Abstract
Major histocompatibility complex, class II, DQ alpha 2, also named BOLA-DQA2, belongs to the Bovine Leukocyte Antigen (BOLA) class II genes which are involved in the immune response. To explore the variability of the BOLA-DQA2 gene and resistance to mastitis in cows, the splice variants (SV), targeted microRNAs (miRNAs), and single nucleotide polymorphisms (SNPs) were identified in this study. A new SV (BOLA-DQA2-SV1) lacking part of exon 3 (195 bp) and two 3′-untranslated regions (UTR) (52 bp+167 bp) of the BOLA-DQA2 gene was found in the healthy and mastitis-infected mammary gland tissues. Four of 13 new SNPs and multiple nucleotide polymorphisms resulted in amino acid changes in the protein and SNP (c. +1283 C>T) may affect the binding to the seed sequence of bta-miR-2318. Further, we detected the relative expressions of two BOLA-DQA2 transcripts and five candidated microRNAs binding to the 3′-UTR of two transcripts in the mammary gland tissues in dairy cattle by using the quantitative real-time polymerase chain reaction. The result showed that expression of the BOLA-DQA2-SV1 mRNA was significantly upregulated 2.67-fold (p<0.05) in mastitis-infected mammary tissues (n=5) compared with the healthy mammary gland mammary tissues (n=5). Except for bta-miR-1777a, miRNA expression (bta-miR-296, miR-2430, and miR-671) was upregulated 1.75 to 2.59-fold (p<0.05), whereas miR-2318 was downregulated in the mastitis cows. Our findings reveal that BOLA-DQA2-SV1 may play an important role in the mastitis resistance in dairy cattle. Whether the SNPs affect the structure of the BOLA-DQA2 gene or association with mastitis resistance is unknown and warrants further investigation.
Introduction
Mastitis is a prevalent and complex infectious disease affected by genetics and pathogens that can result in significant economic losses to dairy herds (Nash et al., 2003). The primary defense against pathogens relies on the appropriate expression of antigen presenting molecules triggering the release of effecter molecules of the innate immune system.
In all mammals, major histocompatibility complex (MHC) plays a central role in the immune response, including disease susceptibility and resistance (Kennedy et al., 2011). The genetic variation of the bovine MHC, also named bovine leukocyte antigen (BOLA), has been associated with the susceptibilities, resistance, and immune responses of several bovine diseases, for example, bovine leukaemia virus, dermatophilosis, foot-and-mouth disease (Glass et al., 2000; Maillard et al., 2003; Juliarena et al., 2008), and regarded as an important genomic target for manipulation to improve bovine health and productivity.
Major histocompatibility complex, class II, DQ alpha 2 (BOLA-DQA2) is a gene of the BOLA class II which encode α and β chains of DR and DQ dimer molecules (Zimin et al., 2009). The α and β chains of DR and DQ dimer molecules can present antigenic peptides to the helper T cells and thereby initiate an adaptive immune response. The MHC region contains a diverse array of genes which are crucial for the initiation of adaptive immune responses and encompasses a large chromosomal region that maps to chromosome 23 in cattle (Fries et al., 1986). They are prominently expressed in antigen-presenting cells, notably macrophages and dendritic cells (Fritz, 2009). The genomic sequence of the BOLA-DQA2 gene consists of three exons and two introns spanning 5.467 kbp. Four new alleles were found in exon 2 of the BOLA-DQA2 gene and the AA −289 haplotype may serve as a marker for lower somatic cell score in cows (López-Benavides, 2004).
