This paper show that inter-subspecies hybridization among certain Vigna unguiculata subspecies, occurred during the course of evolution. This has affected several regions of the genome and is interfering with the dependable assessment of sub-species relationships using single (rRNA regions) or multilocus markers.
Abstract
Background and aims
Intra-species hybridization and incompletely homogenized ribosomal RNA repeat units have earlier been reported in 21 accessions of Vigna unguiculata from six subspecies using internal transcribed spacer (ITS) and 5S intergenic spacer (IGS) analyses. However, the relationships among these accessions were not clear from these analyses. We therefore assessed intra-species hybridization in the same set of accessions.
Methodology
Arbitrarily primed polymerase chain reaction (AP-PCR) analysis was carried out using 12 primers. The PCR products were resolved on agarose gels and the DNA fragments were scored manually. Genetic relationships were inferred by TREECON software using unweighted paired group method with arithmetic averages (UPGMA) cluster analysis evaluated by bootstrapping and compared with previous analyses based on ITS and 5S IGS.
Principal results
A total of 202 (86 %) fragments were found to be polymorphic and used for generating a genetic distance matrix. Twenty-one V. unguiculata accessions were grouped into three main clusters. The cultivated subspecies (var. unguiculata) and most of its wild progenitors (var. spontanea) were placed in cluster I along with ssp. pubescens and ssp. stenophylla. Whereas var. spontanea were grouped with ssp. alba and ssp. tenuis accessions in cluster II, ssp. alba and ssp. baoulensis were included in cluster III. Close affinities of ssp. unguiculata, ssp. alba and ssp. tenuis suggested inter-subspecies hybridization.
Conclusions
Multi-locus AP-PCR analysis reveals that intra-species hybridization is prevalent among V. unguiculata subspecies and suggests that grouping of accessions from two different subspecies is not solely due to the similarity in the ITS and 5S IGS regions but also due to other regions of the genome.
Introduction
Vigna unguiculata is an important legume crop belonging to section Catiang in the subgenus Vigna. Vigna comprises a heterogeneous assemblage of 39 species of African origin that are divided into six sections, viz. Vigna, Comosae, Macrodontae, Reticulatae, Liebrechtsia and Catiang (Maxted et al. 2004). Disagreement still exists over the primary centres of domestication of V. unguiculata; however, based on the studies, different centres have been proposed such as Northeast Africa, West Africa, Ethiopia, Asia, etc. (Baudoin and Maréchal 1985; Pasquet 1996, 1999; Garba and Pasquet 1998; Coulibaly et al. 2002; Ba et al. 2004; Timko and Singh 2008). These include both wild and cultivated taxa of perennial and annual species, having high variation in their morphological characterization (Pasquet 1999). Cultivated species differ from wild species in having non-dehiscent pods, larger pods and non-dormant larger seeds (Lush and Evans 1981). Apart from these characters associated with domestication, some traits such as rhomboid leaves and anthocyanin pigmentation of the internodes, the length of the floral peduncle, photosensitivity and morphology of the seeds and pods are the principal variations in the cultivated forms.
Vigna unguiculata has 11 subspecies that differ from one another with respect to various morphological characteristics (Pasquet 1999; Maxted et al. 2004). Five of the subspecies, viz. baoulensis, burundiensis, letouzeyi, aduensis and pawekiae, are perennial, allogamously adapted to humid environments and are mainly recognized by their floral characteristics. Five other subspecies, viz. alba, pubescens, tenuis, stenophylla and dekindtiana, are wild, perennial, autogamous and are recognized by their vegetative traits showing their adaptation to drier and coastal environments. Only one subspecies is annual (ssp. unguiculata), comprising wild (var. spontanea) and cultivated (var. unguiculata) forms. The var. spontanea is a savanna taxon and often grows as a weed in and around cultivated fields. The cultivated forms of V. unguiculata are classified into five cultivar groups (cv.-gr.): Unguiculata, Biflora, Sesquipedalis, Textilis and Melanophthalmus (Pasquet 1998).
The relationship between the subspecies from subgenus Vigna has been analysed in the recent past by using different parameters, including morphological (Padulosi 1993) and biochemical parameters (seed storage proteins: Fotso et al. 1994; isozymes: Panella and Gepts 1992; Vaillancourt et al. 1993; Pasquet 1999, 2000). Molecular marker techniques, viz. AFLP (amplified fragment length polymorphism; Coulibaly et al. 2002), RAPD (randomly amplified polymorphic DNA; Ba et al. 2004), SSR (simple sequence repeat; Asare et al. 2010) and nrRNA (nuclear ribosomal RNA) spacer sequences (Saini 2005; Saini et al. 2008; Saini and Jawali 2009; Vijaykumar et al. 2010, 2011), have also been used to help understand relationships among the wild and cultivated accessions of V. unguiculata. Relationships among subspecies of most of the Vigna species could be analysed with the exception of V. unguiculata. The relationships among taxa belonging to V. unguiculata subspecies inferred by 18S-5.8S-26S rRNA ITS (internal transcribed spacer) did not show subspecies-specific clustering, but an intragenomic ITS variant was detected in an accession belonging to V. unguiculata ssp. tenuis (NI 1637; Vijaykumar et al. 2010). In addition, some accessions of V. unguiculata have very intriguing relationships among the 5S IGS (intergenic spacer) variants in relation to subspecies. This indicates extensive hybridization among the V. unguiculata subspecies, which is generally uncommon in rRNA gene units (Vijaykumar et al. 2011).
