Abstract
Bitter taste perception is an important sensory input warning against the ingestion of toxic and noxious substances. Bitter receptors, a family of ∼30 highly divergent G-protein-coupled receptors, are exclusively expressed in taste receptor cells that contain the G-protein α-subunit gustducin, bind to α-gustducin in vitro, and respond to bitter tastes in functional expression assays. We generated a taste receptor type 2 member 5 (T2R5)-Cre/green fluorescent protein reporter transgenic mouse to investigate the tissue distribution of T2R5. Our results showed that Cre gene expression in these mice was faithful to the expression of T2R5 in taste tissue. More surprisingly, immunostaining and X-gal staining revealed T2R5 expression in the testis. Ablation of T2R5 + cells led to a smaller testis and removed the spermatid phase from most of the seminiferous tubules. The entire taste transduction cascade (α-gustducin, Ggamma13, phospholipase Cβ2) was detected in spermatogenesis, whereas transient receptor potential, cation channel subfamily M member 5 (Trpm5), was observed only in the later spermatid phase. In short, our results indicate that the taste transduction cascade may be involved in spermatogenesis.
Keywords: bitter receptors, taste transduction, spermatogenesis, mouse, α-gustducin
Introduction
Taste receptor type 2 (T2R) is a family of ∼30 highly divergent G-protein-coupled receptors initially identified in taste tissues. They are selectively expressed in subsets of taste receptor cells distinct from those containing sweet and umami receptors. T2R genes are linked to the bitter taste in humans and mice, and are clustered in regions of the genome (Adler et al., 2000; Chandrashekar et al., 2000; Mueller et al., 2005). Heterologous expression assays have shown that many T2Rs function as bitter taste receptors (Chandrashekar et al., 2000; Bufe et al., 2002). Knockout and misexpression studies have further confirmed that T2Rs are necessary and sufficient for bitter taste perception. Mice lacking mT2R5 (the candidate cycloheximide receptor) markedly and selectively lose their ability to taste the cognate bitter compound (Mueller et al., 2005). Therefore, T2Rs are currently considered to be the bitter taste receptors in mammals.
More recent evidence shows that taste transduction-related signaling is found in various cell types from the stomach to the large intestine (Kaske et al., 2007). Furthermore, it has been proposed that solitary chemosensory cells are present throughout the upper respiratory system and express the entire suite of taste-related signaling molecules, including T2R receptors, phospholipase Cβ2 (PLCβ2), α-gustducin and the transduction channel Trpm5 (Tizzano et al., 2010, 2011). Interestingly, cultured human airway epithelium expresses some T2Rs along with associated downstream elements, and the T2Rs are present on the cilia of the ciliated epithelial cells (Shah et al., 2009). Another report shows that the smooth muscle cells of human airways express T2R (bitter) taste receptors along with gustducin and some components of the taste-associated PLCβ2 signaling cascade (Deshpande et al., 2010). Expression of both T1R3 and T1R2 has been detected in gut (Margolskee et al., 2007), pancreatic beta cells (Nakagawa et al., 2009) and testis (Max et al., 2001; Iwatsuki et al., 2010); thus, the T1R3/T1R2 receptor complex is thought to detect nutrients such as sugar and amino acid in non-taste tissues.
Although taste receptors and their associated downstream signaling components are widely distributed in diverse organ systems, the function of the receptors in many tissues remains unclear. In addition, limited availability of reliable antibodies against T2Rs makes their study more complicated and confusing. Transgenesis with bacterial artificial chromosomes (BACs) has been used in various biological, medical and biotechnological applications and is considered a major breakthrough in the generation of transgenic animals (Giraldo and Montoliu, 2001). In the present study, the use of BACs in transgenesis is likely to ensure the position-independence of the expression of the transgenes, and precisely mark T2R5-expressing cells in vivo.