Alternative splicing (AS) of eukaryotic pre-mRNAs is a key mechanism for potentially generating many transcript isoforms from a single gene. It serves versatile regulatory functions in controlling major developmental decisions and fine-tuning of gene function (Lopez, 1998). Many recent studies have pointed to the importance of detection and measurement of AS. For example, more genetic variations in the CEU HapMap population manifest themselves through changes in transcript structure, including splicing, than changes in gene transcription (Kwan et al., 2008). Many immunologically related mutiple-exon gene transcripts generated by AS play important roles in innate immunity (Lynch, 2004; Zikherman and Weiss, 2008; Zhang et al., 2009). Mastitis can be caused by many bacteria, including Staphylococcus aureus and Escherichia coli. The BOLA-DQA2 gene is a multiple exon gene and is predicted to contain various splice sites. We hypothesized that BOLA-DQA2 is regulated via AS. MicroRNAs (miRNAs) are a class of single-strand, endogenous, noncoding small RNAs molecules 18–26 nucleotides in size. Diverse miRNA expression patterns and the abundance of potential miRNA targets suggest that miRNAs are likely to be involved in diseases (Kloosterman and Plasterk, 2006). However, our knowledge of the differential expression of specific splicing events, targeted miRNAs, and characterization of BOLA-DQA2 gene in the cattle mastitis resistance is limited.
The aim of this study was as follows: (1) to investigate whether the different splice variants (SV) of the BOLA-DQA2 gene are present in bovine tissues; (2) to analyze the differential expression in the healthy and mastitis infected mammary gland tissues; (3) to investigate the expression of candidate miRNAs of the BOLA-DQA2 gene; (4) to explore genetic variants of the BOLA-DQA2 gene.
Materials and Methods
Animals
Samples were collected from five healthy and five mastitis-infected mammary gland tissues of first lactation Chinese Holstein cows from a commercial bovine slaughter farm. The initial selection of mastitis cows was based on clinical symptoms. One of the tissue samples was collected and stored in the liquid nitrogen for RNA isolation; other tissues were collected and the pathogen identified. No pathogen was observed in the healthy cow's mammary tissues (n=5). Only mammary tissues (n=5) from S. aureus caused mastitis cases were used for this study. Mammary glands, spleen, liver, and kidney tissues from two healthy and two mastitis-infected cows were used for SV identification. All ten mammary tissue samples were used for analysis of the relative expression of BOLA-DQA2 mRNA.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from the mammary tissue using Trizol reagent (Invitrogen) according to the manufacturer's recommendation. Samples were treated with RQ1 RNase-free DNase (Promega) to remove contaminating genomic DNA. RNA purity and concentration were measured with the Biophotometer (Eppendorf). First strand cDNA synthesis was performed in a 20 μL volume using Quantscript RT kit (Tiangen). The reaction was incubated for 10 min at 30°C, followed by inactivation of the RTase at 99°C for 5 min.
To identify novel SV of the BOLA-DQA2 gene, primers were designed for reverse transcription-polymerase chain reaction (RT-PCR) amplification based on two existing BOLA-DQA2 sequences deposited in GenBank (Accession number: No.D50049 and No.BC102953). During primer design, Mfold (http://frontend.bioinfo.rpi.edu/applications/mfold/cgi-bin/dna-form1.cgi) and BLAST (www.ncbi.nlm.nih.gov/blast) were used to check for possible secondary structures and primer specificity, respectively. One set of primers (F: 5′ CACTGCTGAGTCCACCTTGA 3′, R: 5′ GAAGAGAGGAGGGGCAGAGT 3′, product size=1345 bp) were used to amplify bovine BOLA-DQA2 mRNA. The fragment covers part of the 5′-untranslated region (5′-UTR), exon 1 - exon 3 and part of the 3′-UTR. PCR was performed in a total volume of 25 μL, containing 50 ng of cDNA, 2.5 μL 10X PCR Buffer, 2.1 mM MgCl2, 0.1 mM dNTPs, 0.25 mM of each primer (BGI), 0.2 μL Easy Taq DNA Polymerase (TransGen Biotech), and ddH2O and run for 35 cycles of 94°C for 40 s, 60°C for 40 s, and 72°C for 40 s, followed by incubation at 72°C for 10 min. PCR products were gel-purified, ligated into the pMD18-T vector (TaKaRa), and then transformed into competent E. coli DH5α. Finally, fifteen randomly selected positive clones from one RT-PCR product were sequenced. To confirm the result, the experiment was repeated twice.
