Abstract
Corticotropin-releasing factor (CRF) is well known for its role in regulating the stress response in vertebrate species by controlling release of glucocorticoids. CRF also influences other physiological processes via two distinct CRF receptors (CRF-Rs) and is co-regulated by a CRF binding protein (CRFBP). Although CRF was first discovered in mammals, it is important for the regulation of the stress response, motor behavior, and appetite in all vertebrates. However, it is unclear how the actions of CRF, CRF-Rs and CRFBP are coordinated. To approach this problem, we cloned and identified CRF, CRF-Rs and CRFBP in a teleost fish model system, Astatotilapia burtoni and mapped their expression patterns in the body and brain. We found that CRF, CRFBP and CRF-Rs gene sequences are highly conserved across vertebrates, suggesting that the CRF system plays an essential role in survival. Members of the CRF system are expressed widely in the brain, retina, gill, spleen, muscle and kidney, thereby implicating them in a variety of bodily functions, including vision, respiration, digestion and movement. We also found that following long-term social stress, mRNA expression of CRF in the brain and CRF type 1 receptor in the pituitary decrease whereas CRFBP in the pituitary increases via a homeostatic mechanism.
Keywords: CRF, CRFBP, CRF receptors, mRNA, teleost
Introduction
In the course of evolution, highly conserved signaling molecules have been exploited by vertebrates to integrate stress responses, reflecting their important role(s) in survival. In particular, the hypothalamo-pituitary-adrenal/interrenal (HPA/I) axis plays a central part in the adaptive response to stress, predominantly via its role in coordinating corticotropin-releasing factor (CRF) release. CRF is a 41-amino acid peptide that stimulates the release of adrenocorticotropic hormone (ACTH) and β-endorphin from the anterior pituitary gland (Vale et al., 1981). In addition to its well studied effects in mediating the whole body stress response, CRF influences a wide spectrum of processes in both the central nervous system and in the periphery, underscoring its role in integrating diverse physiological systems in mammals (Turnbull and Rivier, 1997; Bale and Vale, 2004). The CRF-related family includes the other subtypes of CRF and three forms of urocortin (1, 2 and 3). Urocortin-1 is an ortholog of urotensin-1 in fish and sauvagen in amphibians (Lovejoy and Balment, 1999; Boorse et al., 2005). The effects of CRF and CRF-like peptides are mediated via two receptor subtypes, the CRF type 1 (CRF-R1) and CRF type 2 (CRF-R2) receptors. Both receptors are expressed in a variety of locations in the brain and body where they act to maintain homeostatic balance in response to stress (Bale and Vale, 2004)
Another regulator of CRF, corticotropin-releasing factor binding protein (CRFBP), has been shown to modulate the effects of CRF or CRF-related ligands in both the central nervous system and in peripheral tissue (Potter et al., 1992; Behan et al., 1993; Seasholtz et al., 2002; Bale and Vale, 2004; Huising et al., 2005; Boorse et al., 2006; Westphal and Seasholtz, 2006). CRFBP decreases cortisol release by inhibiting the effects of CRF (Behan et al., 1995; Chan et al., 2000) and regulates ACTH release by blocking CRF in the pituitary (Westphal and Seasholtz, 2006).This effect of CRFBP on cortisol levels implies that there is a direct link between CRF and cortisol.
Teleost fish, especially cichlids, are well suited for analysis of stress and behavior because many species exhibit remarkable behavioral and neural plasticity, and complex social interactions that modify the stress axis (Krause et al., 2000; Bass and Grober, 2001; Fernald, 2003). The African cichlid fish, Astatotilapia burtoni (A. burtoni) is a particularly well developed model system in which males display dramatic plasticity in their reproductive and stress systems in response to changes in social status (Fernald, 2003). In the wild, A. burtoni males that lose their territories change their behavior dramatically (Fernald and Hirata, 1977b) and rapidly exhibit an increase in circulating cortisol levels (Fox et al., 1997). Growing evidence suggests that the CRF system acts on both HPI axis and other systems to regulate the stress response. However, it is not known whether the CRF system functions in teleosts as it does in mammals or whether it is regulated by social behavior similar to the regulation of the HPG axis.
Here we report the cloning and analysis of the regulation of CRF, CRFBP and the two CRF receptors in A. burtoni. We found the CRF family is widely expressed in peripheral tissues, as well as in the central nervous system, including the brain, pituitary gland and spinal cord. Interestingly, CRF and CRF-R1 mRNA levels are higher in the brains and pituitary of territorial A. burtoni male than in those of non-territorial males. These data suggest that the CRF system not only controls cortisol release but also maintains homeostasis under long term social stress.
Material and Methods
Animals
Astatotilapia burtoni derived from a wild-caught population were raised in aquaria under conditions matched to their native equatorial habitat in Lake Tanganyika, Africa (pH 8, 28°C). Fish were kept in a 12-h light, 12-h dark cycle that included 10 min of transitional twilight in the morning and evening. Light and dark onsets were at 8 AM and 8 PM, respectively. Two males and three females were kept in each aquarium with a terra cotta shelter for at least five weeks. All work was performed in compliance with the animal care and use guidelines of the Stanford University Administrative Panel on Laboratory Animal Care.
Behavioral observations and analysis
To allow identification of individuals, males were marked with colored beads attached just below the dorsal fin. Each male was observed three times per week for three minutes at the same time (five hours after light onset) for four weeks. Using these observations, males were classified as either territorial (T) or non-territorial (NT) males based on their reproductive activity and behavior. Territorial males are large, brightly colored, and reproductively competent. They also establish and defend territories containing a food resource used to entice females to spawn. Non-territorial males are smaller, cryptically colored, have regressed gonads, and spend their time schooling with females (Hirata and Fernald, 1975; Fernald and Hirata, 1977a; Fernald and Hirata, 1977b; Fraley and Fernald, 1982). To quantify behavioral social status, a “dominance index” (DI = [aggressive acts + sexual acts – fleeing acts]/minutes observed) was calculated as the number of dominant acts minus the number of submissive acts that occurred during a given observation period. We also measured the relative gonad size (gonadosomatic index; GSI=[gonad mass/body mass] ×100). To be classified as a T, a male must have had daily mean DI values greater than 2 during all observation periods and GSI values greater than 0.5 (White et al., 2002). To be classified as an NT, a male must have had negative daily mean DI values during all observation periods and GSI values less than 0.2.
Cloning of CRF, CRFBP and CRF receptors
Partial sequences of all CRF family members were amplified using degenerate primers on A. burtoni cDNA derived from RNA isolated from brain tissue. CRF primers were based on conserved regions of CRF from other teleosts: Carassius auratus (AF098629), Carostomus commersoni (S65264), Danio rerio (BC085458), and Oncorhynchus mykiss (AF296672). CRFBP degenerate primers were based on conserved regions of CRFBP from: Cyprinus carpio (CRFBP1 - AJ490880 and CRFBP2- AJ490881), Oncorhynchus masous (CRFBP1, 2, and 3 - AY898808, AY905544 and AY911309), Oncorhynchus mykiss (AY363677). CRF-R1 primers were based on conserved regions of CRF-R1 from the following species: Cyprinus carpio (AJ576244), Epinephelus coioides (AY820281), Gallus gallus (NM204321), Homo sapiens (NM004382), Rana catesbeiana (AB188110). CRF-R2 primers were based on conserved regions of CRF-R2 from the following species: of Gallus gallus (NM204454), Homo sapiens (BC096830), Oncorhynchus keta (AJ277158), Mus musculus (NM009953), Rana catesbeiana (AB188111).