Materials and Methods
BAC-mT2R5-IRES-Cre construction and generation of transgenic mouse lines
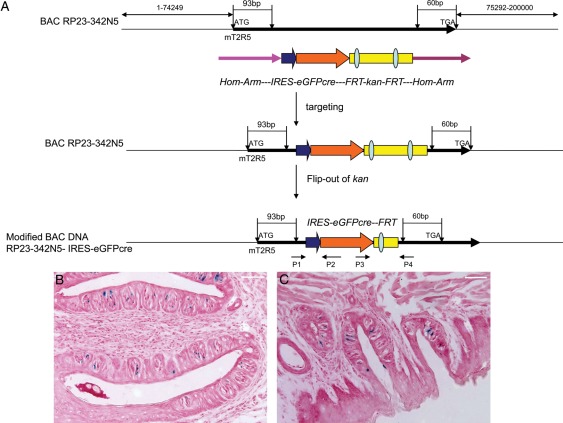
BAC RP23-342N5 containing the entire mT2R5 gene was purchased from BACPAC Resources, Oakland, USA. The IRES-Cre/green fluorescent protein (GFP)-frt-KanR-frt fragment was obtained from plasmid pICGN21 (Lee et al., 2001) by PCR. Two homologous arms of 48 bp from the mT2R5 gene were synthesized into both sides of the primers used to amplify the IRES-Cre/GFP-frt-KanR-frt cassette in the pICGN21 plasmid (forward primer 5′-GAAGCTGGGCTGGGAGTTTTAGGGAACACATTTATTGCACTGGTAAACGCCAAGCTATCGAATTCCGCC-3′, reverse primer 5′-TAATCCTTGCCGTACCTTTACAAAGGCTTGCTTTAGCTGGCTGTTAGACTATTCCAGAAGTAGTGAGGA-3′). The IRES-Cre/GFP-frt-KanR-frt cassette was introduced into the T2R5 gene downstream of the start codon (93 bp) and upstream of its stop codon (60 bp) by Red-mediated homologous recombination (Lee et al., 2001), followed by flp-mediated removal of the KanR selectable marker from the BAC-mT2R5-IRESCre/GFP construct (Fig. 1A). Restriction digestion with pulsed-field gel electrophoresis and sequencing was used to confirm the correct insertion of the IRES-Cre/GFP gene into the mT2R5 BAC (data not shown).
Figure 1.
T2R5-Cre/GFP construction. BAC DNA (RP23-342N5) contains more than 70 kb 3′ and 5′ sequence of T2R5 gene. The IRES-GFP/Cre-frt-KanR-frt fragment is obtained from plasmid pICGN21 by PCR. Two homologous arms of 48 bp from the mT2R5 gene are synthesized into both sides of the primers used to amplify the IRES-eGFPcre-frt-KanR-frt cassette in the pICGN21 plasmid. The IRES-eGFPcre-frt-KanR-frt cassette is introduced into the T2R5 gene downstream of the start codon (93 bp) and upstream of its stop codon (60 bp) by Red-mediated homologous recombination, followed by flp-mediated removal of the KanR selectable marker from the BAC-mT2R5-IRESCre/GFP construct. Final construct was verified by pulse field gel electrophoresis analysis and sequence. P1/P2 and P3/P4 primers are used for genotyping (A). In order to verify the function of Cre, we crossed the T2R5-Cre/GFP transgenic mouse with RosA-LoxP-LacZ mouse and got double transgenic mouse. After X-gal staining of sections of mouse tongue, we found positive cells in circumvallate papillae (B) and foliate papillae (C). Scale bar 12.5 μm.
The BAC-mT2R5-IRES-Cre/GFP construct was purified and microinjected into the pronuclei of B6 (C57BL/6J) mouse zygotes, which were implanted into pseudo-pregnant foster mothers using standard techniques. The transgenic founder mice and their progeny were identified by PCR with Cre/GFP-specific primers (P1 or P3) and mT2R5-specific primers (P2 or P4) (Fig. 1A). The expected PCR products were 384 bp with P1 and P2 primer, 345 bp with P3 and P4 (P1 5′-ACTTCAGATTCCCCCACAACA-3′; P2 5′-TGCTTCCTTCACGACATTCAA-3′; P3 5′-GATACCTGGCCTGGTCTGGA-3′; P4 5′-CGCATGTGACCCTGAGAGCT-3′).