Arbitrarily amplified DNA markers are used extensively for reconstructing relationships among various species (Bussel et al. 2005). Randomly amplified polymorphic DNA has the ability to amplify DNA from dispersed polymorphic loci from the genome (Baral and Bosland 2002; Rasul et al. 2007; Wang et al. 2011) and has been used to assess genetic diversity among and between species/subspecies/cultivars of Vigna species (Kaga et al. 1996; Santalla et al. 1998; Lakhanpaul et al. 2000; Ba et al. 2004; Saini et al. 2004; Raturi et al. 2011). There are several studies where the same set of taxa has been analysed by single-locus sequence analysis as well as multi-locus techniques such as RAPD to get more insight into relationships at population, subspecies and species levels (Hess et al. 2000; Ortíz-Dorda et al. 2005; Qi et al. 2008; Wang et al. 2011).
The objective of the present study was to assess the genetic relationships among wild and cultivated V. unguiculata germplasm using a multi-locus marker technique, AP-PCR (arbitrarily primed polymerase chain reaction).
Materials and methods
Plant material
Twenty-one accessions of V. unguiculata, belonging to six subspecies (ssp. unguiculata, ssp. tenuis, ssp. alba, ssp. pubescens, ssp. stenophylla and ssp. baoulensis) obtained from the National Botanic Garden, Belgium (Meise collection), were used for the present study. The details of Vigna accessions used, along with the longitude and latitude coordinates are given in Table 1. The same set of accessions has also been used in previous studies (Vijaykumar et al. 2010, 2011).
Table 1.
Vgna unguiculata accessions along with their country of origin and germplasm accession numbers.
| No. | Vigna accessionsa | Section | Germplasm accession no.b | Country of originc | Longitude and latitude |
|---|---|---|---|---|---|
| 1 | V. unguiculata (L.) Walp. ssp. ung. cv.-gr. Unguiculata | Catiang | NI 479 | DR Congo | 023 57 E, 06 45 S |
| 2 | V. unguiculata (L.) Walp. ssp. ung. cv.-gr. Sesquipedalis | Catiang | NI 269 | China | NA |
| 3 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea# | Catiang | NI 1405 | Tanzania | 039 13 E, 06 00 S |
| 4 | V. unguiculata (L.) Walp. ssp. ung. cv.-gr. Textilis | Catiang | NI 816 | Togo | NA |
| 5 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea# | Catiang | NI 1639 | Namibia | 021 40 E, 18 10 S |
| 6 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea | Catiang | NI 1668 | Kenya | 040 54 E, 02 17 S |
| 7 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea | Catiang | NI 1687 | Yemen | 044 00 E, 13 58 N |
| 8 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea | Catiang | NI 1475 | Malawi | 034 07 E, 10 35 S |
| 9 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea | Catiang | NI 1384 | Botswana | 027 25 E, 21 00 S |
| 10 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea | Catiang | NI 945 | Niger | 003 26 E, 12 23 N |
| 11 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea | Catiang | NI 319 | D.R. Congo | 023 57 E, 06 45 S |
| 12 | V. unguiculata (L.) Walp. ssp. ung. var. spontanea | Catiang | NI 1507 | Zimbabwe | 032 05 E, 19 57 S |
| 13 | V. unguiculata (L.) Walp. ssp. tenuis | Catiang | NI 1712 | South. Africa | 030 50 E, 30 10 S |
| 14 | V. unguiculata (L.) Walp. ssp. tenuis | Catiang | NI 1636 | Zambia | 027 35 E, 13 38 S |
| 15 | V. unguiculata (L.) Walp. ssp. tenuis* | Catiang | NI 1637 | Mozambique | 032 50 E, 26 03 S |
| 16 | V. unguiculata (L.) Walp. ssp. alba | Catiang | NI 1652 | Angola | 013 15 E, 09 50 S |
| 17 | V. unguiculata (L.) Walp. alba | Catiang | NI 1388 | Congo | 011 51 E, 04 43 S |
| 18 | V. unguiculata (L.) Walp. ssp. baoulensis | Catiang | NI 1651 | Ivory Coast | 005 50 W, 06 43 N |
| 19 | V. unguiculata (L.) Walp. ssp. stenophylla | Catiang | NI 1478 | Botswana | 026 14 E, 23 55 S |
| 20 | V. unguiculata (L.) Walp. ssp. pubescens | Catiang | NI 856 | Tanzania | 038 23 E, 06 43 S |
| 21 | V. unguiculata (L.) Walp. ssp. pubescens | Catiang | NI 989 | Kenya | 039 46 E, 03 55 S |
NA, information not available.
Hashes indicate Vigna accessions harbouring single 5S IGS (Vijaykumar et al. 2011).
An asterisk indicates Vigna accession harbouring intra-genomic ITS variant (Vijaykumar et al. 2010).
aList of Vigna accessions used. ssp., subspecies; ung., unguiculata; var., variety; cv.-gr., cultivar-group.
bAccession numbers of the National Botanic Garden, Meise, Belgium.
cDR Congo, Democratic Republic of Congo.