Sequence information from these original clones was used to design gene-specific primers for 5’ RACE and 3’ RACE, and subsequently to clone the full-length cDNAs. 5’ RACE and 3’ RACE cDNA were prepared according to the manufacturer’s instructions (SMART RACE cDNA Amplification Kit, BD Biosciences, USA) by Adventage polymerase mix (BD Biosciences, USA) containing a proofreading polymerase. A. Burtoni cDNAs are derived from multiple RNA extractions from two sexually mature male brains and one female brain. Specific primers based on partial A. burtoni sequences of each target gene were used in combination with the 5’ or 3’ universal (UPM) and nested universal (NUP) primers to amplify the end of each gene from the RACE A. burtoni brain cDNA. The full sequences of CRF, CRFBP, and CRF-Rs were cloned. The reaction products were purified, subcloned (TOPO TA cloning kit, Invitrogen), and sequenced (Sequentech, Mountain View, CA). We could not identify any other types of CRF, CRF-Rs or CRFBP using these techniques.
Phylogenetic analysis of CRF, CRFBP and CRF receptors
The sequences of the A. burtoni CRF, receptors, and binding protein were aligned with those of other species (Clustal W; http://www.ch.embnet.org/software/ClustalW.html), identical amino acids were identified (Box shade; http://www.ch.embnet.org/software/BOX_form.html), and phylogenetic analyses were performed to situate members of the A. burtoni CRF family among previously known sequences. The predicted sequences of CRF family polypeptides were aligned and a phylogenetic tree generated by MEGA3.1 (Kumar et al., 2004). The tree was generated by neighbor-joining methods using only the coding regions of the cloned sequences. Bootstrap values were also calculated (MEGA 3.1). Full species names and GenBank accession numbers for the cDNAs in Fig. 2 and Fig. 3 are as follows. For the CRF cDNAs: A. burtoni, Astatotilapia burtoni; African clawed frog or Xenopus, Xenopus laevis: AAB24277; Chicken, Gallus gallus: CAF18561; Common carp I, Cyprinus carpio CRF type 1: CAC84859; Common carp II, Cyprinus carpio CRF type 2: CAE11291; European flounder, Platichthys flesus: CAD88277; Human, Homo sapiens: CAA23834; Mozambique tilapia or Tilapia, Oreochromis mossambicus: CAB77056; Orange-spotted grouper, Epinephelus coioides: AAV71132; Rainbow trout UT I, Oncorhynchus mykiss urotensin I: CAA06461; Rat, Rattus norvegicus, NP112281; Zebrafish, Danio rerio: AAH85458. For the CRFBP cDNAs: A. burtoni, Astatotilapia burtoni; Carp 1, Cyprinus carpio type 1 CRFBP: CAD35748; Chicken, Gallus gallus: XP_424801; Fugu, Takifugu rubripes: CAF18402; Human, Homo sapiens: AAH18038; Rainbow trout, Oncorhynchus mykiss: AAR12888; Rat, Rattus norvegicus: NP_631922; Zebrafish, Danio rerio: XP_683328. For the CRF-R1 cDNAs: A. burtoni or A. burtoni 1, Astatotilapia burtoni; African clawed frog 1 or Xenopus, Xenopus laevis: CAA74363; Brown bullhead 1, Ameiurus nebulosus: AAK01068; Bullfrog 1, Rana catesbeiana: BAD36783; Carp 1, Cyprinus carpio: CAE11292; Chicken 1, Cyprinus carpio: NP_989652; Chum salmon or Chum salmon1, Oncorhynchus keta: CAC81753; Fugu , Takifugu rubripes: CAC82924; Goldfish or Goldfish 1, Carassius auratus: AAV98392; Human or Human 1, Homo sapiens: NP_004373; Orange-spotted grouper, Epinephelus coioides: AAV71133; Rainbow trout 1, Oncorhynchus mykiss: AAT38872; Rat or Rat 1, Rattus norvegicus: NP_112261; Zebrafish 1, Danio rerio: XP_696346. For the CRF-R2 cDNAs: A. burtoni or A. burtoni 2, Astatotilapia burtoni; African clawed frog2 or Xenopus, Xenopus laevis: CAA74364; Brown bullhead or Brown bullhead 2, Ameiurus nebulosus: AAK01069; Bullfrog 2, Rana catesbeiana: BAD36784; Chicken 2, Gallus gallus: NP_989785; Chum salmon or Chum salmon 2, Oncorhynchus keta: CAC81754; Human or Human 2, Homo sapiens: NP_001874; Rat or Rat 2, Rattus norvegicus: NP_073205; Zebrafish 2, Danio rerio: XP_686454.
Figure 2.
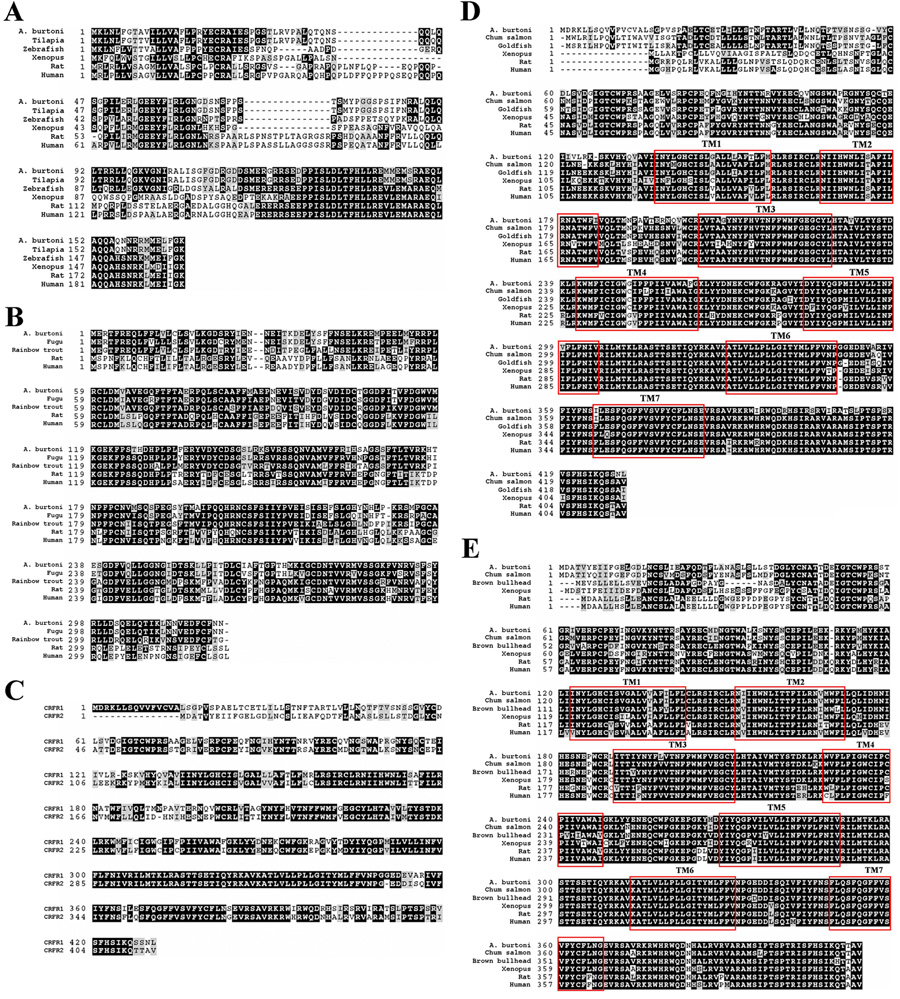
Amino acid alignments of A. burtoni CRF (A), CRFBP (B), CRF-R1 (D), and CRF-R2 (E) sequences with other vertebrate species, including tilapia, zebrafish, xenopus, rat and human. (C) CRF-R1 and CRF-R2 amino acid sequence comparison. Identical residues are shaded black and similar residues (e.g., same charge) are shaded gray. Hyphens indicate gaps in the sequence among the species. Gray boxes indicate the seven transmembrane domain regions (TM) for each receptor sequence.