Sequence analysis
Sequence alignment was performed to search for the SV and single nucleotide polymorphisms (SNPs) of the BOLA-DQA2 gene using DNAMAN (version 6.0) software. Exon splice enhancer was predicted using ESE finder3.0 (Cartegni et al., 2003). Regulatory RNA motifs and elements finder was predicted by online software (Huang et al., 2006). The conservative domain of protein was predicted with the Sanger Datebase (http://pfam.sanger.ac.uk/search/sequence). Target sites of miRNA were predicted using the MicroInspector (http://bio2server.bioinfo.uni-plovdiv.bg/microinspector/) and RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html).
Quantitative real-time PCR
To determine the relative expression of the two BOLA-DQA2 SV, quantitative-PCR (Q-PCR) was carried out using the SYBRGreen PCR Master Mix (TakaRa) according to the manufacturer's protocol. The primers of the target bovine BOLA-DQA2-SV1 gene (F: 5′ TGAGACCAGCTTCCTCCCTA 3′; R: 5′ GGCTCCCAGTGTTTCAAAAG 3′; Size=141 bp), BOLA-DQA2-complete gene (F: 5′ TGAGTCGCACCCTAGAAAGG 3′; R: 5′ GGTTCCCAATTCTCCCACTT 3′; Size=128 bp), and housekeeping internal control gene (β-actin, GenBank accession no. BT030480.1; Primer sequences. F: 5′ GCACAATGAAGATCAAGATCATC 3′; R: 5′ CTAACAGTCCGCCTAGAAGCA 3′; Size=173 bp) were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/). The Q-PCR protocol and calculation of relative expression were described by Huang et al. (2010).
Quantitative analysis of miRNAs
The relative expression of miRNAs was determined by Q-PCR using the miScript PCR system (Qiagen) according to the manufacturer's protocol. Total RNA was extracted and converted to cDNA with the miScript reverse transcriptase mix (Qiagen). The miRNA specific primer is the same as the reference mature miRNA sequence. Bta-let-7g was used as an endogenous control (Huang et al., 2011). Q-PCR was carried out using the miScript SYBR Green PCR kit (Qiagen) with the specific primer and the universal primer from the kit following the included instructions. The relative expression of miRNAs was shown as fold change.
Statistical analysis
The value of the relative quantity was presented as fold change. The means of two groups were compared by Student's paired-samples t-test. The analysis was performed using SPSS software (v.10.0, SPSS Inc.). A value of p<0.05 was regarded as significant.
Results
SV of the BOLA-DQA2 gene
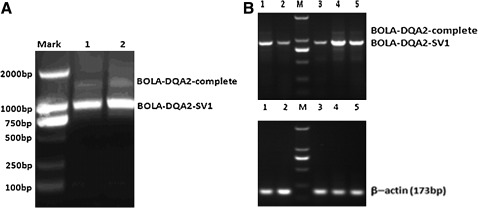
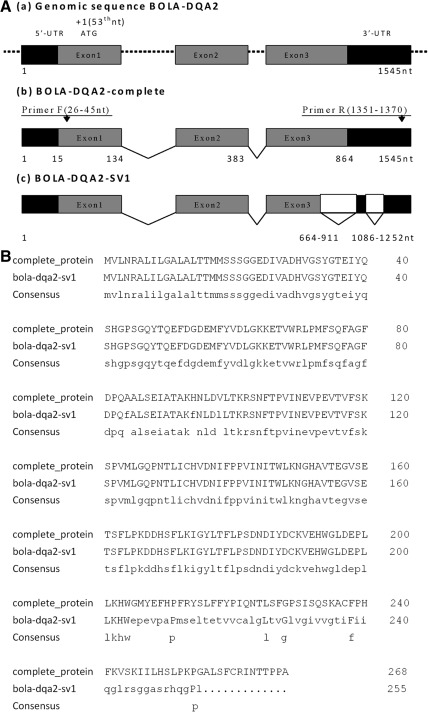
To identify the SV of the BOLA-DQA2 gene, RT-PCR was used to amplify the BOLA-DQA2 gene fragment including the partial 5′-UTR, coding region and partial 3′-UTR region. As shown in Figure 1A, RT-PCR of healthy and mastitis-infected mammary glands resulted in two fragments that included the expected amplicon (1345 bp) and a shorter amplicon (931 bp). Sequence analysis showed that part of exon 3 (195 bp) and the 3′-UTR (52 bp+167 bp) of the bovine BOLA-DQA2 gene were spliced out, which resulted in the new 931 bp SV (Fig. 2A). Sequence alignment showed that the two SV include the 139 and 501 bp 3′-UTR, respectively (Fig. 2, Supplementary Fig. S1, Supplementary Tables S1 and S2; Supplementary Data are available online at