To the Editors:
Rheumatic fever (RF) and rheumatic heart disease (RHD) have become rare in the developed world,1 but remain major causes of childhood diseases in developing countries and in some indigenous communities.2 In populations endemic for RF/RHD, additional factors other than those currently included in the Revised Jones Criteria would increase diagnostic sensitivity.3 Moreover, identification of robust markers for disease activity could provide better monitoring and delivery of health care for patients.
It has been established that immune responses against group A streptococci (GAS) and heart tissue correlate with antibody and T-cell responses against cardiac myosin. Recently, in samples obtained from rheumatic carditis patients from India, Hawaii, and mainland United States, it was found that IgG antibodies targeted cardiac myosin epitopes in the S2 subfragment containing amino acid residues 842 to 992 and 1164 to 1272.4 It has been suggested that, regardless of the GAS M serotype involved in infection, S2 reactivity is similar among populations with rheumatic carditis. In the current exploratory study, we determined IgG reactivity in Australian serum samples to 32 S2 (rod region) and 50 LMM (light meromyosin) overlapping peptides from cardiac myosin using an enzyme-linked immunosorbent assay.4 Sera samples were obtained from 12 RF/RHD patients from the Townsville Hospital and the Royal Darwin Hospital (6 patients with acute rheumatic fever (ARF) with confirmed carditis and 6 patients with ARF/RHD on penicillin prophylaxis for more than a year) and 10 healthy controls. We also assessed IgG reactivity to the entire length of GAS M5 protein using 25 overlapping peptides (N-terminal, n = 10; B region, n = 8; and C region, n = 7). Reactivity to each peptide was determined in serum samples of each individual, and the cumulative optical density values were categorized into the different groups for comparison by either unpaired 2-tailed t tests (Fig. 1A, C) or ANOVA with Newman-Keuls multiple comparison post hoc test (Fig. 1B) on transformed data.
FIGURE 1.
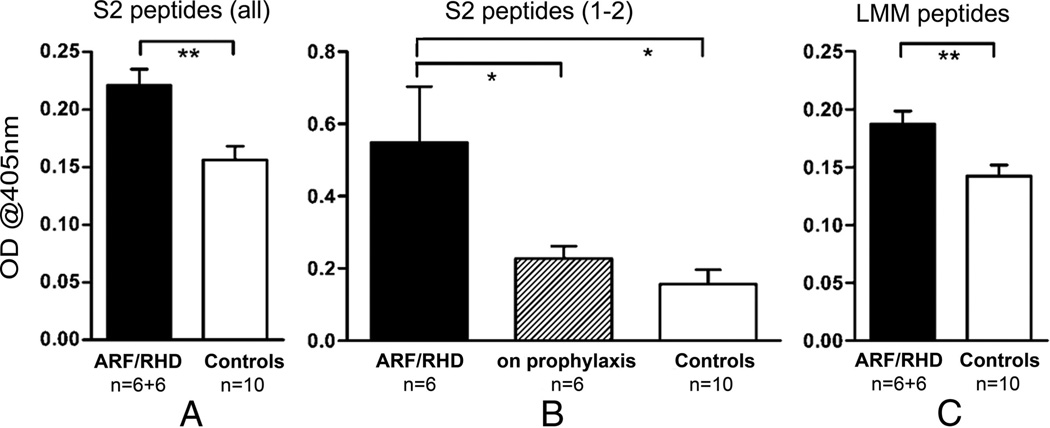
Immunoreactivity of Australian serum samples with peptides from the S2 and LMM regions of human cardiac myosin. A, ARF/RHD patients had higher reactivity to peptides from the S2 region of cardiac myosin compared with healthy controls (**P < 0.01). B, After prophylaxis for >12 months, patient reactivity to S2 1 and 2 (842–880) was comparable to healthy controls (*P < 0.05). C, Higher reactivity to the LMM region of cardiac myosin was also observed in ARF/RHD patients compared with healthy controls (**P < 0.01). Shown are mean optical density values + standard error of the mean.
Significant differences between the patient and control group were observed in their antibody reactivity to myosin peptides. These observations are further proof of our previous suggestion that, regardless of the GAS M serotype involved in infection, S2 reactivity may be similar among populations with ARF.4 Significantly higher mean combined IgG reactivity to S2 (Fig. 1A) peptides was observed in patients. More importantly, within the patient group, we also found that IgG reactivity to S2 1 and 2 (842–880) was significantly higher in patients with ARF and confirmed carditis than in patients who have had prophylaxis for 12 months or longer (Fig. 1B). This observation warrants further assessment of antibody kinetics in patients with ARF undergoing treatment.
Significantly higher mean combined IgG reactivity to LMM peptides was also observed in patients (Fig. 1C). IgG reactivity to GAS M5 N-terminal and B-repeat region peptides were minimal in patient and control groups, indicating that these subjects were most probably not exposed to GAS M5 (data not shown; GAS M5 is not a common isolate in circulation in Australia). However, the significantly high mean combined IgG reactivity observed in both patients and controls to GAS M protein C region peptides (data not shown), which have demonstrated high homology across the different GAS M types,1 indicated that both groups had been exposed to GAS.
The assessment of antibody kinetics in patients with ARF undergoing treatment to determine whether antibody reactivity to S2 could be used as a marker of disease activity and the effectiveness of treatment and prophylaxis regimens warrants further investigation.
Acknowledgments
Supported in part by an Australian National Health and Medical Research Council Project Grant (540419) (to N.K., R.N., and D.G.).
Footnotes
The authors have no additional funding or conflicts of interest to disclose.
Contributor Information
Davina E. Gorton, Microbiology and Immunology, School of Veterinary and Biomedical Sciences, James Cook University, Townsville, Queensland, Australia.
Brenda L. Govan, Microbiology and Immunology, School of Veterinary and Biomedical Sciences, James Cook University, Townsville, Queensland, Australia.
Natkunam Ketheesan, Microbiology and Immunology, School of Veterinary and Biomedical Sciences, James Cook University, Townsville, Queensland, Australia.
Alan A. Sive, Paediatrics/Pathology, Townsville Hospital, Townsville, Queensland, Australia.
Robert E. Norton, Paediatrics/Pathology, Townsville Hospital, Townsville, Queensland, Australia.
Bart J. Currie, Tropical and Emerging Infectious, Diseases Division, Menzies School of Health Research, Darwin, Northern Territory, Australia.
Rebecca J. Towers, Tropical and Emerging Infectious, Diseases Division, Menzies School of Health Research, Darwin, Northern Territory, Australia.
Adita I. Mascaro-Blanco, Department of Microbiology and Immunology, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
Madeleine W. Cunningham, Department of Microbiology and Immunology, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
REFERENCES
- 1.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parnaby MG, Carapetis JR. Rheumatic fever in indigenous Australian children. J Paediatr Child Health. 2010;46:527–533. doi: 10.1111/j.1440-1754.2010.01841.x. [DOI] [PubMed] [Google Scholar]
- 3.Cann MP, Sive AA, Norton RE, et al. Clinical presentation of rheumatic fever in an endemic area. Arch Dis Child. 2009;95:455–457. doi: 10.1136/adc.2008.157107. [DOI] [PubMed] [Google Scholar]
- 4.Ellis NM, Kurahara DK, Vohra H, et al. Priming the immune system for heart disease: a perspective on group A streptococci. J Infect Dis. 2010;202:1059–1067. doi: 10.1086/656214. [DOI] [PubMed] [Google Scholar]