DNA isolation, PCR amplification and agarose gel electrophoresis
Total DNA was isolated, purified and quantitated by procedures described in Vijaykumar et al. (2010). Arbitrarily primed PCR was carried out according to Saini et al. (2004) using 12 primers 18–23 bases in length (Table 2). The PCR mixture (25 μL) contained 1× reaction buffer (10 mM Tris-HCl pH 9.0, 2 mM MgCl2) from Bangalore Genei Pvt Ltd (Bangalore, India), 0.2 μM primer (BRIT, Mumbai, India), 0.2 mM each dNTP (Roche Applied Science, Mannheim, Germany), 1.0 unit of Taq DNA polymerase (Bangalore Genei Pvt Ltd) and 100 ng of template genomic DNA. Amplified products were separated on a 2 % agarose (Sigma, Saint Louis, MO, USA) gel in 1× TBE at a constant voltage of 8 V/cm, stained with ethidium bromide according to Sambrook and Russell (2001) and photographed under UV light on a Gel-doc system from Syngene, Inc. (Cambridge, UK). Sizes of the PCR products were estimated by using GeneTools software of the Gel-doc system and comparing with the DNA size standards.
Table 2.
Characteristics of primers used for AP-PCR analysis along with the number of total and polymorphic bands obtained.
| No. | Primer | Sequence | Length (bases) | Tm (°C) | G + C (%) | Total no. of bands | No. of polymorphic bands |
|---|---|---|---|---|---|---|---|
| 1 | SS 1.2 | CTCGTCTGAGATCGGAGG | 18 | 68 | 60 | 21 | 18 |
| 2 | SS 5.1 | GGAAGATGGTCATGGTGG | 18 | 63 | 50 | 17 | 15 |
| 3 | SS 9.1 | GTACAGGACAAGATGCTT | 18 | 58 | 45 | 23 | 18 |
| 4 | SS 13.2 | CAGGATGAGAGTTGGTTGGTAG | 22 | 69 | 50 | 14 | 11 |
| 5 | SS 19.1 | GACATCTCTAGTGCACACAT | 20 | 60 | 45 | 25 | 21 |
| 6 | SS 24.1 | TTTAATATCACCACCACACC | 20 | 50 | 46 | 13 | 12 |
| 7 | VM 3.2 | GAGCCAGGGCACAGGTAGT | 19 | 55 | 63 | 18 | 16 |
| 8 | VM 5.1 | AGCGACGGCAACAACGAT | 18 | 50 | 56 | 25 | 23 |
| 9 | VM 11.1 | CGGGAATTAACGGAGTCACC | 20 | 54 | 55 | 21 | 17 |
| 10 | VM 13.2 | GTCCCCTCCCTCCCACTG | 18 | 57 | 72 | 22 | 20 |
| 11 | VM 19.1 | TATTCATGCGCCGTGACACTA | 21 | 52 | 48 | 17 | 15 |
| 12 | VM 71.1 | TCGTGGCAGAGAATCAAAGACAC | 23 | 55 | 48 | 17 | 16 |
Data analysis
The AP-PCR was carried out twice and profiles were analysed manually; the amplified products were scored as present (1) or absent (0) for each primer–accession combination. Molecular data were used to compute genetic distances (Nei and Li 1979) within and between V. unguiculata subspecies using TREECON (version 1.3b; Van de Peer and De Watcher 1994; http://bioc-www.uia.ac.be/u/yvdp/treeconw.html) and a dendrogram was generated using the unweighted paired group method with arithmetic averages (UPGMA). Statistical analysis was carried out by the bootstrap method (Felsenstein 1985).
Results
DNA fingerprinting of V. unguiculata accessions
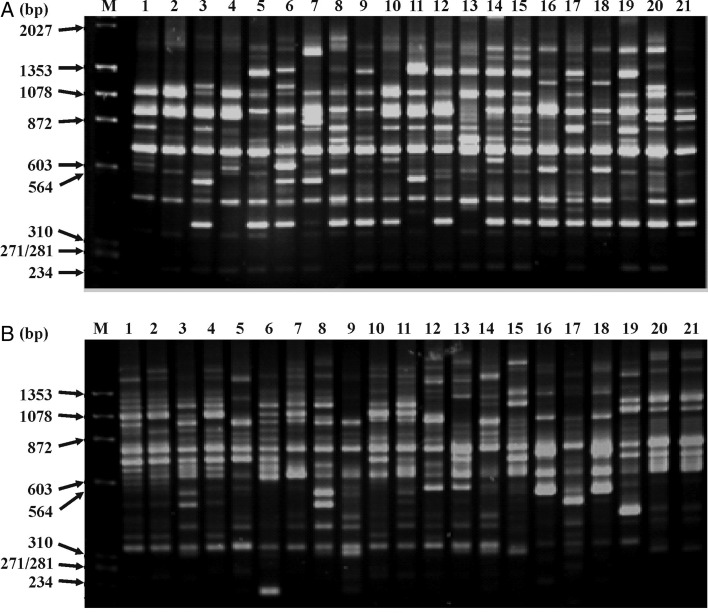
Initially, several long primers were screened for their utility in detecting polymorphism in two V. unguiculata accessions, viz. NI 479 and NI 1405 and 12 primers were selected for further studies. These primers detected intra-specific variations generating scorable amplicons, reproducible patterns and generated 233 markers in the range of 100–3500 bp. Among these, 202 markers were polymorphic, amounting to 86.6 % polymorphism. Arbitrarily primedPCR amplification in the 21 V. unguiculata accessions using primers SS 9.1 and SS 5.1 is shown in Fig. 1A and B. The number of polymorphic bands obtained ranged from 11 (SS 13.2) to 23 (VM 5.1) (Table 2). On average, each AP-PCR primer generated ∼19.4 scorable fragments and 16.8 polymorphic fragments.
Fig. 1.