www.liebertonline.com/dna). Further, miRNA target sites of BOLA-DQA2-SV1 were altered (Supplementary Fig. S1, Supplementary Tables S1 and S2). The alternatively splicing pattern of the BOLA-DQA2 gene belongs to the N-possibility that includes the exon skipping and alternative polyadenation. The complete BOLA-DQA2 gene encodes 268 amino acids (aa), whereas the novel SV (designated as BOLA-DQA2-SV1, GenBank accession: JN225517) putatively encodes 255 aa (Fig. 2B). The first 23 aa of the two proteins (BOLA-DQA2-SV1 and BOLA-DQA2-complete) is the signal peptide. The domain MHC II alpha (aa 29–110) and C1-set (aa 120–200) were found in the two SV proteins. Therefore, two SV are expected to have similar primary function. Except for the individual aa mutation, the first 204 aa of BOLA-DQA2-SV1 is identical to the complete protein; however, the sequence is different from 205 aa to 255 aa (Fig. 2B). This may affect the function of the predicted BOLA-DQA2-SV1 protein. The boundary of the BOLA-DQA2-SV1 is AG-GC or GG-CA splice sites, instead of the classical GT-AG rule. Either one or two variants were found in the tissues of mammary gland, liver, spleen, and kidney (Fig. 1B). Moreover, the novel SV is the main transcript. Interestingly, the complete transcript was not detected in the liver.
FIG. 1.
(A) Agarose gel showing the two amplicons in mammary glands. Marker: DL2000; 1, Healthy cow; 2, Mastitis cow. (B) Agarose gel showing the one or two amplicons in various tissues. 1, mammary gland; 2, 3, liver; 4, spleen; 5, kidney; M-DL2000 marker.
FIG. 2.
(A) Map of the genomic and splice variant sequences of the bovine BOLA-DQA2 gene. Gray boxes represent the exons; Black boxes represent the UTR regions. Blank boxes represent the regions spliced out. Dotted line represents the introns. Arrows indicate the position of the primers. Number represents the nucleotide sequence position relative to the reference sequence. (B) BOLA-DQA2 complete protein and comparison with the putative aa sequence of BOLA-DQA2-SV1. The lower case letters represent the consensus sequence of the BOLA-DQA2 complete and BOLA-DQA2-SV1 protein sequences. SV, splice variant; UTR, untranslated region.
Expression of the SV in the healthy and mastitis tissues
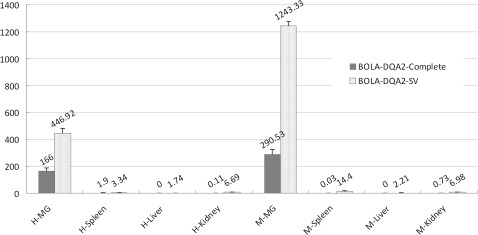
Differential expression of the SV of BOLA-DQA2 mRNA in healthy and infected mammary glands was investigated using Q-PCR. The expression of BOLA-DQA2-SV1 mRNA in the mastitis group was upregulated 2.67-fold compared with the healthy group (Fig. 3, p<0.05). The expression of the BOLA-DQA2-complete mRNA in the mastitis mammary gland tissues was significantly higher (1.75-fold) than that in the healthy mammary gland tissues (p<0.05). It is noteworthy that the expression of the BOLA-DQA2-SV mRNA was significantly higher than that of the BOLA-DQA2-complete mRNA in the spleen, liver, and kidney tissues of the healthy and mastitis cows (Fig. 3, p<0.05), which suggests that the novel SV is the main transcript of the BOLA-DQA2 gene. The result is consistent with the previous RT-PCR result. The relative expression of BOLA-DQA2 gene in the mammary gland tissue was significantly higher than those of the other three tissues (Fig. 3).