Figure 3.
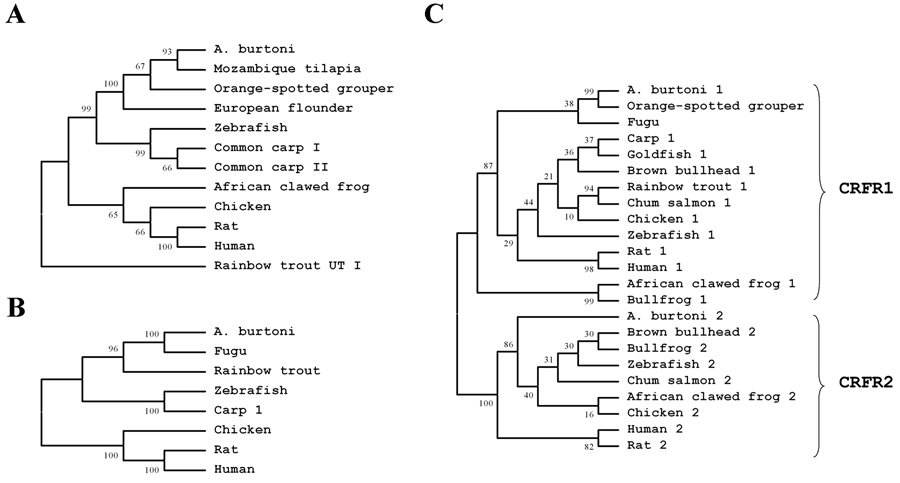
Phylogenetic trees of CRF (A), CRFBP (B), and CRF-R1 and CRF-R2 peptides (C) including various vertebrate species. The predicted sequences of those polypeptides were aligned and a phylogenetic tree generated using neighbor-joining methods based on the coding regions. Bootstrap values estimate the evolutionary distance between the species and are shown at each branch point.
CRF, CRFBP and two types of CRF receptors mRNA localization
To understand where CRF family genes were expressed, polymerase chain reaction (PCR) was performed on cDNA from tissues taken from adult A. burtoni (brain, pituitary gland, spinal cord, retina, gill, heart, intestine, kidney, liver, muscle, ovaries, retina, spleen, and testes) using primers specific to the CRF family members. The cornea and lens were removed from the eye and the retina extracted from its attachment to the sclera. The tissues were homogenized and RNA extracted (Rneasy; Qiagen Inc., Valencia, CA) from both males and females (n = 3). To obtain cDNA in each tissue sample, rapid amplification of 3′-cDNA ends was performed with total RNA (SMART cDNA synthesis; Clontech Laboratories Inc., Palo Alto, CA). Primers specific to the A. burtoni CRF were designed, 5' (5’-CCT TGA CAT GAA GCT CAA TTT ATT CGG TA-3’) and 3′ (5’-ACA GAA GAA TGA TGG AGC TCT TCG GG-3’), which generated a 505-bp PCR product. Primers specific to the A. burtoni CRFBP were designed, 5′ (5’-GCT TTC TTC ATG GCA GAG CCC AAT-3’) and 3′ (5’-TCG CTT TCA CTG GAC CAA CTC ACA-3’), which generated a 568-bp PCR product. Primers specific to the A. burtoni CRF-R1 were designed, 5′ (5’-ACA ACA CCA CCA ATC GGG TCT ACA-3’) and 3′ (5’-TCC TGA TGA CCA AAC TCA GGG CAT-3’), which generated a 757-bp PCR product. CRF-R2 primers were designed, 5′ (5’-AGG ATG CTA CCT TCA CAC AGC CAT TG-3’) and 3′ (5’- TGC TTT CTA AAC GGA GAG GTT CGC T-3’), which generated a 488-bp PCR product. PCR was performed with a 68 – 60°C touchdown protocol as follows: 3-min denaturation at 95° C, followed by 16 cycles of 30-sec denaturation at 95°C, 30-sec annealing (68 – 60°C), and 15-min extension at 72°C. Each of these reactions yielded a single product as revealed by gel electrophoresis and no significantly difference between individual animals.
Negative controls were performed using the same procedure as for the experimental group without adding cDNA from any tissue and none of these reactions produced products. As a positive control, specific primers were used to amplify the housekeeping gene, actin, for quality control in each cDNA sample.
RNA extraction and PCR sample preparation for Real time-PCR
Total RNA was extracted from whole brain (T: n = 8; NT: n = 8), pituitaries (T: n = 12; NT: n = 12) and spinal cords (T: n = 12; NT: n = 12) following a standard protocol (RNeasy Micro Kit, Qiagen Inc., Valencia, CA). 1.0 µg total RNA was reverse transcribed (SuperScript II RNase H, Invitrogen, Carlsbad, CA) to cDNA in each sample. To examine whether social status influenced the gene expression patterns of the CRF family, we used real-time PCR (RT-PCR). Territorial or non-territorial status was determined as described above and animals were sacrificed by rapid cervical transection. The tissues were immediately put into lysis buffer (RNeasy Micro Kit, Qiagen Inc., Valencia, CA) and stored at −80°C after homogenization. Total RNA was extracted from samples following a standard protocol (RNeasy Micro Kit, Qiagen Inc., Valencia, CA). 1.0 µg total RNA was reverse transcribed (SuperScript II RNase H, Invitrogen, Carlsbad, CA) to cDNA in each sample. Primers for the CRF ligand were designed: 5′ (5’-CGA ACT CTT TCC CAT CAA CGT CCA-3’) and 3′ (5’-TAA AGT TGG GAA CAT CAG GGC GCT-3’), which generated a 121-bp PCR product. Similarly, CRFBP primers were designed, 5′ (5’-ACT GAC CTC TGC ATC GCT TTC ACT-3’) and 3′ (5’-AGGC ATG GTG TCC AGT GGG AAG TTT-3’), which generated 90-bp PCR products. Primers specific to the A. burtoni CRF-R1 were also designed, 5′ (5’-TTG GTG AAG GCT GTT ACC TCC ACA-3’) and 3′ (5’-TCC TGA TGA CCA AAC TCA GGG CAT-3’), which generated a 282 bp PCR product. Finally, primers specific to the A. burtoni CRF-R2 were designed, 5′ (5’-TGC CAC AAC CGA TGA GAT TGG AAC-3’) and 3′ (5’-GTG AAG TAC AAC ACA ACG AGG AGC G-3’), which generated a 113 bp PCR product. A housekeeping gene, actin, previously cloned from A. burtoni, was used to control for sample differences in total cDNA content. Polymerase chain reactions were performed (iCycler; Bio-Rad, Hercules, CA) and the reaction progress in 30 µl reaction volumes was monitored by fluorescence detection at 490 nm during each annealing step. Reactions contained 2x IQ SYBR® Green SuperMix (Bio-Rad), 10 µM of each primer, and 1 ng cDNA (RNA equivalent). Reaction conditions were 1 min at 95°C; then 35 cycles of 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C; followed by a melting curve analysis over the temperature range from 95°C to 4°C. All reactions were performed in duplicate.