AP-PCR profiles of V. unguiculata genotypes obtained with primers SS9.1 (A) and SS5.1 (B). Lane 1: V. u. ssp. unguiculata cv.-gr. Unguiculata (NI 479); Lane 2: V. u. ssp. unguiculata cv.-gr. Sesquipedalis (NI 269); Lane 3: V. u. ssp. unguiculata var. spontanea (NI 1405); Lane 4: V. u. ssp. unguiculata cv.-gr. Textilis (NI 816); Lane 5: V. u. ssp. unguiculata var. spontanea (NI 1639); Lane 6: V. u. ssp. unguiculata var. spontanea (NI 1668); Lane 7: V. u. ssp. unguiculata var. spontanea (NI 1687); Lane 8: V. u. ssp. unguiculata var. spontanea (NI 1475); Lane 9: V. u. ssp. unguiculata var. spontanea (NI 1384); Lane 10: V. u. ssp. unguiculata var. spontanea (NI 945); Lane 11: V. u. ssp. unguiculata var. spontanea (NI 319); Lane 12: V. u. ssp. unguiculata var. spontanea (NI 1507); Lane 13: V. u. ssp. tenuis (NI 1712); Lane 14: V. u. ssp. tenuis (NI 1636); Lane 15: V. u. ssp. tenuis (NI 1637); Lane 16: V. u. ssp. alba (NI 1652); Lane 17: V. u. ssp. alba (NI 1388); Lane 18: V. u. ssp. baoulensis (NI 1651); Lane 19: V. u. ssp. stenophylla (NI 1478); Lane 20: V. u. ssp. pubescens (NI 856); Lane 21: V. u. ssp. pubescens (NI 989); Lane M contains a mixture of ϕX174 DNA (HaeIII digest) and λ (HindIII digest).
Cluster analysis
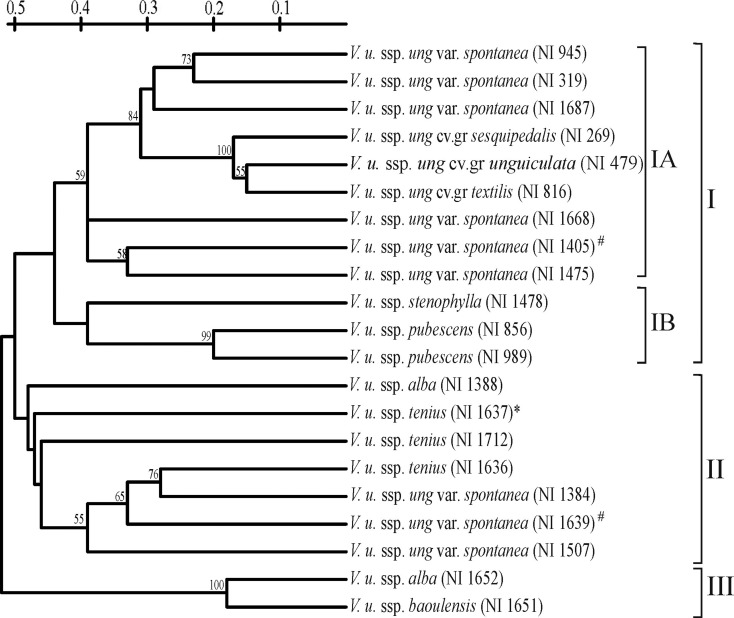
The dendrogram obtained from the combined data of 12 primers delineated all the V. unguiculata accessions into three main clusters with low bootstrap values (Fig. 2). Cluster I was further divided into two subclusters (IA and IB). Subcluster IA comprised accessions belonging to V. unguiculata ssp. unguiculata. This subcluster included three cv.-gr. from V. unguiculata ssp. unguiculata, viz. cv.-gr. Unguiculata, cv.-gr. Sesquipedalis and cv.-gr. Textilis, along with six accessions of V. unguiculata ssp. unguiculata var. spontanea, the wild progenitors of cultivated V. unguiculata (V. unguiculata ssp. unguiculata var. unguiculata). The evolved cultivars, viz. cv.-gr. Unguiculata, cv.-gr. Sesquipedalis and cv.-gr. Textilis, grouped together with high (100 %) bootstrap values. Six out of the nine V. unguiculata ssp. unguiculata var. spontanea accessions were placed in subcluster IA and they clustered closely with the cultivated V. unguiculata accessions supported by high bootstrap values (84 and 59 %). In subcluster IA, the three var. spontanea from Eastern African countries, viz. NI 1686 (Kenya), NI 1405 (Tanzania) and NI 1475 (Malawi), were relatively divergent. Subcluster IB in cluster I included accessions belonging to two different subspecies of V. unguiculata: ssp. pubescens (NI 856, NI 989) and ssp. stenophylla (NI 1478). The clustering of the accessions of both these subspecies was supported by high (99 %) bootstrap values.
Fig. 2.
UPGMA cluster analysis of 21 V. unguiculata genotypes based on the AP-PCR marker data. Numbers at nodes indicate bootstrap values (in %) for a 1000-replicate analysis. An asterisk indicates the V. u. ssp. tenuis accession that showed intra-genomic ITS (Vijaykumar et al. 2010) and hashes indicate the V. u. ssp. unguiculata var. spontanea accessions that showed single type 5S IGS (Vijaykumar et al. 2011).
Cluster II comprised seven taxa belonging to three subspecies, viz. V. unguiculata ssp. unguiculata, V. unguiculata ssp. tenuis and V. unguiculata ssp. baoulensis. Three V. unguiculata ssp. unguiculata var. spontanea accessions from South African countries, viz. NI 1507 (Zimbabwe), NI 1639 (Namibia) and NI 1384 (Botswana), in cluster II were found to be divergent from the six V. unguiculata ssp. unguiculata var. spontanea accessions placed in cluster I (Fig. 2). Cluster III included only two accessions belonging to two different subspecies: ssp. alba (NI 1652, Angola) and ssp. baoulensis (NI 1651, Ivory Coast).