R26:loxP-Stop-loxP LacZbpA mice were purchased from the Jackson Laboratory, Bar Harbor, USA. The R26:lacZbpAflox diphtheria toxin A (DTA) line was a gift from Brockschnieder et al. (2004, 2006).
Histology and immunostaining procedure
For immunocytochemistry, mice were perfused transcardially with 2–4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; pH 7.2–7.4). The testis tissues were dissected, post-fixed in PFA for 2–12 h and cryoprotected in 30% sucrose in PBS at 4°C overnight. After sectioning on a cryostat, 10–12 µm sections were collected onto Superfrost Plus Microscope slides (Fisher Scientific, Loughborough, UK). The polyclonal primary antibodies used were specific for GFP (goat ab-5450, rabbit ab-6556; Abcam, Cambridge, UK), PLCβ2 (rabbit sc-206; Santa Cruz Biotechnology, Santa Cruz, USA), Ggamma13 (goat sc-26781; Santa Cruz Biotechnology) and α-gustducin (rabbit sc-395; Santa Cruz Biotechnology) T1R3 (Goat sc-22458; Santa Cruz Biotechnology). The rabbit polyclonal antibody against Trpm5 (1028–1049 amino acids) was as described by Perez et al. (2002). Staining was performed using the Tyramide signal amplification Plus (modified horse-radish peroxidase) system from Perkin Elmer, Waltham, USA according to the manufacturer's instructions. Fluorescent images were captured using a Leica TCS SP2 Spectral Confocal Microscope (Leica Microsystems Inc., Mannheim, Germany). Staining against GFP was performed using the standard immunocytochemical procedure according to the manufacturer's instructions (VECTASTAIN Elite ABC Kit; Vector Laboratories, Burlingame, USA).
Standard hematoxylin and eosin staining was conducted and bright field images of the sections were captured digitally. Biometric morphometric data were analyzed quantitatively using ImagePro Plus (Media Cybernetics, Inc., Silver Spring, MD, USA) in serial sections of testis (at least seven sections) as described by Franca et al. (2006). The length density of the seminiferous tubule was obtained by dividing the tubule volume by the squared tubule radius (R2) multiplied by π. Morphometric data are presented as the mean ± SEM. These data were analyzed by one way analysis of variance using SPSS11.0 software. Differences were considered to be significant when P < 0.05.
LacZ staining
Animals were perfused with 2% PFA in PBS. Testis tissue was then fixed in 2% PFA for 1 h, after which it was cryoprotected in 30% sucrose in PBS at 4°C overnight. The following morning, tissue was cryosectioned at 20 µm thickness. The sections were washed three times for 20 min in PBS and stained in X-gal solution (5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 0.02% detergent NP-40, 0.01% Na deoxycholate, 1 mg/ml X-gal) at 37°C overnight. Stained sections were washed three times for 20 min in PBS and counterstained with nuclear fast red. Bright field images were captured using a SPOT digital camera (Diagnostic Instruments, Inc., Sterling Heights, USA) attached to a Nikon SA Microphot microscope and minimally processed using ImagePro Plus image analysis software (Media Cybernetics, Inc.).