PCR data analysis
Fluorescence readings for each sample were baseline subtracted and suitable fluorescence thresholds were automatically measured (MyiQ™ software). To determine the number of cycles needed to reach threshold, the original fluorescence reading data were analyzed using a curve-fitting real time PCR algorithm (Zhao and Fernald, 2005). This algorithm calculates reaction efficiency and the fractional cycle number at threshold (CT) of RT-PCR amplify curve for more accurate computation of mRNA levels. The relative amount of mRNA were normalized using the level of a housekeeping gene, actin. All RT-PCR data before normalization were analyzed for normality and homogeneity of variance.
Statistical analysis
Data are expressed as mean ± standard errors. The data which were normally distributed with equal variance were tested statistically using a T-test. The data which were not normally distributed were tested statistically using the Mann-Whitney Rank Sum test, with p<0.05 set as the significance threshold.
Results
Cloning, sequencing and characterization of A. burtoni CRF family
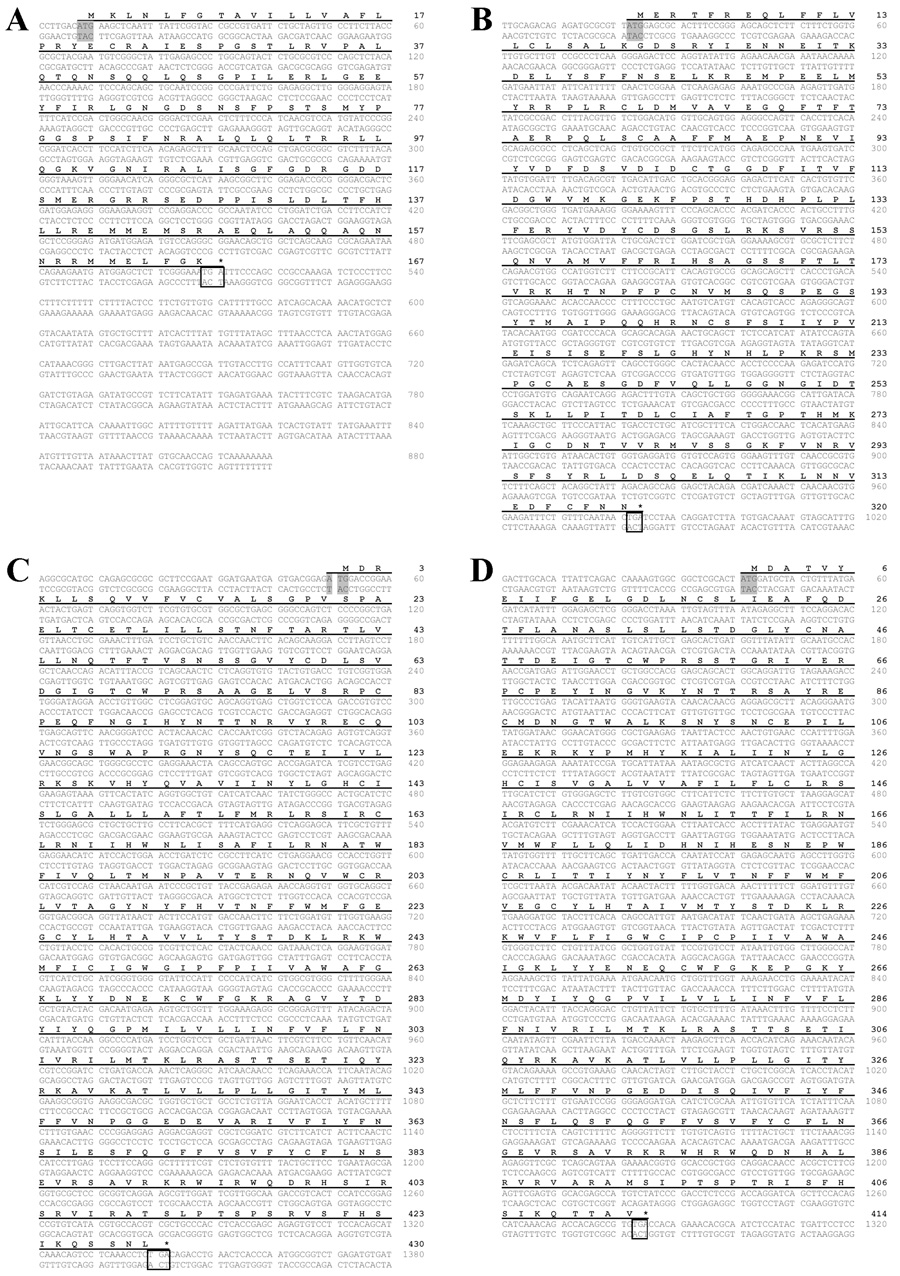
The complete coding sequences of the A. burtoni CRF family members were obtained from A. burtoni brains. The message that encodes the CRF ligand is 880 base pairs (bp) composed of a 7 bp 5′-untranslated region (UTR), a 504 bp open reading frame (ORF) that translates to 167 amino acids, and a 369 bp 3′-UTR containing the polyadenylation signal (Fig. 1A). The CRFBP coding sequence is 963 bp, which translates to 320 amino acids (Fig. 1B). CRF-R1 is 1293 bp long (430 amino acids; Fig. 1C) and the CRF-R2 coding sequence is 1245 bp (414 amino acids; Fig. 1D).
Figure 1.
Nucleotide and deduced amino acid sequences of A. burtoni CRF (A), CRFBP (B), CRF-R1(C), and CRF-R2 (D) cDNA. Start codons are labeled by the shaded box and the stop codons are indicated by the asterisk and box. The predicted peptide is indicated in bold and corresponding nucleotides are underlined.
All nucleotide sequences were converted to deduced amino acid sequences (Bendtsen et al., 2004) and compared with sequences in both nucleotide and protein sequence databases (Altschul et al., 1990). Comparing the amino acid sequences of A. burtoni with those from other species revealed extremely high conservation of CRF (Fig. 2A), CRFBP (Fig. 2B) and CRF-Rs (Fig. 2D & 2E) across vertebrates. The predicted transmembrane (TM) domain regions of the G protein coupled receptors, CRF-R1 and CRF-R2, were identified and are shown (Huising et al., 2004).The two A. burtoni CRF receptors share similar regions close to the N terminal, but not near the C terminal (Fig. 2C). As expected, the sequences of CRF family members were conserved across all vertebrates and A. burtoni fits phylogenetically with other teleosts, including tilapia and flounder (Fig. 3).
