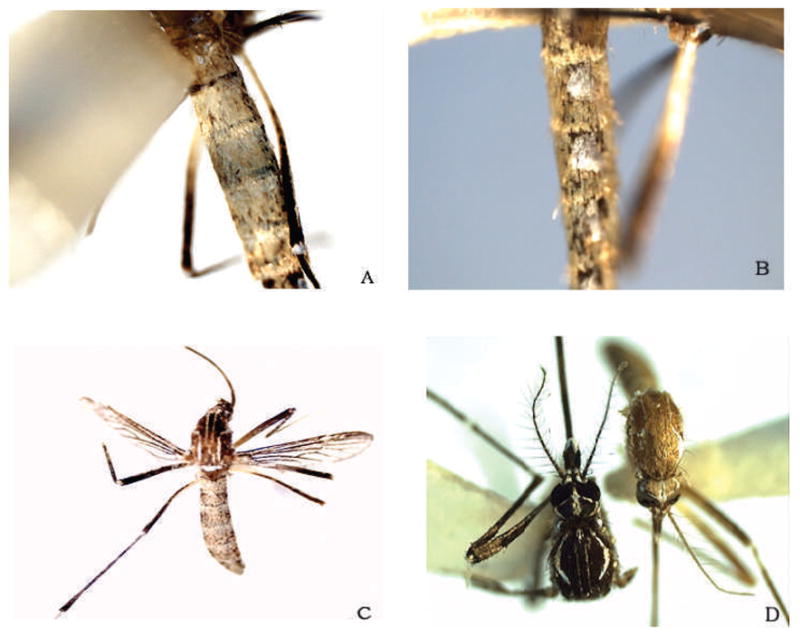
Reared Aedes aegypti from northern Antigua. (A) Specimen 1, dorsal view of the abdomen with dull white to gold scales. Portions of the dark cuticle can be seen through the colorless scales. (B) Specimen 1, lateral view of the abdomen with normal silver-white spots surrounded by dull white and gold scales. (C) Specimen 2, similar abdominal scale color to specimen 1, pale scales are less dense in distribution. (D) (left) Type form reared from field-collected egg (Bradenton, FL) with dark occipital and mesonotal scales compared to (right) specimen 3 with narrow, gold mesonotal scales that partially obscure the centrad lyre markings, and occipital scales are bronze.
Preserved adult females and slides of exuviae of 4th instars originating from Antigua during the MOMA project were obtained from the Smithsonian Institution in Washington, DC, and compared with the specimens of the current study. The MOMA specimens were in excellent condition and were indistinguishable from the type form in the current collection. Adults showed similar abdominal banding, scale coloration, and integument color. However, none of the MOMA specimens showed the abdominal scaling seen in the reared variants described above.
The MOMA slides of exuviae of 4th instars were examined to determine the lengths of seta 1–X in relation to saddle lengths. Saddle measurements were taken from the margin area closest to the origin of seta 1–X, and to the adjacent margin. Lengths ranged from 0.73 to 1.10, significantly longer than those found on our specimens. Measurements of 4th instars in specimens from the ROCK strain, Bahamas, and Florida populations were also within this range or slightly below.
A discernable amount of color variation among some of the studied populations was present. The adult specimens from Antigua were palest, with an overall light brown appearance. The ROCK strain was slightly darker, whereas those collected from the Bahamas and Florida were significantly darker, displaying a dark brown to almost black coloration.
The Antigua variants demonstrated morphology comparable to previously described mutations in Craig and Vandehey (1962) and which have been genetically characterized and mapped (Craig and Hickey 1967, Munstermann and Craig 1979, Munstermann 1993). The mutant phenotypes as described in the above reports appeared to fit the forms recovered in the current collection:
In specimens 1 and 2 (Fig. 1A–C), the amount of white or light yellow scaling on the abdomen is increased by the mutation White abdomen (W). Females that have the abdomen covered with matte-white to ivory scales are homozygous for W. The W gene is located on chromosome 2, the chromosome that bears other color mutations including yellow larva (y), Gold mesonotum (G), spot abdomen (s), and several genes for insecticide resistance (DDT, dieldrin, and malathion). Resistance to insecticides in Antigua is relatively high (Rawlins and Wan 1995), and selection pressures for these genes may have driven the linked color mutant genes to high frequencies in localized instances. White abdomen is clearly distinguished from s phenotypes in that the lateral silver patches retain the type form size and coloration. In contrast to the mutant color forms, the type form has abdominal pale scales limited to basal banding and lateral spots.