FIG. 3.
Relative expressions of the two transcripts of the BOLA-DQA2 gene. H-MG, Healthy mammary gland; M-MG, Mastitis-infected mammary gland; H denotes healthy cow group; M represents mastitis-infected cow group. Number above the box represents mean quantitative values. The vertical bar represents standard error.
Expression of the target miRNAs in the healthy and mastitis cows' mammary gland tissues
The binding of five candidate miRNAs predicted in silico, including bta-miR-296, bta-miR-2430, bta-miR-671, bta-miR-2318, and bta-miR-1777a, to the 3' UTR was determined using Q-PCR. When comparing the relative expression of the four miRNAs between the healthy and mastitis cow's mammary gland tissues, bta-miR-296, bta-miR-2430, and bta-miR-671 were upregulated 1.75, 2.59, and 1.92-fold (p<0.05), respectively, in the tissues from cows with mastitis. Bta-miR–1777a showed no significant difference. Incontrast, bta-miR-2318 in mastitis gland tissue was downregulated 1.43-fold.
Identification of SNPs in the coding and UTR regions of the BOLA-DQA2 gene
Thirteen SNPs/multiple nucleotide length polymorphisms were identified in the coding and partial UTR regions (Table 1 and Supplementary Fig. S2). Four were missense mutations, changing the corresponding amino acid located in the domain structure (29th −110th amino acids). One SNP (c.+1283 C>T) in the 3′-UTR region may affect binding to the seed sequence of bta-miR-2318 (Fig. 4).
Table 1.
Single Nucleotide Polymorphisms in Coding Region and Analysis of Function
| Region | SNPs | Function (wild/mutant) |
|---|---|---|
| 5′-UTR | c.-2 G>C | GATA-1>IK-2 |
| CDS | c.+18 C>T | p.Ala6Ala |
| c.+27 A>G | p.Leu9Leu | |
| c.+90 C>T | p.His30His | |
| c.+250 GC>TT | p.Ala84Phe | |
| c.+280 CA>TT | p.His94Phe | |
| c.+292 G>C | p.Val98Leu | |
| c.+330 T>C | p.Asn110Asn | |
| c.+375 G>C | p.Leu125Leu | |
| c.+425 T>C | p.Val142Ala | |
| c.+601 C>T | p.Leu201Leu | |
| 3′-UTR | c.+1262 T>C | — |
| c.+1283 C>T | bta-miR-2318 |
SNP, single nucleotide polymorphisms; UTR, untranslated region.
FIG. 4.
One single nucleotide polymorphism (c. +1283 C>T) located in the seed region of bta-miR-2318 binding to the 3′-UTR of BOLA-DQA2.
Discussion
Identification and expression analysis of the SV of the BOLA-DQA2 gene
In the present study, two BOLA-DQA2 SVs were identified in the mammary gland, spleen, and kidney tissues in cows and the complete SV was not detected in the liver. The expression pattern of the SVs was tissue-specific, which may indicate functional differences. The ratio of alternatively spliced isoforms is tissue-specific and nearly all genes have differentially spliced regions (Pan et al., 2008; Wang et al., 2008). However, it is impossible to discuss the effects on its functionality without further analysis.
The results of differential expression of the SV in healthy and mastitis-infected mammary glands indicate that the two SV may play an important role on the response to pathogens by altering gene expression via the alternative splicing mechanism. There could be a relationship between the BOLA-DQA2 gene and mastitis in Chinese Holstein cattle. However, the exact nature of this needs to be elucidated.
Relationship between miRNAs expression and target 3′-UTR
In the present study, sequence alignment showed that the two BOLA-DQA2 SV include different length 3′-UTR. Animal miRNAs are a large class of small regulatory RNAs that regulate target genes by binding to 3′-UTRs of target mRNAs, and multiple binding sites for the same miRNA in 3′-UTRs can strongly enhance the degree of regulation (Fang and Rajewsky, 2011). miRNA target sites of BOLA-DQA2-SV1 were fewer than those of BOLA-DQA2-complete. We have investigated the differential expression of several target miRNAs between the healthy and mastitis-infected mammary glands by Q-PCR, which suggested that some of the predicted target miRNAs, including bta-miR-296, bta-miR-2430, and bta-miR-671 were upregulated. bta-miR-1777a, a common target miRNA of both transcripts, was not.