Discussion
Multi-locus marker techniques such as RAPD, AP-PCR, inter simple sequence repeat, AFLP, etc. have been extensively used for a variety of genetic analyses, including investigating genetic relationships; they can also provide valuable information on phylogenetics and systematics, if used appropriately (Bussell et al. 2005). These multi-locus markers have also been extensively used for analysis of species of Asian and African origin from the genus Vigna (Li et al. 2001; Coulibaly et al. 2002; Ba et al. 2004; Dikshit et al. 2005; Ruchi et al. 2009; Raturi et al. 2011). In the present study, the AP-PCR profiles of 21 taxa belonging to different subspecies of V. unguiculata showed moderate divergence and were used to determine their genetic relationships. The same set of taxa was previously analysed using ITS (Vijaykumar et al. 2010) and 5S IGS (Vijaykumar et al. 2011) regions. Hence, the phylogenetic trees obtained from these analyses were compared to understand how some of the taxa have evolved.
In general, the analyses of species/subspecies using multi-locus genome-wide marker analyses show clustering at the species as well subspecies level. On the contrary, the dendrogram obtained from the AP-PCR analyses of the taxa belonging to V. unguiculata did not show clear-cut grouping of taxa at the subspecies level. Out of the 12 taxa belonging to V. unguiculata ssp. unguiculata, nine were placed in cluster 1A, and the other three were grouped with other subspecies, albeit at low bootstrap values. Among the other subspecies (ssp. pubescens, ssp. tenuis and ssp. alba) where more than one taxa was analysed, two accessions of ssp. pubescens were placed together (in subcluster IB) along with ssp. stenophylla. Accessions of both ssp. tenuis and ssp. alba also showed divergence and did not cluster together. Accession NI 1388 of ssp. alba was placed in cluster II along with V. unguiculata ssp. tenuis and V. unguiculata var. spontanea, while NI 1652 was placed in cluster III with ssp. baoulensis. The three accessions (NI 1636, NI 1637 and NI 1712) of ssp. tenuis, though placed in cluster II, were highly divergent. Accession NI 1637, which harboured intra-genomic ITS variant (Vijaykumar et al. 2010) was placed in cluster II and showed more affinity towards ssp. unguiculata var. spontanea (NI 1384) compared with the other two ssp. tenuis accessions (NI 1637, NI 1712) (Fig. 2). Our results are also in agreement with previous reports (Pasquet 1999, 2000; Coulibaly et al. 2002).
The present AP-PCR analysis clearly delineated cultivated V. unguiculata accessions from wild types. The present analyses placed the majority of the ssp. unguiculata var. spontanea accessions with the cultivated V. unguiculata in cluster I. However, three ssp. unguiculata var. spontanea accessions, viz., NI 1384, NI 1507 and NI 1639, were highly divergent and grouped with ssp. tenuis and ssp. alba in cluster II. Two of these accessions, i.e. NI 1384 and NI 1639 (=SP 160), were part of the var. spontanea BWA group (includes accessions from Botswana) in Pasquet (1999); however, NI 1507 (=MT 76) was not, which reinforces the hybridization origin of this group of var. spontanea accessions from southern Africa. A close relationship between NI1639 and NI 1384 was also observed based on the ITS analysis (Vijaykumar et al. 2010). The clustering of var. spontanea with ssp. tenuis and ssp. alba may be the result of hybridization between these taxa. These results confirm that, in general, the genomes of ssp. unguiculata var. spontanea, ssp. tenuis and ssp. alba have indeed undergone hybridization, and this has led to their convergence with accessions of other subspecies. Accession NI 1637 of ssp. tenuis was grouped with two other accessions of ssp. tenuis (NI 1712, NI 1636); however, this accession harboured intra-individual ITS and 5S IGS variants (Vijaykumar et al. 2010, 2011). In the case of ITS analysis, the two variants were found to have originated as a result of hybridization between ssp. tenuis and ssp. pubescens. However, AP-PCR analysis revealed that the major proportion of the genome of the NI 1637 accession is close to ssp. tenuis. This indicates that although rRNA genes (18S-5.8S-26S rRNA and 5S rRNA) have not been homogenized subsequent to subspecies hybridization in NI 1637 (Vijaykumar et al. 2010, 2011), the genome is homogenized towards ssp. tenuis. The present analysis grouped one accession each from ssp. baoulensis (NI 1651) and ssp. alba (NI 1652) in cluster III (Fig. 2). However, this accession of ssp. alba, based on ITS, was found to be close to ssp. unguiculata var. spontanea (NI 1384 and NI 1639). This is in accordance with previous studies based on AFLP (Coulibaly et al. 2002) and isozymes (Pasquet 1999). The three ssp. unguiculata var. spontanea accessions, viz. NI 1384, NI 1507 and NI 1639, also appear to be a product of hybridization between ssp. unguiculata and ssp. tenuis.
The accession NI 1478 belonging to ssp. stenophylla showed close affinity to NI 1475 from ssp. unguiculata on the basis of rRNA gene analyses (Vijaykumar et al. 2010, 2011). Under the present investigation it grouped with ssp. pubescens, suggesting that NI1478 may have undergone hybridization with ssp. unguiculata and ssp. pubescens, during the course of evolution. However, more detailed studies would be required to prove these aspects, as perfect assessment of positions of distant accessions may not be possible solely on the basis of AP-PCR marker analysis.