Location of CRF, CRFBP, CRF-R1 and CRF-R2 in the periphery tissues
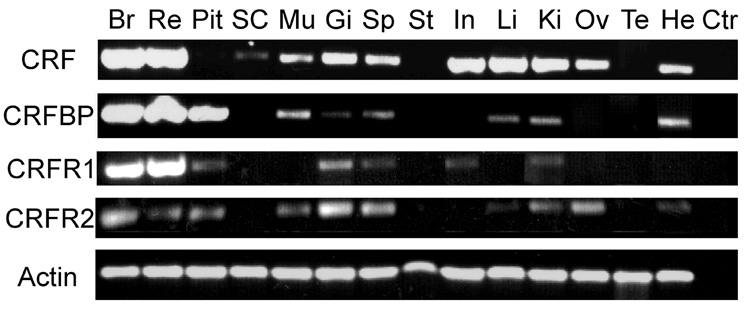
To localize CRF family gene expression in central and peripheral tissues, we used PCR with specific primers to amplify A. burtoni CRF, CRFBP and CRF-Rs from cDNA derived from different tissues. CRF is expressed widely in brain (Br), retina (Re), spinal cord (SP), muscle (Mu), gill (Gi), spleen (Sp), intestine (In), liver (Li), kidney (Ki), ovaries (Ov), and heart (He; Fig. 4). We found CRF mRNA expression in the brain, retina, kidney, intestine, liver, gill, spleen, ovaries, heart, spinal cord, and muscle. The localization of CRF indicates its influence on the digestive, reproductive, immune, and circulatory systems. CRF mRNA could not be detected in pituitary (Pit), stomach (St), or testis (Te). These data suggest that CRF could act as a local modulator in peripheral tissue, but does not exclude the possibility that CRF influences peripheral tissue via the circulatory system.
Figure 4.
Distribution of the CRF, CRFBP, CRF-R1, CRF-R2 transcripts in A. burtoni tissue as shown by PCR. Semi-quantitative gel analysis was performed on representative PCR products of cDNA which was isolated and reverse transcribed from tissues, including brain (Br), retina (Re), pituitary (Pit), spinal cord (SC), muscle (Mu), gill (Gi), spleen (Sp), stomach (St), intestine (In), liver (Li), kidney (Ki), ovaries (Ov), testis (Te) and heart (He). Control (Ctr) column is the PCR reaction without tissue cDNA. The upper rows are the PCR products of specific primers for A. burtoni CRF, CRFBP, CRF-R1 and CRF-R2. The lowest row is the actin PCR product, which is the positive control. Approximately equal amounts of cDNA were added to each PCR reaction.
We found CRFBP gene expression in the brain, retina, and pituitary (Pit;Fig. 4), as well as in the muscle, gill, spleen, liver, kidney, and heart. CRFBP is therefore positioned to influence the digestive, immune, and circulatory systems. CRFBP transcripts were co-expressed with CRF in brain, retina, muscle, gill, spinal cord, liver, kidney, and heart. These data suggest that CRFBP could interact with CRF, not only in the HPI axis, but also in the retina, and peripheral tissues. CRFBP mRNA could not be detected in the spinal cord, stomach, intestine, ovary, or testis.
CRF receptor types are co-expressed in the brain, retina, pituitary, gill, spleen, and kidney. CRF-R2 is also expressed in the muscle, liver, ovaries, and heart and CRF-R1 is expressed in the intestine. Interestingly, we found that CRF-R2 is wider expressed than CRF-R1 is (Fig. 4). CRF-R1 mRNA could not be detected in the spinal cord, muscle, stomach, liver, ovaries, testis, or heart. Likewise, CRF-R2 mRNA could not be detected in the spinal cord, stomach, intestine, or testis.
The abundance of CRF family mRNA as a function of social status
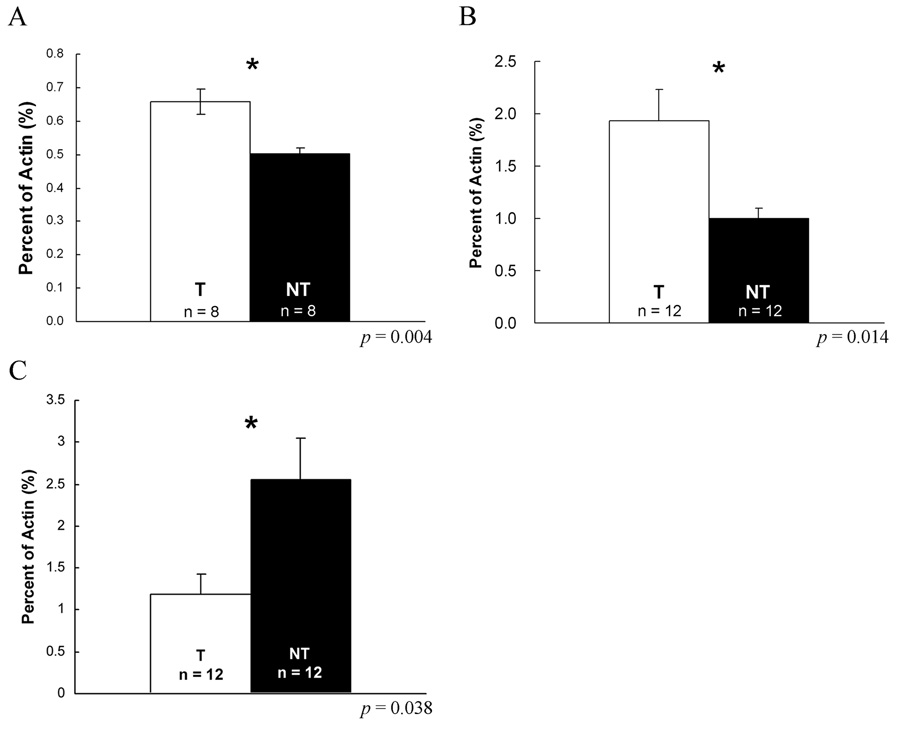
To understand how mRNA abundance of CRF family members changes as a function of social status in the nervous and endocrine systems, we used RT-PCR to compare expression between individuals of different social status in the brain, pituitary and spinal cord. In the brain, we found CRF mRNA was significantly higher in territorial males than non-territorial males (t = 3.447, p = 0.004; Fig. 5A), but that expression levels of CRFBP, CRF-R1 and CRF-R2 were not significantly different (p = 0.37, 0.18 or 0.52; Data not shown). Furthermore, pituitary CRF-R1 was twice as abundant in T as in NT males (Mann-Whitney Rank Sum Test, p = 0.014; Fig. 5B) and pituitary CRFBP was half as much in T as in NT males (Mann-Whitney Rank Sum Test, p = 0.038; Fig. 5C). In the spinal cord, CRF transcript abundance was similar between T and NT males (p = 0.84; Data not shown).
Figure 5.