The impact of SNPs on miRNA and exonic splicing enhancer element
It is reported that up to 50% point mutations frequently cause genetic diseases by disrupting the correct pattern of pre-mRNA splicing (Cartegni et al., 2002). Some of point mutations have much more severe effects on the structure of the encoded protein, for example, when they inactivate an exonic splicing enhancer (ESE), thereby resulting in exon skipping. In the present study, one novel SV was found to be spliced in exon 3 and 3′-UTR of the BOLA-DQA2 gene. We analyzed whether the exonic SNPs influence the putative ESE element using the ESEfinder (http://exon.cshl.edu/ESE/). The predicted result showed that no SNP affect the ESE element.
During the course of the immune response, the miRNA–Argonaute complex interacts with the 3′-UTR of target mRNAs through complementary binding of the miRNA to the mRNA. This binding either blocks translation initiation, induces the endonucleolytic cleavage of the target mRNA, or both (Tili et al., 2008). In the present study, one SNP (c. +1283 C>T) in the 3′-UTR region may affect the imperfect complementary binding of the seed sequence and bta-miR-2318. Our Q-PCR result suggests the expression of bta-miR-2318 was downregulated in the mastitis mammary gland tissues. We speculate that the SNP maybe affect the binding of the bta-miR-2318 to the 3′-UTR of the BOLA-DQA2 gene though this requires confirmation. Overall, our findings suggest that the SV, miRNAs, and SNPs analyzed in this study may be significant in the BOLA-DQA2 gene function.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 31000543), the Support Program of the Ministry of Science and Technology, China (2011BAD19B02; 2011BAD19B04), Major Project of National Transgene in China (2011ZX08007-001), Program of National Cow Industrial Technology System (No. nycytx-0107), the Youth Science Foundation from Shandong Academy of Agriculture Science (No. 2006YQN030), and Projects of the Department of Science and Technology of Shandong Province (2007LZ10-04; Y2007D72).
Disclosure Statement
No competing financial interests exist.
References
- Cartegni L. Chew S.L. Krainer A.R. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet. 2002;3:285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- Cartegni L. Wang J. Zhu Z. Zhang M.Q. Krainer A.R. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z. Rajewsky N. The impact of miRNA target sites in coding sequences and in 3’-UTRs. PLoS One. 2011;6:e18067. doi: 10.1371/journal.pone.0018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries R. Hediger R. Stranzinger G. Tentative chromosomal localization of the bovine major histocompatibility complex by in situ hybridization. Anim Genet. 1986;17:287–294. doi: 10.1111/j.1365-2052.1986.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Fritz K.L. Texas A&M University; Texas, USA: 2009. Analysis of haplotype structure in the bovine major histocompatibility complex [Ph.D. thesis] [Google Scholar]
- Glass E.J. Oliver R.A. Russell G.C. Duplicated DQ haplotypes increase the complexity of restriction element usage in cattle. J Immunol. 2000;165:134–138. doi: 10.4049/jimmunol.165.1.134. [DOI] [PubMed] [Google Scholar]
- Huang H.Y. Chien C.H. Jen K.H. Huang H.D. RegRNA: a regulatory RNA motifs and elements finder. Nucleic Acids Res. 2006;34:W429–W434. doi: 10.1093/nar/gkl333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.M. Ju Z.H. Li Q.L. Hou Q.L. Wang C.F. Li J.B. Li R.L. Wang L.L. Sun T. Hang S.Q. Gao Y.D. Hou M.H. Zhong J.F. Solexa sequencing of novel and differentially expressed microRNAs in testicular and ovarian tissues in Holstein cattle. Int J Biol Sci. 2011;7:1016–1026. doi: 10.7150/ijbs.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.M. Liu G. Liu Y.P. Yao Y.C. Wu K.L. Fang M.Y. Splice variant identification and expression analysis of the fat mass and obesity-associated (FTO) gene in intact and castrated male pigs. DNA Cell Biol. 2010;29:729–733. doi: 10.1089/dna.2009.1004. [DOI] [PubMed] [Google Scholar]