Results
Generation and identification of T2R5-Cre/GFP transgenic mice
It is well known that T2R5 is expressed in taste buds and thought to be a bitter receptor (Chandrashekar et al., 2000; Zhang et al., 2003; Mueller et al., 2005). Recently, it was reported that T2R is expressed in nose (Tizzano et al., 2010) and lung (Deshpande et al., 2010) tissues. To further study the expression of T2R, we replaced the T2R5 sequence with the IRES-Cre/GFP sequence using the Red recombinase system (Fig. 1A). After modified BAC DNA had been microinjected into C57BL/6 oocytes, we obtained three transgenic founders. To confirm the expression of Cre/GFP, we crossed these transgenic mice with R26:loxP-Stop-loxP LacZbpA transgenic mice (Supplementary data, Fig. S1A). β-Gal staining showed that LacZ was expressed as expected in circumvallate and foliate papillae (Fig. 1B and C). However, we failed to observe LacZ expression in fungiform papillae, suggesting selective expression in taste buds as reported by Adler et al. (2000; Chandrashekar et al., 2000). We then crossed the transgenic mice with R26:lacZbpAflox DTA (Supplementary data, Fig. S1B). A two-bottle taste preference test was performed to determine whether ablation of T2R5 + cells (co-expression of the DTA gene leads to cell death) resulted in the tasteless phenotype. As expected, the double transgenic mice did not show any preference for normal water over a bitter compound (data not shown), indicating the successful generation of T2R5-Cre/GFP/DTA double transgenic mice.
T2R5 expression in testis
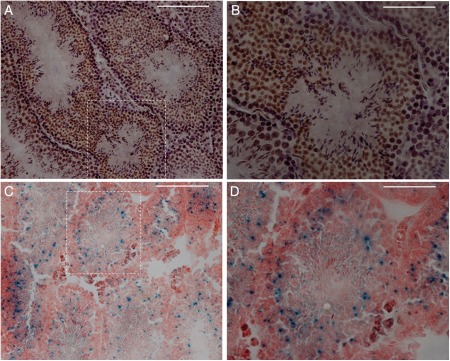
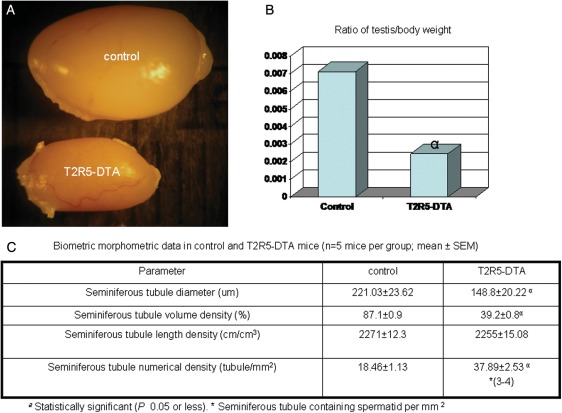
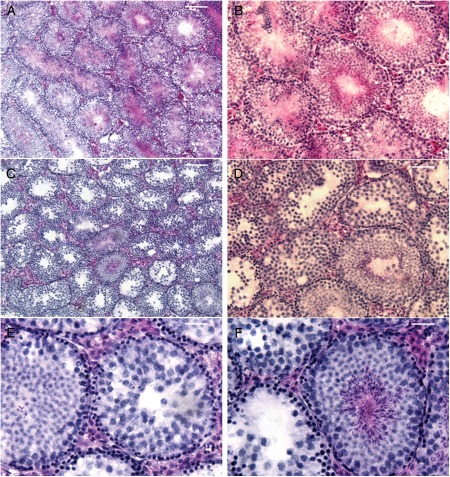
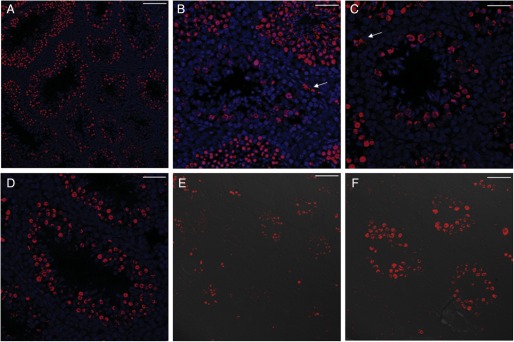
When we crossed T2R5-Cre/GFP transgenic mice with R26:lacZbpAfloxDTA (Supplementary data, Fig. S1B), it was observed that the male double transgenic mice were infertile, indicating that T2R5 was expressed in the testis. To verify this hypothesis, we subjected testis sections to immunohistochemistry and β-gal staining. On immunostaining with anti-GFP, T2R5 expression was observed in the round spermatid phase (Fig. 2A and B). β-Gal staining also showed T2R5 expression in the round spermatid phase (Fig. 2C and D). Dissection of the testis of T2R5-Cre/GFP/DTA double transgenic mice showed that the testes were smaller than those of control mice (Fig. 3A). These control mice are R26lacZbpAfloxDTA mice, which are backcrossed with C57BL/6J at least five generations. The testis:body weight ratio differed significantly from that of the controls (n = 5, 0.007, >0.0023, P < 0.05; Fig. 3B). Compared with control mice, the seminiferous