Expression level of CRF mRNA between T and NT in the brains and pituitaries. mRNA levels are expressed as a percentage of actin mRNA. (A) CRF mRNA fraction of actin mRNA amount in T brains (n = 8; open bar) is significant higher than CRF amount in NT brains (n = 8; closed bar; p = 0.004). (B) CRF-R1 mRNA fraction of actin transcripts in T pituitaries (n = 12; open bar) is twice more than the amount in NT pituitaries (n = 12; closed bar; p = 0.014). (C) CRFBP mRNA fraction of actin transcripts in NT pituitaries (n = 12; open bar) is twice more than the amount in T pituitaries (n = 12; closed bar; p = 0.038). *: p < 0.05.
Discussion
We have identified the coding sequences of CRF, CRFBP, and two types of CRF receptors in A. burtoni and shown that the deduced amino acid sequences are highly conserved across all vertebrates. As expected, the coding sequences of A. burtoni CRF family members are closest to those from other teleost species. The widespread expression of CRF, CRFBP and CRF receptors in the brain, pituitary gland, retina and internal organs, indicates that the CRF system in addition to its well known role in controlling cortisol in HPA/I axis also plays a role in mediating physiological functions in various organs. We also found that long term social stress regulates the HPI axis and that social status regulates the transcription of CRF and CRF-R1, both of which are more highly expressed in the brains and pituitaries of territorial males than in those of non-territorial males.
CRF systems are expressed in central nervous system
CRF, CRFBP, and both CRF receptor types are expressed in the brain and pituitary in A. burtoni and in the other teleosts (Olivereau and Olivereau, 1988; Arai et al., 2001; Pepels et al., 2002; Pepels and Balm, 2004; Alderman and Bernier, 2007; Alderman et al., 2008) suggesting that CRF acts in the central nervous system along with its role in the HPI axis. The present of CRFBP and both types of CRF-Rs, not CRF mRNA in the pituitary gland implicates that the outside CRF neurons, possibly in the hypothalamus innervate the pituitary via CRF receptors and the ligands are regulated by CRFBP. On the other hand, CRF is known to play other roles in the brain, such as regulating appetite (Bernier and Peter, 2001), aggression (Gammie and Stevenson, 2006) and locomotion (Carpenter et al., 2007). The decreased amount of CRF in NT male brains could be also related to its behaviors, such as less aggression, less movement, and more growth than T males do (Fernald and Hirata, 1977b; Hofmann et al., 1999), although it is reasonable to presume that parallel functions are served by the CRF system in fish, which our whole brain data do not provide this level of detail.
Although the CRF protein has been immunocytochemically detected in the retina of mammals, birds, reptiles and teleosts (Skofitsch and Jacobowitz, 1984; Kiyama et al., 1985; Sakanaka et al., 1987; Williamson and Eldred, 1989; Zhang and Yeh, 1990), we present the first evidence that CRF, CRFBP, and the two types of CRF receptors are transcribed directly in a teleost retina. These data suggest that the local CRF system may play a role in modulating visual processing in retina.
We detected CRF mRNA in A. burtoni spinal cord suggesting the existence of the caudal neurosecretory system (CNSS), a unique fish neuroendocrine organ in the most caudal spinal cord which is known to control release of cortisol from the interrenal glands (Lovejoy and Balment, 1999; Winter et al., 2000; Lu et al., 2004; Craig et al., 2005). However, we did not find any difference in spinal cord CRF expressing levels between T and NT males, suggesting that this part of the CRF system may not be involved in regulating stress responses under long term social stress.
CRF systems are expressed in the peripheral tissues
We found CRF, CRFBP, and CRF-R transcripts in multiple organs as has also been reported in mammals, amphibians and teleosts (De Souza et al., 1991; Baigent and Lowry, 2000; Muramatsu et al., 2000; Kimura et al., 2002; Boorse and Denver, 2004; Boorse and Denver, 2006; Huising et al., 2007). Since urocortin, a CRF-related peptide, can interact with CRF-R2 to protect the heart from ischemia in rats and humans (Baigent and Lowry, 2000; Kimura et al., 2002), it is particularly interesting that we also found CRF, CRFBP and CRF-R2 mRNA expressed in skeletal muscle and cardiac muscle in the teleost. This suggests that the cardiac CRF system may mediate the adaptive response of the heart to stress in all vertebrates, from teleosts to mammals (Coste et al., 2002). CRFBP has been found to play a role in other muscles such as tail muscle cells during spontaneous metamorphosis where increased CRFBP expression increases the loss of cells (Seasholtz et al., 2002; Boorse et al., 2006). Although the function of the CRF system in teleost skeletal muscle is still unclear, our findings point that in skeletal muscle CRF could be a local modulator regulated by CRFBP via CRF-R2. CRF, CRFBP and both CRF-Rs are also expressed in gills which play a key role in oxygen exchange and are altered by the stress response in fish (Flik et al., 2006). Increased CRF and CRF-R1 mRNA expression in gill tissue in response to physical stress may contribute to increases in ventilation rate and the oxygen transport capacity of the blood (Wendelaar Bonga, 1997; Mazon et al., 2006).
Endogenously produced CRF exerts its effects through CRF-R1 or CRF-R2 in an autocrine or paracrine manner in the spleen, intestine, liver, kidney and ovary of A. burtoni. As in mammals, the expression of both CRF-Rs in the spleen and the present of CRFBP and CRF-R2 in the liver provide additional evidence for a physiological role of CRF in coordinating the stress response, immune system, and energy homeostatic system (De Souza et al., 1991; Baigent and Lowry, 2000). The CRF system, especially CRF and CRF-R1, in the gastrointestinal tract locally influences intestinal mobility (Sehringer et al., 2004; Tache and Perdue, 2004), while the CRF system expressed in the kidney may be involved in metabolic function or cortisol regulation via the glucocorticoid-secreting interrenal cells (Wendelaar Bonga, 1997; Huising et al., 2007). CRF also plays a role in suppressing reproduction and is involved in follicle maturation in the mammalian ovary (Nappi and Rivest, 1995; Asakura et al., 1997), which is consistent with our finding that the local CRF and CRF-R2 mRNA is expressed in the ovaries. Taken together, our data show that the molecular conservation of the CRF family is likely reflected in conserved functions based on its distribution in A. burtoni
CRF systems are regulated by social stress
During short-term physical stress, CRF mRNA and CRF-R1 mRNA levels increase in the mammalian hypothalamus or pituitary (Imaki et al., 1998; Qahwash et al., 2002; Doyon et al., 2005). In the teleost, CRF and CRFBP expression also increase in the hypothalamus, but CRF-R1 expression decreases in the pituitary during acute stress (Huising et al., 2004). Our study shows that after one month of social stress, the CRF system in both brain and pituitary is down-regulated. CRF mRNA and CRF-R1 mRNA both decrease in the brain and pituitary, respectively, and CRFBP mRNA, which could block CRF effect in the pituitary decreases in NT males.