- Juliarena M. Poli M. Sala L. Ceriani C. Gutierrez S. Dolcini G. Rodriguez E. Marino B. Rodriguez-Dubra C. Esteban E. Association of BLV infection profiles with alleles of the BoLA-DRB3.2 gene. Anim Genet. 2008;39:432–438. doi: 10.1111/j.1365-2052.2008.01750.x. [DOI] [PubMed] [Google Scholar]
- Kennedy L.J. Modrell A. Groves P. Wei Z. Single R.M. Happ G.M. Genetic diversity of the major histocompatibility complex class II in Alaskan caribou herds. Int J Immunogenet. 2011;38:109–119. doi: 10.1111/j.1744-313X.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- Kloosterman W.P. Plasterk R.H.A. The diverse functions of MicroRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Kwan T. Benovoy D. Dias C. Gurd S. Provencher C. Beaulieu P. Hudson T.J. Genome-wide analysis of transcript isoform variation in humans. Nat Genet. 2008;40:225–231. doi: 10.1038/ng.2007.57. [DOI] [PubMed] [Google Scholar]
- López-Benavides M.G. Lincoln University; New Zealand, USA: 2004. BoLA-DQA2 haplotypes and resistance to bovine mastitis [Ph.D. thesis] [Google Scholar]
- Lopez A.J. Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu Rev Genet. 1998;32:279–305. doi: 10.1146/annurev.genet.32.1.279. [DOI] [PubMed] [Google Scholar]
- Lynch K.W. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4:931–940. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- Maillard J. Berthier D. Chantal I. Thevenon S. Sidibé I. Stachurski F. Belemsaga D. Razafindraïbé H. Elsen J. Selection assisted by a BoLA-DR/DQ haplotype against susceptibility to bovine dermatophilosis. Genet Sel Evol. 2003;35:193–200. doi: 10.1186/1297-9686-35-S1-S193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C.P. He L. Tsai Y.C. Peng S. Kang T.H. Pang X. Monie A. Hung C.F. Wu T.C. In vivo microRNA-155 expression influences antigen-specific T cell-mediated immune responses generated by DNA vaccination. Cell Biosci. 2011;18:3. doi: 10.1186/2045-3701-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D.L. Rogers G.W. Cooper J.B. Hargrove G.L. Keown J.F. Heritability of intramammary infections at first parturition and relationships with sire transmitting abilities for somatic cell score, udder type traits, productive life, and protein yield. J Dairy Sci. 2003;86:2684–2695. doi: 10.3168/jds.S0022-0302(03)73864-8. [DOI] [PubMed] [Google Scholar]
- Pan Q. Shai O. Lee L.J. Frey B.J. Blencowe B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Tili E. Michaille J.J. Costinean S. Croce C.M. MicroRNAs, the immune system and rheumatic disease. Nat Clin Pract Rheumatol. 2008;4:534–541. doi: 10.1038/ncprheum0885. [DOI] [PubMed] [Google Scholar]
- Wang E.T. Sandberg R. Luo S.J. Khrebtukova I. Zhang L. Mayr C. Kingsmore S.F. Schroth G.P. Burge C.B. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Wang L. Song L. Zhao J. Qiu L. Gao Y. Song X. Li L. Zhang Y. Zhang L. The genomic structure, alternative splicing and immune response of Chlamys farreri thioester-containing protein. Dev Comp Immunol. 2009;33:1070–1076. doi: 10.1016/j.dci.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Zikherman J. Weiss A. Alternative splicing of CD45: the tip of the iceberg. Immunity. 2008;29:839–841. doi: 10.1016/j.immuni.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Zimin A.V. Delcher A.L. Florea L. Kelley D.R. Schatz M.C. Puiu D. Hanrahan F. Pertea G. Van Tassell C.P. Sonstegard T.S. Marçais G. Roberts M. Subramanian P. Yorke J.A. Salzberg S.L. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009;10:R42. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.