In general, different taxa of the same species/genus are analysed by different approaches and the results inferred from such studies are often not directly comparable. If done appropriately, studies on the same set of taxa using a multiple marker system help in understanding the genetic relationships at subspecies/population level, along with evolutionary/phylogenetic relationships at species level, and also allow us to decipher how different loci vis-á-vis genome have evolved. Furthermore, multi-locus markers are also useful when single-locus regions fail to resolve phylogenetic/genetic relationships (Després et al. 2003). Despite these advantages, studies on the same set of taxa using multiple markers are not often carried out and only a few molecular phylogenetic studies have been reported where the rRNA gene sequences and multi-locus marker analysis have been performed using the same set of taxa (Blattner et al. 2001). Arbitrarily primed-PCR techniques have also been explored to study the genetic relationships among species in combination with sequence-based analysis (Hess et al. 2000; Jorgensen et al. 2003; Ortíz-Dorda et al. 2005; Qi et al. 2008; Wang et al. 2011). The same set of accessions has been used for assessment of phylogenetic relationships using both multi-locus and single-locus markers in different species such as Olea europaea complex (Hess et al. 2000), Trollius species complex (Després et al. 2003), Atriplex halimus (Ortíz-Dorda et al. 2005), genus Rehmannia (Qi et al. 2008), genus Zea (Wang et al. 2011) and Vigna (Raturi et al. 2011).
In the present investigation we have used the same set of V. unguiculata accessions and the results were very useful in understanding the evolution of some of the species/taxa analysed. Our previous studies (Vijaykumar et al. 2010, 2011) suggest that intra-species hybridization has affected the rRNA gene loci during the course of evolution. Consequently, it was difficult to infer phylogenetic relationships among certain V. unguiculata subspecies. Hence, the present analysis was carried out to infer the relationships among the same set of taxa using information generated from multi-locus markers that can analyse many regions of the genome. The results presented in this study suggest that regions of the V. unguiculata genome other than ITS and 5S IGS also show evidence of inter-subspecies hybridization.
Our previous study on the analysis of Vigna species from subgenus Vigna (African Vigna) using the 18S-5.8S-26S nrDNA ITS region gave evidence of hybridization and slow molecular drive of the repeat units. An accession of ssp. tenuis harboured an additional ITS variant (more similar to ssp. pubescens); however, it was not active transcriptionally. Furthermore, certain accessions of ssp. unguiculata, ssp. tenuis and ssp. alba showed close relationships with other subspecies. This suggested that these accessions have also undergone hybridization but subsequently the ITS sequences have homogenized towards the other subspecies (Vijaykumar et al. 2010). Analysis of the same set of taxa by 5S IGS region also suggested hybridization among several V. unguiculata subspecies (Vijaykumar et al. 2011). The 5S IGS sequences were homogenized to a much lower extent as compared with the ITS sequences, although the number of 5S loci in V. unguiculata were reported to be lower than 18S-5.8S-25S genes (Galasso et al. 1995).
The evidence for inter-subspecies hybridization among the V. unguiculata accessions came from the fact that despite using a large number of markers, relationships among accessions of certain subspecies (ssp. unguiculata var. spontanea, ssp. alba and ssp. tenuis) in cluster II were not supported by high bootstrap values. The low bootstrap values have been attributed to very frequent intercrossing among the taxa analysed (Barkley et al. 2006; Chao et al. 2007; Chapuis et al. 2008). Our results show that subspecies relationships within V. unguiculata cannot be inferred and this could be due to intra-subspecies hybridization. Similar findings were recorded in our previous studies using rRNA gene analysis (Vijaykumar et al. 2011).
The results from the present analyses confirm that the V. unguiculata subspecies have undergone inter-subspecies hybridization and introgression, with most taxa maintaining the variant 5S IGS sequences while very few maintain variant ITS sequences. Hence, irrespective of the actual multi-locus marker analysis, the relationships among the subspecies could not be clearly inferred.
Conclusions and forward look
The present analysis of V. unguiculata subspecies using AP-PCR markers clearly shows that extensive hybridization has occurred among certain subspecies. The same set of taxa when previously analysed by ITS and 5S IGS sequences had shown evidence of hybridization and incomplete homogenization of rRNA repeat units. The present study based on multi-locus analysis further substantiates the previous findings on intra-subspecies hybridization events among certain V. unguiculata subspecies. The present study thus advocates further in-depth analysis of such interactions among the V. unguiculata subspecies, along with eco-geographical parameters.
Contributions by the authors
The project was conceived and planned by N.J. The major experimental part of the study was carried out by A.V. as part of her PhD thesis. Some parts of the experiments and data analysis were done by A.S.
Conflict of interest statement
None declared.
Acknowledgements
We thank Dr Thierry Vanderborght, Seed Bank Manager, National Botanic Garden, Belgium, for providing plant materials used in this study. We thank Dr S. K. Apte, Bhabha Atomic Research Centre, Mumbai, India, for encouragement and support.
