Abstract
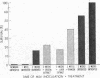
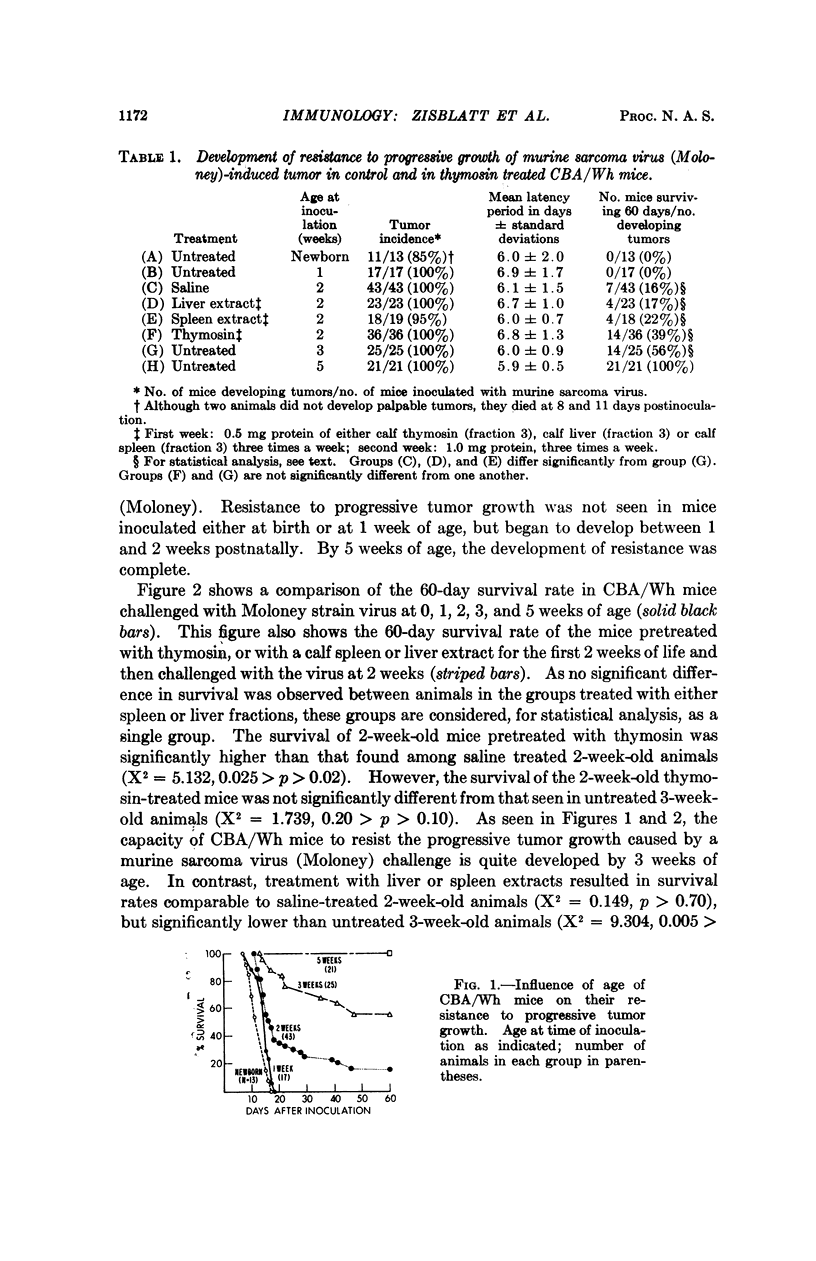
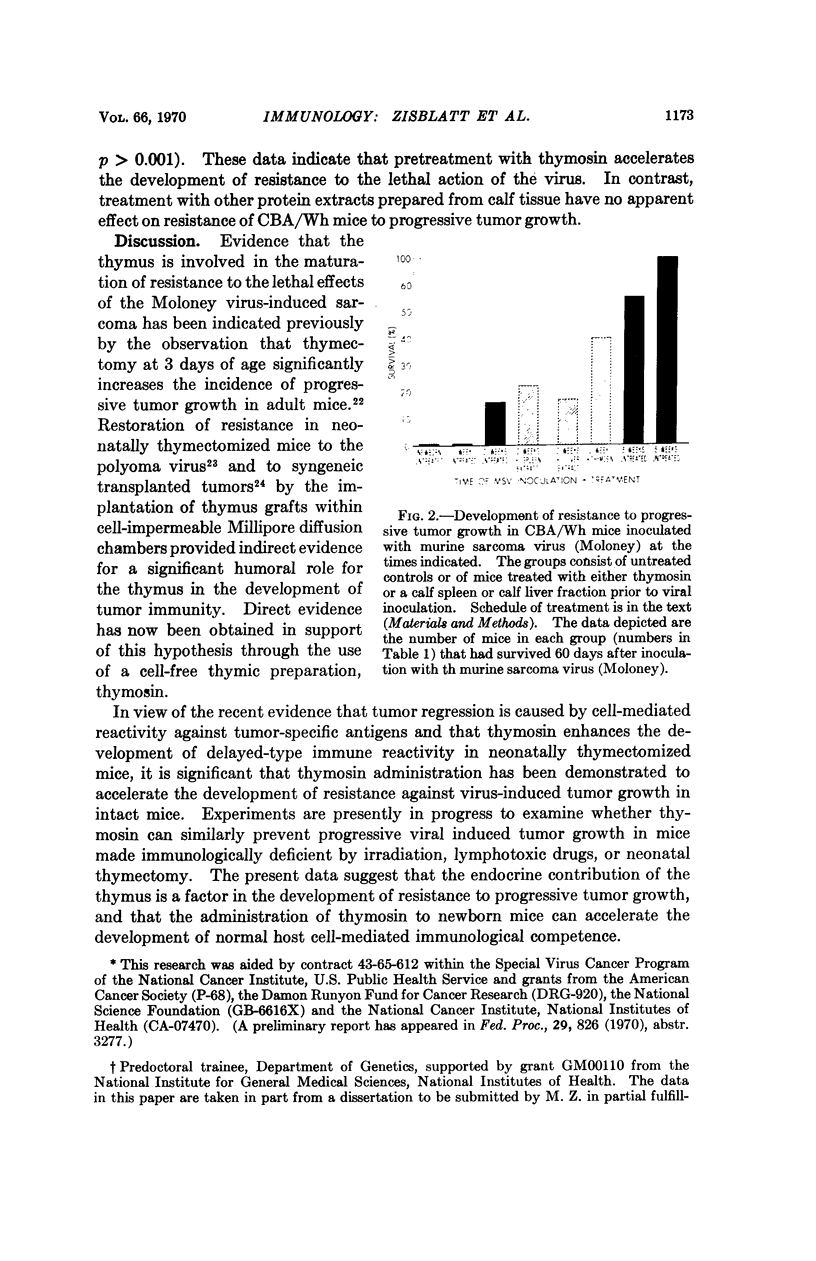
The development of resistance to progressive tumor growth and the effect of a thymic extract upon this developmental process were examined in CBA/Wh mice. Inoculating mice of various ages with murine sarcoma virus (Moloney) permitted the assessment of the period of time postnatally at which the animals developed a threshold level of resistance to progressive tumor growth. Resistance in CBA/Wh mice, at the virus dose used, was first detected at approximately two weeks of age and was completely developed at five weeks.