Fox et al (1997) showed that NT males have high levels of circuiting cortisol over one month.We hypothesize that NT males experience more stress because they are constantly attacked and chased by T males. The high cortisol level can decrease CRF mRNA in the hypothalamus and CRF-R1 mRNA in the pituitary (Imaki et al., 1995; Pozzoli et al., 1996; Bernier et al., 1999; Doyon et al., 2006), and CRF simulation can increase CRF-R1 mRNA in the pituitary (Pozzoli et al., 1996). The less active CRF system in brain-pituitary-interrenal axis during long term stress reflects a homeostatic balance, rather than a rapid stress regulation. In sum, long term social stress decreases CRF system activation in the brain and pituitary, thereby preventing cortisol from overshooting its target value and maintain the cortisol level stable.
Acknowledgments
Supported by Lucille P. Markey Biomedical Research Fellowship to CCC and NINDS J. Javits Award (NS34950) to RDF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderman SL, Bernier NJ. Localization of corticotropin-releasing factor, urotensin I, and CRF-binding protein gene expression in the brain of the zebrafish, Danio rerio. J Comp Neurol. 2007;502:783–793. doi: 10.1002/cne.21332. [DOI] [PubMed] [Google Scholar]
- Alderman SL, Raine JC, Bernier NJ. Distribution and regional stressor-induced regulation of corticotrophin-releasing factor binding protein in rainbow trout (Oncorhynchus mykiss) J Neuroendocrinol. 2008 doi: 10.1111/j.1365-2826.2008.01655.x. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arai M, Assil IQ, Abou-Samra AB. Characterization of three corticotropin-releasing factor receptors in catfish: a novel third receptor is predominantly expressed in pituitary and urophysis. Endocrinology. 2001;142:446–454. doi: 10.1210/endo.142.1.7879. [DOI] [PubMed] [Google Scholar]
- Asakura H, Zwain IH, Yen SS. Expression of genes encoding corticotropin-releasing factor (CRF), type 1 CRF receptor, and CRF-binding protein and localization of the gene products in the human ovary. J Clin Endocrinol Metab. 1997;82:2720–2725. doi: 10.1210/jcem.82.8.4119. [DOI] [PubMed] [Google Scholar]
- Baigent SM, Lowry PJ. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J Mol Endocrinol. 2000;25:43–52. doi: 10.1677/jme.0.0250043. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bass AH, Grober MS. Social and neural modulation of sexual plasticity in teleost fish. Brain Behav Evol. 2001;57:293–300. doi: 10.1159/000047247. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Behan DP, Potter E, Sutton S, Fischer W, Lowry PJ, Vale WW. Corticotropin-releasing factor-binding protein. A putative peripheral and central modulator of the CRF family of neuropeptides. Ann N Y Acad Sci. 1993;697:1–8. doi: 10.1111/j.1749-6632.1993.tb49918.x. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bernier NJ, Lin X, Peter RE. Differential expression of corticotropin-releasing factor (CRF) and urotensin I precursor genes, and evidence of CRF gene expression regulated by cortisol in goldfish brain. Gen Comp Endocrinol. 1999;116:461–477. doi: 10.1006/gcen.1999.7386. [DOI] [PubMed] [Google Scholar]
- Bernier NJ, Peter RE. The hypothalamic-pituitary-interrenal axis and the control of food intake in teleost fish. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:639–644. doi: 10.1016/s1096-4959(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Crespi EJ, Dautzenberg FM, Denver RJ. Urocortins of the South African clawed frog, Xenopus laevis: conservation of structure and function in tetrapod evolution. Endocrinology. 2005;146:4851–4860. doi: 10.1210/en.2005-0497. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Denver RJ. Expression and hypophysiotropic actions of corticotropin-releasing factor in Xenopus laevis. Gen Comp Endocrinol. 2004;137:272–282. doi: 10.1016/j.ygcen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Denver RJ. Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol. 2006;146:9–18. doi: 10.1016/j.ygcen.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Kholdani CA, Seasholtz AF, Denver RJ. Corticotropin-releasing factor is cytoprotective in Xenopus tadpole tail: coordination of ligand, receptor, and binding protein in tail muscle cell survival. Endocrinology. 2006;147:1498–1507. doi: 10.1210/en.2005-1273. [DOI] [PubMed] [Google Scholar]
- Carpenter RE, Watt MJ, Forster GL, Overli O, Bockholt C, Renner KJ, Summers CH. Corticotropin releasing factor induces anxiogenic locomotion in trout and alters serotonergic and dopaminergic activity. Horm Behav. 2007;52:600–611. doi: 10.1016/j.yhbeh.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein "ligand inhibitor" in rat brain. Neuroscience. 2000;101:115–129. doi: 10.1016/s0306-4522(00)00322-5. [DOI] [PubMed] [Google Scholar]
- Coste SC, Quintos RF, Stenzel-Poore MP. Corticotropin-releasing hormone-related peptides and receptors: emergent regulators of cardiovascular adaptations to stress. Trends Cardiovasc Med. 2002;12:176–182. doi: 10.1016/s1050-1738(02)00157-3. [DOI] [PubMed] [Google Scholar]
- Craig PM, Al-Timimi H, Bernier NJ. Differential increase in forebrain and caudal neurosecretory system corticotropin-releasing factor and urotensin I gene expression associated with seawater transfer in rainbow trout. Endocrinology. 2005;146:3851–3860. doi: 10.1210/en.2005-0004. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Grigoriadis DE, Webster EL. Role of brain, pituitary and spleen corticotropin-releasing factor receptors in the stress response. Methods Achiev Exp Pathol. 1991;14:23–44. [PubMed] [Google Scholar]
- Doyon C, Leclair J, Trudeau VL, Moon TW. Corticotropin-releasing factor and neuropeptide Y mRNA levels are modified by glucocorticoids in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. 2006;146:126–135. doi: 10.1016/j.ygcen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Doyon C, Trudeau VL, Moon TW. Stress elevates corticotropin-releasing factor (CRF) and CRF-binding protein mRNA levels in rainbow trout (Oncorhynchus mykiss) J Endocrinol. 2005;186:123–130. doi: 10.1677/joe.1.06142. [DOI] [PubMed] [Google Scholar]
- Fernald RD. How does Behavior Change the Brain? Multiple Methods to Answer Old Questions. Integrative and Comparative Biology. 2003;43:771–779. doi: 10.1093/icb/43.6.771. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni: habitats and co-habitant. Environmental Biology of Fishes. 1977a;2:299–308. [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni: Quantitative behavioural observations. Animal Behaviour. 1977b;25:964–975. [Google Scholar]
- Flik G, Klaren PH, Van den Burg EH, Metz JR, Huising MO. CRF and stress in fish. Gen Comp Endocrinol. 2006;146:36–44. doi: 10.1016/j.ygcen.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Fox HE, White SA, Kao MH, Fernald RD. Stress and dominance in a social fish. J Neurosci. 1997;17:6463–6469. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley NB, Fernald RD. Social control of developmental rate in the African cichlid, Haplochromis burtoni. Zeitschrift für Tierpsychologie. 1982;60:66–82. [Google Scholar]