tubule diameter was smaller and more seminiferous tubules were found. However, the length density of the seminiferous tubules was not increased (Fig. 3C). Standard H&E staining showed ablation of spermatids in most of the seminiferous tubules from mutant mice (Fig. 4C and D), though spermatids were seen in 10% of the tubules, indicating that T2R5 was not expressed in all spermatids (Fig. 4E and F). We still observed spermatogonia and the spermatocyte phase (Fig. 4E and F). In summary, our data suggested that T2R5 was expressed in the spermatid phase, not all spermatids were T2R5 positive, and ablation of T2R5 + spermatids did not completely block spermatogenesis.
Figure 2.
Immunostaining with anti-GFP showed that T2R5 was expressed in early to mid-stage round spermatids, but not in spermatocytes or spermatogonia (A and B) in T2R5-CreGFP transgenic mice. (A) Low magnification. (B) High magnification from dotted frame in (A). After X-gal staining, positive signals were also observed in spermatids (C and D). (C) Low magnification. (D) High magnification from dotted frame in (C). Scale bar: 50 μm.
Figure 3.
To confirm the expression of T2R5 in testis, we crossed T2R5-GFP/Cre transgenic mice with R26:lacZbpAfloxDTA transgenic mice. These male double transgenic mice are infertile. After dissection, their testes were observed to be smaller than those of control mice (A). The testis:body weight ratio differed significantly from that of the controls (n = 5, 0.0071 ± 0.0003, >0.0024 ± 0.0007) (B). Compared with control mice (R26:lacZbpAfloxDTA transgenic mice). The seminiferous tubule diameter was smaller and there were more seminiferous tubules. The length densities of the seminiferous tubules were similar. We observed fewer tubules containing spermatids (3–4/37.89) (C).
Figure 4.
Compared with control mice (A and B), H&E staining showed that expression of DTA in T2R5 + cells ablate spermatids from most of the seminiferous tubules (C and D). In a few tubules, we still observed the spermatid phase (E and F). Scale bars: (A and C) 25 µm; (B and D) 12.5 µm; (E and F) 6.5 µm.
Taste signal transduction is expressed in spermatogenesis
It is well known that the transduction of bitter, sweet and amino acid tastes uses elements of a common pathway (Margolskee, 2002; Zhang et al., 2003). It has been reported that T1R3 (Max et al., 2001) and T1R2 (Iwatsuki et al., 2010) are expressed in testis. Interestingly, α-gustducin is also expressed in testis and spermatozoa (Fehr et al., 2007). Our results also showed the expression of T2R5 in testis. The next question is whether the common elements of taste transduction are found in the testis.