References
- Asare TA, Gowda BS, Galyuon IKA, Aboagye LL, Takrama JF, Timko MP. Assessment of genetic diversity in cowpea (Vigna unguiculata L. Walp.) germplasm from Ghana using simple sequence repeat markers. Plant Genetic Resources. 2010;8:142–150. [Google Scholar]
- Ba FS, Pasquet RS, Gepts P. Genetic diversity in cowpea [Vigna unguiculata (L.) Walp.] as revealed by RAPD markers. Genetic Resources and Crop Evolution. 2004;51:539–550. [Google Scholar]
- Baral J, Bosland PW. Genetic diversity of a Capsicum germplasm collection from Nepal as determined by randomly amplified polymorphic DNA markers. Journal of the American Society for Horticultural Science. 2002;127:316–324. [Google Scholar]
- Barkley NL, Roose ML, Krueger R, Frederici CT. Assessing genetic diversity and population structure in a citrus germplasm collection utilizing simple sequence repeat markers (SSRs) Theoretical and Applied Genetics. 2006;112:1519–1531. doi: 10.1007/s00122-006-0255-9. [DOI] [PubMed] [Google Scholar]
- Baudoin JP, Maréchal R. Genetic diversity in Vigna. In: Singh SR, Rachie KO, editors. Cowpea research, production, and utilization. New York: John Wiley & Sons; 1985. pp. 3–11. [Google Scholar]
- Blattner FR, Weising K, Banfer G, Maschwitz U, Fiala B. Molecular analysis of phylogenetic relationships among myrmecophytic Macaranga species (Euphorbiaceae) Molecular Phylogenetics and Evolution. 2001;19:331–344. doi: 10.1006/mpev.2001.0941. [DOI] [PubMed] [Google Scholar]
- Bussell JD, Waycott M, Chappill JA. Arbitrarily amplified DNA markers as characters for phylogenetic inference. Perspectives in Plant Ecology, Evolution and Systematics. 2005;7:3–26. [Google Scholar]
- Chao S, Zhang W, Dubcovsky J, Sorrells M. Evaluation of genetic diversity and genome-wide linkage disequilibrium among U.S. wheat (Triticum aestivum L.) germplasm representing different market classes. Crop Science. 2007;47:1018–1030. [Google Scholar]
- Chapuis M-P, Lecoq M, Michalkis Y, Loiseau G, Sword A, Piry S, Estoup A. Do outbreaks affect genetic population structure? A worldwide survey in Locusta migratoria, a pest plagued by microsatellite null alleles. Molecular Ecology. 2008;17:3640–3653. doi: 10.1111/j.1365-294X.2008.03869.x. [DOI] [PubMed] [Google Scholar]
- Coulibaly S, Pasquet RS, Papa R, Gepts P. AFLP analysis of the phenetic organization and genetic diversity of Vigna unguiculata L. Walp. reveals extensive gene flow between wild and cultivated types. Theoretical and Applied Genetics. 2002;104:358–366. doi: 10.1007/s001220100740. [DOI] [PubMed] [Google Scholar]
- Després L, Gielly L, Redoutet B, Taberlet P. Using AFLP to resolve phylogenetic relationships in a morphologically diversified plant species complex when nuclear and chloroplast sequences fail to reveal variability. Molecular Phylogenetics and Evolution. 2003;27:185–196. doi: 10.1016/s1055-7903(02)00445-1. [DOI] [PubMed] [Google Scholar]
- Dikshit HK, Jhang T, Singh NK, Koundal KR, Bansal KC, Chandra N, Tickoo JL, Sharma TR. Genetic differentiation of Vigna species by RAPD, URP and SSR markers. Biologia Plantarum. 2005;51:451–457. [Google Scholar]
- Felsenstein J. Confidence limits of phylogenies: an approach using bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fotso M, Azanza JL, Pasquet R, Raymond J. Molecular heterogeneity of cowpea (Vigna unguiculata, Fabaceae) seed storage proteins. Plant Systematics and Evolution. 1994;191:39–56. [Google Scholar]
- Galasso I, Schmidt T, Oignone D, Heslop-Harrison JS. The molecular genetics of Vigna unguiculata (L.) Walp: the physical organization of 18S-5.8S-25S rRNA genes, 5S rRNA genes, telomere-like sequences and family of centromeric repetitive DNA sequences. Theoretical and Applied Genetics. 1995;91:928–935. doi: 10.1007/BF00223902. [DOI] [PubMed] [Google Scholar]
- Garba M, Pasquet RS. Isozyme diversity in Vigna vexillata (L.) A. Rich (Fabaceae) complex. South African Journal of Botany. 1998;64:163–175. [Google Scholar]
- Hess J, Kadereit JW, Vargas P. The colonization history of Olea europaea L. in Macaronesia based on internal transcribed spacer 1 (ITS-1) sequences, randomly amplified polymorphic DNAs (RAPD), and intersimple sequence repeats (ISSR) Molecular Ecology. 2000;9:857–868. doi: 10.1046/j.1365-294x.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- Kaga A, Tomooka N, Egawa Y, Hosaka K, Kamijima O. Species relationship in the subgenus Ceratotropis (genus Vigna) as revealed by RAPD analysis. Euphytica. 1996;88:17–24. [Google Scholar]
- Lakhanpaul S, Chadha S, Bhat KV. Random amplified polymorphic DNA (RAPD) analysis in Indian mungbean (Vigna radiata (L.) Wilczek) cultivars. Genetica. 2000;109:227–234. doi: 10.1023/a:1017511918528. [DOI] [PubMed] [Google Scholar]
- Li C-D, Fatokun CA, Ubi B, Singh BB, Scoles GJ. Determining genetic similarities and relationships among cowpea breeding lines and cultivars by microsatellite markers. Crop Science. 2001;41:189–197. [Google Scholar]
- Lush WM, Evans LT. The domestications and improvement of cowpeas (Vigna unguiculata (L.)Walp.) Euphytica. 1981;30:579–87. [Google Scholar]