- Gammie SC, Stevenson SA. Intermale aggression in corticotropin-releasing factor receptor 1 deficient mice. Behav Brain Res. 2006;171:63–69. doi: 10.1016/j.bbr.2006.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata NR, Fernald RD. Non-intentional sound production in a Cichlid fish (Haplochromis burtoni, Gunther) Experientia. 1975;31:299–300. doi: 10.1007/BF01922548. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: consequences for life-history strategies. Proc Natl Acad Sci U S A. 1999;96:14171–14176. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huising MO, Metz JR, De Mazon AF, Verburg-van Kemenade BM, Flik G. Regulation of the stress response in early vertebrates. Ann N Y Acad Sci. 2005;1040:345–347. doi: 10.1196/annals.1327.057. [DOI] [PubMed] [Google Scholar]
- Huising MO, Metz JR, van Schooten C, Taverne-Thiele AJ, Hermsen T, Verburg-van Kemenade BM, Flik G. Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. J Mol Endocrinol. 2004;32:627–648. doi: 10.1677/jme.0.0320627. [DOI] [PubMed] [Google Scholar]
- Huising MO, van der Aa LM, Metz JR, de Fatima Mazon A, Kemenade BM, Flik G. Corticotropin-releasing factor (CRF) and CRF-binding protein expression in and release from the head kidney of common carp: evolutionary conservation of the adrenal CRF system. J Endocrinol. 2007;193:349–357. doi: 10.1677/JOE-07-0070. [DOI] [PubMed] [Google Scholar]
- Imaki T, Naruse M, Harada S, Chikada N, Nakajima K, Yoshimoto T, Demura H. Stress-induced changes of gene expression in the paraventricular nucleus are enhanced in spontaneously hypertensive rats. J Neuroendocrinol. 1998;10:635–643. doi: 10.1046/j.1365-2826.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest. 1995;96:231–238. doi: 10.1172/JCI118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab. 2002;87:340–346. doi: 10.1210/jcem.87.1.8160. [DOI] [PubMed] [Google Scholar]
- Kiyama H, Katayama-Kumoi Y, Kimmel J, Steinbusch H, Powell JF, Smith AD, Tohyama M. Three dimensional analysis of retinal neuropeptides and amine in the chick. Brain Res Bull. 1985;15:155–165. doi: 10.1016/0361-9230(85)90132-7. [DOI] [PubMed] [Google Scholar]
- Krause J, Butlin RK, Peuhkuri N, Pritchard VL. The social organization of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol Rev Camb Philos Soc. 2000;75:477–501. doi: 10.1111/j.1469-185x.2000.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Briefings in Bioinformatics. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- Lu W, Dow L, Gumusgoz S, Brierley MJ, Warne JM, McCrohan CR, Balment RJ, Riccardi D. Coexpression of corticotropin-releasing hormone and urotensin i precursor genes in the caudal neurosecretory system of the euryhaline flounder (Platichthys flesus): a possible shared role in peripheral regulation. Endocrinology. 2004;145:5786–5797. doi: 10.1210/en.2004-0144. [DOI] [PubMed] [Google Scholar]
- Mazon AF, Verburg-van Kemenade BM, Flik G, Huising MO. Corticotropin-releasing hormone-receptor 1 (CRH-R1) and CRH-binding protein (CRH-BP) are expressed in the gills and skin of common carp Cyprinus carpio L. and respond to acute stress and infection. J Exp Biol. 2006;209:510–517. doi: 10.1242/jeb.01973. [DOI] [PubMed] [Google Scholar]
- Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Rivest S. Corticotropin-releasing factor (CRF) and stress-related reproductive failure: the brain as a state of the art or the ovary as a novel clue? J Endocrinol Invest. 1995;18:872–880. doi: 10.1007/BF03349836. [DOI] [PubMed] [Google Scholar]
- Olivereau M, Olivereau J. Localization of CRF-like immunoreactivity in the brain and pituitary of teleost fish. Peptides. 1988;9:13–21. doi: 10.1016/0196-9781(88)90004-6. [DOI] [PubMed] [Google Scholar]
- Pepels PP, Balm PH. Ontogeny of corticotropin-releasing factor and of hypothalamic-pituitary-interrenal axis responsiveness to stress in tilapia (Oreochromis mossambicus; Teleostei) Gen Comp Endocrinol. 2004;139:251–265. doi: 10.1016/j.ygcen.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Pepels PP, Meek J, Wendelaar Bonga SE, Balm PH. Distribution and quantification of corticotropin-releasing hormone (CRH) in the brain of the teleost fish Oreochromis mossambicus (tilapia) J Comp Neurol. 2002;453:247–268. doi: 10.1002/cne.10377. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzoli G, Bilezikjian LM, Perrin MH, Blount AL, Vale WW. Corticotropin-releasing factor (CRF) and glucocorticoids modulate the expression of type 1 CRF receptor messenger ribonucleic acid in rat anterior pituitary cell cultures. Endocrinology. 1996;137:65–71. doi: 10.1210/endo.137.1.8536643. [DOI] [PubMed] [Google Scholar]
- Qahwash IM, Cassar CA, Radcliff RP, Smith GW. Bacterial lipopolysaccharide-induced coordinate downregulation of arginine vasopressin receptor V3 and corticotropin-releasing factor receptor 1 messenger ribonucleic acids in the anterior pituitary of endotoxemic steers. Endocrine. 2002;18:13–20. doi: 10.1385/ENDO:18:1:13. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, McMaster D, Chohan K, Shibasaki T, Stell WK, Lederis K. Urotensin I-like immunoreactivity in amacrine cells of the goldfish retina. Neurosci Lett. 1987;76:96–100. doi: 10.1016/0304-3940(87)90199-6. [DOI] [PubMed] [Google Scholar]
- Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol. 2002;175:89–97. doi: 10.1677/joe.0.1750089. [DOI] [PubMed] [Google Scholar]
- Sehringer B, Zahradnik HP, Simon M, Ziegler R, Noethling C, Schaefer WR. mRNA expression profiles for corticotrophin-releasing hormone, urocortin, CRH-binding protein and CRH receptors in human term gestational tissues determined by real-time quantitative RT-PCR. J Mol Endocrinol. 2004;32:339–348. doi: 10.1677/jme.0.0320339. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Corticotropin releasing factor-like immunoreactive neurons in the rat retina. Brain Res Bull. 1984;12:539–542. doi: 10.1016/0361-9230(84)90169-2. [DOI] [PubMed] [Google Scholar]
- Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16 Suppl 1:137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga SE. The stress response in fish. Physiol Rev. 1997;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878–1891. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. J Exp Biol. 2002;205:2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Eldred WD. Amacrine and ganglion cells with corticotropin-releasing-factor-like immunoreactivity in the turtle retina. J Comp Neurol. 1989;280:424–435. doi: 10.1002/cne.902800308. [DOI] [PubMed] [Google Scholar]
- Winter MJ, Ashworth A, Bond H, Brierley MJ, McCrohan CR, Balment RJ. The caudal neurosecretory system: control and function of a novel neuroendocrine system in fish. Biochem Cell Biol. 2000;78:193–203. [PubMed] [Google Scholar]
- Zhang DR, Yeh HH. Histogenesis of corticotropin releasing factor-like immunoreactive amacrine cells in the rat retina. Brain Res Dev Brain Res. 1990;53:194–199. doi: 10.1016/0165-3806(90)90006-k. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fernald R. Comprehensive Algorithm for Quantitative Real-time Polymerase Chain Reaction. Journal of Computional Biology. 2005;12:1045–1062. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]