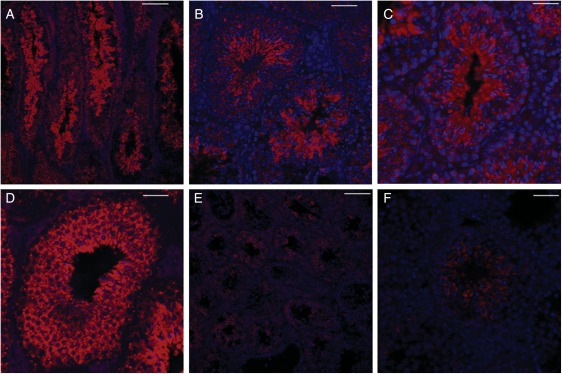
First, we investigated α-gustducin expression by confocal analysis. Immunostaining with anti-α-gustducin showed that α-gustducin was expressed in spermatogenesis, in the spermatogonial, spermatocyte and spermatid phases (Fig. 5A–D). DTA expression in T2R5 + cells ablated the α-gustducin + spermatid phase in most of the seminiferous tubules (Fig. 5E). However, we still observed the α-gustducin + spermatid phase in few seminiferous tubules (Fig. 5F), matching the results of H&E staining. It should be noted that α-gustducin expression was still observed in the spermatogonial and spermatocyte phases after ablation of T2R5 + cells (Fig. 5E and F). Combined with the H&E results, it seems that ablation of T2R5 + cells did not completely remove the spermatogonial or spermatocyte phase. Taken together with the results of another study, which found that α-gustducin is expressed in spermatozoa (Fehr et al., 2007), our data suggest that α-gustducin expression occurs throughout the process of spermatogenesis.
Figure 5.
α-Gustducin expression in testis. Confocal analysis showed α-gustducin expression in spermatogenesis, in the spermatogonial, spermatocyte and spermatid phases (A–D) in control mice (R26:lacZbpAfloxDTA transgenic mice). α-Gustducin expression was not observed in interstitial tissues. DTA expression in T2R5 + cells ablated the spermatid phase from most of the seminiferous tubules (E). In a few tubules, we still observed α-gustducin + spermatids (F). (A and E) Low magnification. (B–D and F) High magnification. Scale bars: (A and E) 80 µm; (B) 30 µm; (C, D and F) 50 µm.
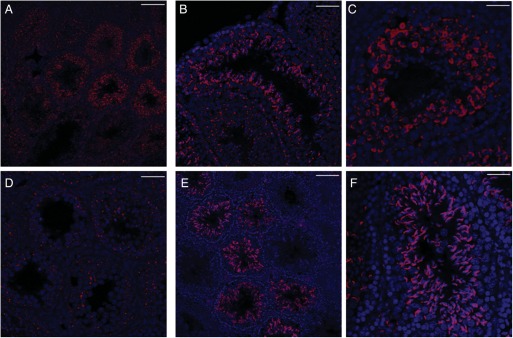
Next, we investigated the expression of PLC-β2, another important element of taste transduction. Surprisingly, PLC-β2 expression was also detected in the three phases of spermatogenesis (Fig. 6A–C). Similar to α-gustducin expression, PLCβ2 expression was still observed in the spermatogonial and spermatocyte phases after ablation of T2R5 + cells (Fig. 6D). We also determined the expression of Trpm5, which is thought to be a component of the channel that mediates taste transduction (Liu and Liman, 2003; Zhang et al., 2003). Confocal analysis showed that Trpm5 was expressed only in the later spermatid phase (Fig. 6E and F), which differed from the pattern of expression of the above components.
Figure 6.
PLC-β2 expression in testis. PLC-β2 expression was observed in spermatogenesis (A–D) in control mice (R26:lacZbpAfloxDTA transgenic mice). DTA expression in T2R5 + cells ablated the spermatid phase from most of the seminiferous tubules (E and F). Trpm5 expression was observed in the later spermatid phase. PLC-β2 and Trpm5 expression were not detected in interstitial tissues. (A and E) Low magnification. (B–D and F) High magnification. Scale bars: (A and E) 80 µm; (D) 30 µm; (B, C and F) 50 µm.