- Maxted N, Mabuza-Diamini P, Moss H, Padulosi S, Jarvis A, Guarino L. An ecogeographic study: African Vigna. Rome, Italy: International Plant Genetic Resources Institute; 2004. [Google Scholar]
- Nei M, Li W. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences of the USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortíz-Dorda J, Martínez-Mora C, Correal E, Simón B, Cenis JL. Genetic structure of Atriplex halimus populations in Mediterranean basin. Annals of Botany. 2005;95:827–834. doi: 10.1093/aob/mci086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padulosi S. Genetic diversity, taxonomy and ecogeographic survey of the wild relatives of cowpea [Vigna unguiculata (L.) Walpers] Louvain la Neuve, Belgium: Thèse Université catholique; 1993. [Google Scholar]
- Panella L, Gepts P. Genetic relationships within V. unguiculata (L.) Walp. based on isozymes analysis. Genetic Resources and Crop Evolution. 1992;39:71–88. [Google Scholar]
- Pasquet RS. Cultivated cowpea (Vigna unguiculata) evolution. In: Pickersgill B, Lock JM, editors. Advances in legume systematics 8: Legumes of economic importance. Kew: Royal Botanic Gardens; 1996. pp. 101–108. [Google Scholar]
- Pasquet RS. Morphological study of cultivated cowpea Vigna unguiculata (L) Walp: importance of ovule number and definition of cv. gr. Melanophthalmus. Agronomie. 1998;18:61–70. [Google Scholar]
- Pasquet RS. Genetic relationships among subspecies of Vigna unguiculata (L.) Walp based on allozyme variation. Theoretical and Applied Genetics. 1999;98:1104–1119. [Google Scholar]
- Pasquet RS. Allozyme diversity of cultivated cowpea V. unguiculata (L.) Walp. Theoretical and Applied Genetics. 2000;101:211–219. [Google Scholar]
- Qi J, Li X, Song J, Eneji E, Ma X. Genetic relationships among Rehmannia glutinosa cultivars and varieties. Planta Medica. 2008;74:1846–1852. doi: 10.1055/s-0028-1088330. [DOI] [PubMed] [Google Scholar]
- Rasul MG, Hirammatsu M, Okubo H. Genetic relatedness (diversity) and cultivar identification by randomly amplified polymorphic DNA (RAPD) markers in teasle gourd (Momordica dioica Roxb.) Scientia Horticulturae. 2007;111:271–279. [Google Scholar]
- Raturi A, Singh SK, Sharma V, Pathak R. Molecular characterization of Vigna radiata (L.) Wilczek genotypes based on nuclear ribosomal DNA and RAPD polymorphism. Molecular Biology Reporter. 2011;29:315–323. doi: 10.1007/s11033-011-0996-7. 10.1007/s11033–011–0996–7. [DOI] [PubMed] [Google Scholar]
- Ruchi V, Bhat KV, Lakhanpaul S. Analysis of population substructure, genetic differentiation and phylogenetic relationships among selected Asiatic Vigna species. Genetic Resources and Crop Evolution. 2009;56:783–795. [Google Scholar]
- Saini A. India.: University of Mumbai; 2005. Ribosomal RNA gene diversity within and between mungbean and other Asiatic Vigna species. PhD Thesis. [Google Scholar]
- Saini A, Jawali N. Molecular evolution of 5S rDNA region in Vigna subgenus Ceratotropis and its phylogenetic implications. Plant Systematics and Evolution. 2009;280:187–206. [Google Scholar]
- Saini A, Reddy KS, Jawali N. Evaluation of long primers for APPCR analysis of mungbean [Vigna radiata (L.) Wilczek]: genetic relationship and fingerprinting of some genotypes. Indian Journal of Biotechnology. 2004;3:511–518. [Google Scholar]
- Saini A, Reddy KS, Jawali N. Intra-individual and intra-species heterogeneity in nuclear rDNA ITS region of Vigna species from subgenus Ceratotropis. Genetics Research. 2008;90:299–316. doi: 10.1017/S001667230800983X. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Santalla M, Power JB, Davey MR. Genetic diversity in mungbean germplasm revealed by RAPD analysis. Plant Breeding. 1998;117:473–478. [Google Scholar]
- Timko MP, Singh BB. Cowpea, a multifunctional legume. In: Moore PH, Ming R, editors. Genomics of tropical crop plants. Berlin: Springer; 2008. pp. 227–258. [Google Scholar]
- Vaillancourt RE, Weeden NF, Barnard J. Isozyme diversity in the cowpea species complex. Crop Science. 1993;33:606–613. [Google Scholar]
- Van de Peer Y, De Watcher R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Computer Applications in the Biosciences. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- Vijaykumar A, Saini A, Jawali N. Phylogenetic analysis of subgenus Vigna species using ribosomal RNA ITS: evidence of hybridization among V. unguiculata subspecies. Journal of Heredity. 2010;101:177–188. doi: 10.1093/jhered/esp084. [DOI] [PubMed] [Google Scholar]
- Vijaykumar A, Saini A, Jawali N. Molecular characterization of intergenic spacer region of 5S ribosomal RNA genes in subgenus Vigna: extensive hybridization among V. unguiculata subspecies. Plant Systematics and Evolution. 2011;294:39–55. [Google Scholar]
- Wang P, Lu Y, Zheng M, Rong T, Jawali N. RAPD and internal transcribed spacer sequence analyses reveal Zea nicaraguensis as a Section Luxuriantes species close to Zea luxurians. PLoS One. 2011;6:e16728. doi: 10.1371/journal.pone.0016728. 10.1371/journal.pone,0016728. [DOI] [PMC free article] [PubMed] [Google Scholar]