In specimen 3 (Fig. 1D), the G factor is a sex-influenced gene that is dominant in females and recessive in males. The phenotype has pale yellowish-golden scales on the dorsum and a light tan cuticle. The lyre markings can be obscured by the pale background. Gold mesonotum mutants frequently have occipital scales entirely golden to bleached white. In the type form, mesonotal and occipital scales as well as the cuticle are dark brown to black.
Five-gallon plastic buckets have many uses, and the local population may have been founded by a contractor or handyman who carried the egg-laden bucket to the rural dump site and thereby created a founder effect. Subsequent inbreeding increased the probability of the appearance of the homozygous mutant forms.
In summary, the morphological mutants of this type are rarely recovered in field populations and their striking appearance can be misinterpreted as representing new forms or species. Certainly, the specimens represent first records of these mutant forms in Antigua. The specimens are deposited at the Yale Peabody Museum Division of Entomology as permanent vouchers.
Acknowledgments
We gratefully acknowledge the following for their valuable input: Walter J. Tabachnick, Florida Medical Entomology Laboratory; Richard F. Darsie Jr., Grove City College; Jeffery R. Powell and Julia E. Brown, Yale University; and Carolyn (Lindy) McBride, Rockefeller University. We wish to thank Jim Pecor of the Walter Reed Biosystematics Unit, US National Museum Natural History, Smithsonian Institution, Washington, DC, for providing MOMA specimens; Scott C. Crans and Linda J. McCuiston of the Center for Vector Biology, Department of Entomology, Rutgers University, New Brunswick, NJ, for providing ROCK specimens; and Christopher Lesser and Debra Kinney of the Manatee County Mosquito Control District, Bradenton, FL, for providing field-collected eggs.
REFERENCES CITED
- Belkin JN, Heinemann SJ. Collection records of the project “Mosquitoes of Middle America” 4. Leeward islands: Anguilla (ANG), Antigua (ANT), Barbuda (BAB), Montserrat (MNT), Nevis (NEV), St. Kitts (KIT) Mosq Syst. 1976;8:123–162. [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (north of Mexico) Berkeley, CA: Univ. Calif. Press; 1955. [Google Scholar]
- Craig GB, Jr, Hickey WA. Genetics of Aedes aegypti. In: Wright JW, Pal R, editors. Genetics of insect vectors of disease. New York, NY: Elsevier; 1967. pp. 67–131. [Google Scholar]
- Craig GB, Jr, Vandehey RC. Genetic variability in Aedes aegypti (Diptera: Culicidae) I. Mutations affecting color pattern. Ann Entomol Soc Am. 1962;55:47–58. [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Mount Laurel, NJ: American Mosquito Control Association; 2005. [Google Scholar]
- Harbach RE, Knight KL. Taxonomists’ glossary of mosquito anatomy. Marlton, NJ: Plexus Publications; 1980. [Google Scholar]
- Kumm HW. The geographical distribution of the yellow fever vectors. Am J Hyg. 1931;12:1–110. [Google Scholar]
- Munstermann LE. Gene map of the yellow fever mosquito, Aedes (Stegomyia) aegypti (2N=6) In: O’Brien SJ, editor. Genetic maps: locus maps of complex genomes. 6. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 3.264–3.268. [Google Scholar]
- Munstermann LE, Craig GB., Jr Genetics of Aedes aegypti: updating the linkage map. J Hered. 1979;70:291–296. [Google Scholar]
- Parker AG, Giglioli MEC, Mussington S, Knudsen AB, Ward RA, Aarons R. Rock hole habitats of a feral population of Aedes aegypti on the island of Anguilla, West Indies. Mosq News. 1983;43:79–81. [Google Scholar]
- Rawlins SC, Wan JO. Resistance in some Caribbean populations of Aedes aegypti to several insecticides. J Am Mosq Control Assoc. 1995;11:59–65. [PubMed] [Google Scholar]
- Reinert JF. New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. J Am Mosq Control Assoc. 2000;16:175–188. [PubMed] [Google Scholar]
- Wallis GP, Tabachnick WJ. Genetic analysis of rock hole and domestic Aedes aegypti on the Caribbean island of Anguilla. J Am Mosq Control Assoc. 1990;6:625–630. [PubMed] [Google Scholar]