Thymosin, a soluble calf thymic fraction, when administered to neonatal mice beginning on the second day of life, significantly accelerated the rate of development of resistance to progressive tumor growth. The data suggest a humoral role for the thymus in the development of tumor immunity, and that the normal development of cell-mediated immunological competence can be accelerated through the use of thymus-derived extracts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., McKenna J. M. Immunologic studies of a spontaneous syngeneic tumor-host system. I. The role of immune mechanisms in tumor transplants in the syngeneic host. Int Arch Allergy Appl Immunol. 1969;35(1):20–34. doi: 10.1159/000230156. [DOI] [PubMed] [Google Scholar]
- Allison A. C. Genetic factors in resistance against virus infections. Arch Gesamte Virusforsch. 1965;17(2):280–294. doi: 10.1007/BF01267912. [DOI] [PubMed] [Google Scholar]
- Blumenschein G. R., Moloney J. B. Quantitative dose-response relationships of murine sarcoma virus (Moloney) in BALB/c mice. J Natl Cancer Inst. 1969 Jan;42(1):123–133. [PubMed] [Google Scholar]
- Duran-Reynals M. L. Inhibiting effect of increasing body-weight on the lethal response of DBA-I mice to vaccinia virus infection. Nature. 1966 Apr 16;210(5033):332–334. doi: 10.1038/210332a0. [DOI] [PubMed] [Google Scholar]
- Fefer A., McCoy J. L., Glynn J. P. Induction and regression of primary moloney sarcoma virus-induced tumors in mice. Cancer Res. 1967 Sep;27(9):1626–1631. [PubMed] [Google Scholar]
- Fefer A., McCoy J. L., Perk K., Glynn J. P. Immunologic, virologic, and pathologic studies of regression of autochthonous Moloney sarcoma virus-induced tumors in mice. Cancer Res. 1968 Aug;28(8):1577–1585. [PubMed] [Google Scholar]
- Goldstein A. L., Asanuma Y., Battisto J. R., Hardy M. A., Quint J., White A. Influence of thymosin on cell-mediated and humoral immune responses in normal and in immunologically deficient mice. J Immunol. 1970 Feb;104(2):359–366. [PubMed] [Google Scholar]
- Goldstein A. L., Banerjee S., Schneebeli G. L., Doughtery T. F., White A. Acceleration of lymphoid tissue regeneration in x-irradiated CBA-W mice by injection of thymosin. Radiat Res. 1970 Mar;41(3):579–593. [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW L. W., TING R. C. IMMUNOLOGIC COMPETENCE AND INDUCTION OF NEOPLASMS BY POLYOMA VIRUS. Proc Soc Exp Biol Med. 1965 Jul;119:823–830. doi: 10.3181/00379727-119-30311. [DOI] [PubMed] [Google Scholar]
- Law L. W., Goldstein A. L., White A. Influence of thymosin on immunological competence of lymphoid cells from thymectomized mice. Nature. 1968 Sep 28;219(5161):1391–1392. doi: 10.1038/2191391a0. [DOI] [PubMed] [Google Scholar]
- Law L. W., Ting R. C., Stanton M. F. Some biologic, immunogenic, and morphologic effects in mice after infection with a murine sarcoma virus. I. Biologic and immunogenic studies. J Natl Cancer Inst. 1968 May;40(5):1101–1112. [PubMed] [Google Scholar]
- Miller J. F., Osoba D. Current concepts of the immunological function of the thymus. Physiol Rev. 1967 Jul;47(3):437–520. doi: 10.1152/physrev.1967.47.3.437. [DOI] [PubMed] [Google Scholar]
- Prasad C. B., Gupta B. M. Influence of increasing body weight and age of mice on their susceptibility to experimental neurovaccinia infection. Indian J Exp Biol. 1968 Jul;6(3):164–165. [PubMed] [Google Scholar]
- Shachat D. A., Fefer A., Moloney J. B. Effect of cortisone on oncogenesis by murine sarcoma virus (Moloney). Cancer Res. 1968 Mar;28(3):517–520. [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: fourth listing. Cancer Res. 1968 Mar;28(3):391–420. [PubMed] [Google Scholar]
- Wheelock E. F., Caroline N. L., Moore R. D. Suppression of established Friend virus leukemia by statolon. I. Demonstration of a latent infection in clinically normal mice. J Virol. 1969 Jul;4(1):1–6. doi: 10.1128/jvi.4.1.1-6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock E. F., Larke R. P. Efficacy of interferon in the treatment of mice with established Friend virus leukemia. Proc Soc Exp Biol Med. 1968 Jan;127(1):230–238. doi: 10.3181/00379727-127-32663. [DOI] [PubMed] [Google Scholar]