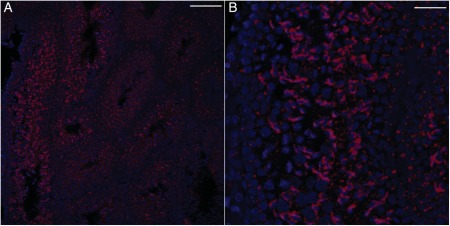
It has previously been reported that T1R3 is expressed in the testis (Max et al., 2001), but its histological distribution is unclear. Our results show T1R3 expression in spermatogenesis (Fig. 7A–D) and in interstitial tissues (Fig. 7B and C; arrow). After ablation of T2R5 + cells, we still observed the expression of T1R3 (Fig. 7E and F). Ggamma13 is thought to be an important gamma subunit of α-gustducin and mediates IP3 responses to bitter compounds (Huang et al., 1999). As expected, Ggamma13 expression was detected in spermatogenesis (Fig. 8A and B). Ggamma13 expression was also observed in the later spermatid phase.
Figure 7.
T1R3 expression in testis. T1R3 expression was observed in spermatogenesis (A–D) and in interstitial tissues (B, C; arrow) in control mice (R26:lacZbpAfloxDTA transgenic mice). After ablation of T2R5 + cells, we still observed the expression of T1R3 (E and F). (A and E) Low magnification. (B–D and F) High magnification. Scale bars: (A and E) 80 µm; (B–D, F) 50 µm.
Figure 8.
Ggamma13 expression in testis. Ggamma13 expression was observed in spermatogenesis (A and B) in control mice (R26:lacZbpAfloxDTA transgenic mice). (A) Low magnification. (B) High magnification. Scale bars: (A) 80 µm; (B) 50 µm.
Our current data collectively suggest that three important components of taste transduction (α-gustducin, Ggamma13, PLC-β2) were present throughout spermatogenesis, whereas Trpm5 was observed only in the later spermatid phase. T1R3 expression was also observed in spermatogenesis.
Discussion
The present study suggests that T2R5 is expressed in the spermatid phase, but not all spermatids express T2R5. We detected the expression of three important components of taste signal transduction (α-gustducin-PLC-β2-Ggamma13) in spermatogenesis. However, Trpm5 expression was found only in the later spermatid phase, indicating that spermatogenesis involves another transient receptor potential cation channel. It is well known that the T2R family comprises ∼30 highly divergent G-protein-coupled receptors (Adler et al., 2000; Chandrashekar et al., 2000). Thus, it is possible that other T2Rs may be expressed in spermatogenesis. However, there are few available antibodies for T2Rs, which makes it difficult to localize their in the body. To detect protein expression reflecting endogenous T2R promoter activity, we need to generate more knock-in mice in the future.
Increasing evidence suggests that taste receptors and components of taste transduction are widely distributed in non-taste tissues (Finger and Kinnamon, 2011). Taste transduction for umami, sweet and bitter compounds, including receptors, has been observed in the gastrointestinal tract (Rozengurt, 2006; Margolskee et al., 2007). The T1R3/T1R2 receptor complex is further suggested to function in sensing sugar and maintaining glucose homeostasis through secretion of incretin (Margolskee et al., 2007). In addition, several studies have shown that T1R3/T1R2 is expressed in testis (Max et al., 2001; Iwatsuki et al., 2010). Interestingly, a segmental distribution of α-gustducin along the flagellum is observed in the sperm of different mammalian species, suggesting a functional role for this G-protein either in signal transduction controlling sperm chemotaxis, or in sperm motility (Fehr et al., 2007). The current study found most elements of the taste transduction cascade, from receptor (T2R5/T1R3) to transduction channel, in spermatogenesis. In short, our findings indicate the taste transduction cascade may be involved in spermatogenesis. The taste signaling cascade may be used to detect chemical elements in the lumen of the seminiferous tubule or the physical compartments established by Sertoli cell-to-Sertoli cell junctions.
Recent studies have demonstrated the expression of T2Rs in the airways (Shah et al., 2009; Deshpande et al., 2010; Tizzano et al., 2011), suggesting that taste transduction may also act by autocrine signaling; that is, the chemical binds to taste receptors on a cell, leading to changes in that cell. Surprisingly, T2R agonists cause airway smooth muscle relaxation in the trachea (Deshpande et al., 2010). However, it is not known how a bitter substance might interact with the smooth muscle cells of the trachea under physiological conditions, as suggested by the relatively tight airway epithelium covering the smooth muscle of the trachea, and it is doubted whether an inhaled bitter substance could rapidly penetrate the epithelium to approach T2R receptors on the muscle (Finger and Kinnamon, 2011). In the present study, the entire bitter taste transduction cascade was detected in spermatogenesis. The Sertoli cell-to-Sertoli cell junctional complex not only provides physical compartmentalization by dividing the seminiferous epithelium into basal and luminal compartments, but also creates a permeability barrier called the blood–testis barrier (Ross et al., 1989). Thus, it is almost impossible that a sweet/bitter substance in the environment (lumen of seminiferous tubule or blood vessel) could penetrate and influence spermatogenesis. A further study of the taste transduction cascade in spermatogenesis will help us to comprehend the role of these receptors in the body.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
F.L. designed the study, carried out and analyzed the experiments and wrote the manuscript. M.Z. screened transgenic mice and performed two-bottle taste preference test.
Funding
This work was supported by grants from the National Institutes of Health (R01 DC007487), the National Science Foundation (DBJ-0216310), Shanghai Municipal Education Commission (Technological Innovation Projects 08YZ42) and Shanghai leading Academic Discipline Project (S30201).
Conflict of interest
There is no financial or other potential conflict of interest.
Supplementary Material
Acknowledgements
We thank Liquan Huang and Robert Margolskee (Monell Chemical Senses Center) for his valuable advice and discussions. We also thank Transgenic and Chimeric Mouse Facility (University of Pennsylvania) for generating transgenic mice.
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Brockschnieder D, Lappe-Siefke C, Goebbels S, Boesl MR, Nave KA, Riethmacher D. Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol Cell Biol. 2004;24:7636–7642. doi: 10.1128/MCB.24.17.7636-7642.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschnieder D, Pechmann Y, Sonnenberg-Riethmacher E, Riethmacher D. An improved mouse line for Cre-induced cell ablation due to diphtheria toxin A, expressed from the Rosa26 locus. Genesis. 2006;44:322–327. doi: 10.1002/dvg.20218. [DOI] [PubMed] [Google Scholar]
- Bufe B, Hofmann T, Krautwurst D, Raguse JD, Meyerhof W. The human TAS2R16 receptor mediates bitter taste in response to beta-glucopyranosides. Nat Genet. 2002;32:397–401. doi: 10.1038/ng1014. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr J, Meyer D, Widmayer P, Borth HC, Ackermann F, Wilhelm B, Gudermann T, Boekhoff I. Expression of the G-protein alpha-subunit gustducin in mammalian spermatozoa. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:21–34. doi: 10.1007/s00359-006-0168-8. [DOI] [PubMed] [Google Scholar]
- Finger TE, Kinnamon SC. Taste isn't just for taste buds anymore. F1000 Biol Reports. 2011;3:20. doi: 10.3410/B3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca LR, Suescun MO, Miranda JR, Giovambattista A, Perello M, Spinedi E, Calandra RS. Testis structure and function in a nongenetic hyperadipose rat model at prepubertal and adult ages. Endocrinology. 2006;147:1556–1563. doi: 10.1210/en.2005-0640. [DOI] [PubMed] [Google Scholar]
- Giraldo P, Montoliu L. Size matters: use of YACs, BACs and PACs in transgenic animals. Transgenic Res. 2001;10:83–103. doi: 10.1023/a:1008918913249. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Nomura M, Shibata A, Ichikawa R, Enciso PL, Wang L, Takayanagi R, Torii K, Uneyama H. Generation and characterization of T1R2-LacZ knock-in mouse. Biochem Biophys Res Commun. 2010;402:495–499. doi: 10.1016/j.bbrc.2010.10.057. [DOI] [PubMed] [Google Scholar]
- Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Ross MH, Reith EJ, Romrell LJ. Histology: A Text and Atlas. 2nd edn. Baltimore: Williams and Wilkins; 1989. [Google Scholar]
- Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.