Abstract
Four types of human T cell lymphotropic viruses (HTLV) have been described (HTLV-1 to HTLV-4) with three of them having closely related simian virus analogues named STLV-1, −2, and −3. To assess the risk of cross-species transmissions of STLVs from nonhuman primates to humans in the Democratic Republic of Congo, a total of 330 samples, derived from primate bushmeat, were collected at remote forest sites where people rely on bushmeat for subsistence. STLV prevalences and genetic diversity were estimated by PCR and sequence analysis of tax-rex and LTR fragments. Overall, 7.9% of nonhuman primate bushmeat is infected with STLVs. We documented new STLV-1 and STLV-3 variants in six out of the seven species tested and showed for the first time STLV infection in C. mona wolfi, C. ascanius whitesidei, L. aterrimus aterrimus, C. angolensis, and P. tholloni. Our results provide increasing evidence that the diversity and geographic distribution of PTLVs are much greater than previously thought.
Like the majority of infectious diseases, human T cell lymphotropic viruses (HTLVs) have also a zoonotic origin.1 HTLVs and their simian analogues (STLVs) are collectively called primate T cell lymphotropic viruses (PTLV). To date four types of HTLV, type 1 to 4, have been described in humans with three of them having simian counterparts.2 No simian virus analogue has yet been identified only for the recently discovered HTLV-4.3 While HTLV-1 has spread worldwide and infects 15 to 20 million people, HTLV-2 is mainly restricted to central Africa, Amerindians in South America, and some intravenous drug user (IDU) populations in Europe and the United States.4,5 HTLV-3 and -4 have been discovered more recently, and today only a few cases have been observed, all in Cameroon among individuals reporting hunting of nonhuman primates (NHP).3,6,7
Most people infected with HTLV-1 remain asymptomatic, but HTLV-1 can cause adult T cell leukemia, neurological disorders such as HTLV-1-associate myelopathy (HAM), also known as tropical spastic paraparesis (TSP), and has also been associated with inflammatory disease.8 HTLV-2 is less pathogenic and only a few cases of a neurological disease similar to HAM/TSP have been documented.9 No information is available yet for the recently described HTLV-3 and HTLV-4, but the viral structure of HTLV-3 suggests a pathogenic potential similar to HTLV-1, and more studies are needed to determine whether molecular features of HTLV-4 are associated with pathogenicity.10,11
Whereas STLV-1 has been documented in a wide variety of old world monkey species and apes from sub-Saharan Africa and Asia, STLV-3 has only been seen in African monkeys and STLV-2 has been detected in captive bonobos, an ape species endemic in the Democratic Republic of Congo (DRC).1,12–15 STLV-1 viruses are interspersed within the different HTLV-1 subtypes and the recently discovered HTLV-3 strains are also closely related to STLV-3 strains from primates in the same geographic areas.2,3,7,8 These observations suggest multiple cross-species transmissions from NHPs to humans. Moreover, the recent identification of new HTLV-1 and HTLV-3 variants in Cameroonian hunters, with high nucleotide identity to STLVs from monkeys hunted in this region, suggests even relatively recent cross-species transmission and ongoing zoonotic infections of STLVs in persons exposed to NHPs.3,7 In contrast to other retroviruses, PTLV-1 and PTLV-3 strains cluster according to geographic location rather than to the host species, suggesting the ease with which STLVs are transmitted among different NHP species.1,13–15
The recent discovery of STLV-3, HTLV-3, and HTLV-4 shows that our knowledge of the diversity of STLV in NHPs and HTLV in humans is still limited and further studies are thus needed to determine the prevalence, geographic distribution, and genetic diversity of PTLVs in humans and NHPs. Although STLV-2 has been identified in two independent troops of captive bonobos (Pan paniscus), this virus has not yet been documented in wild primates. In addition, the zoonotic relationship of this divergent virus to HTLV-2 is less clear than for PTLV-1 and −3.12 Today humans are still hunting and butchering a wide diversity of primate species and the possibility of additional cross-species transfers has to be considered.16,17 To better understand the simian reservoir of HTLV-2 and HTLV-4, and to document the wide diversity of STLVs to which humans are exposed through hunting and butchering, we investigated the diversity of STLV infection in nonhuman primate bushmeat from the DRC, home to a wide diversity of endemic primate species.
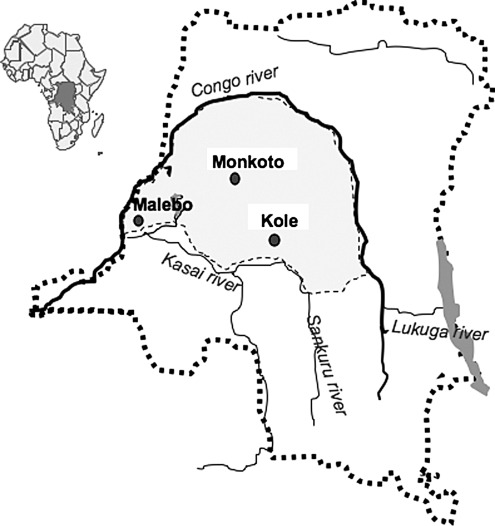
A total of 330 samples from NHP bushmeat were collected between May 2009 and July 2010 as dried blood spots (DBS) around three rural cities in DRC: Kole in Kasai Oriental province (n=258), Malebo in Bandundu province (n=43), and Monkoto in Equateur province (n=29) (Fig. 1). Whole blood was collected from primate bushmeat sold on the market and/or in the villages and was spotted onto a filter 903 FTA card (Whatman plc, Kent, UK). Each paper card was then identified with a sample ID, the site and date of sample collection, as well as the corresponding primate species based on visual identification according to the primate taxonomic classification provided by Groves.18 After air drying at ambient temperature, NHP DBS were wrapped into individual envelopes and stored at ambient temperature. Information provided by the owners (vendors) indicated that the animals died 6–78 h prior to the sampling. All primate samples were obtained with government approval from Congolese Ministry of Environment and Health and the National Ethics Committee. Bushmeat samples were obtained through a strategy specifically designed not to increase demand, i.e., women or men preparing and/or preserving the meat for subsequent sale and hunters already involved in the trade were asked for permission to sample blood from carcasses, which were then returned.17
FIG. 1.
Nonhuman primate sampling sites in the Democratic Republic of Congo.
To ascertain the primate species identification done in the field, we extracted DNA from all the DBS samples with the Biomerieux DNA extraction Kit (Biomerieux, Craponne, France) with minor changes19 to amplify and sequence a fragment of the 12S rRNA gene using primers 12S-L1091 and 12S-H1478 to confirm the NHP species as previously described.14,17 Sequence analysis of the 12S rRNA gene revealed the presence of seven species: 147 yellow nosed red tailed guenons (Cercopithecus ascanius whitesidei), 79 Tshuapa red colobus (Piliocolobus tholloni), 33 Wolf's monkeys (Cercopithecus mona wolfi), 33 black mangabeys (Lophocebus atterrimus atterrimus), 25 Angolan pied colobus (Colobus angolensis), 10 De Brazza monkeys (Cercopithecus neglectus), and 3 Allen swamp monkeys (Allenopithecus negroviridis). Four of the seven species or subspecies are endemic to DRC18.
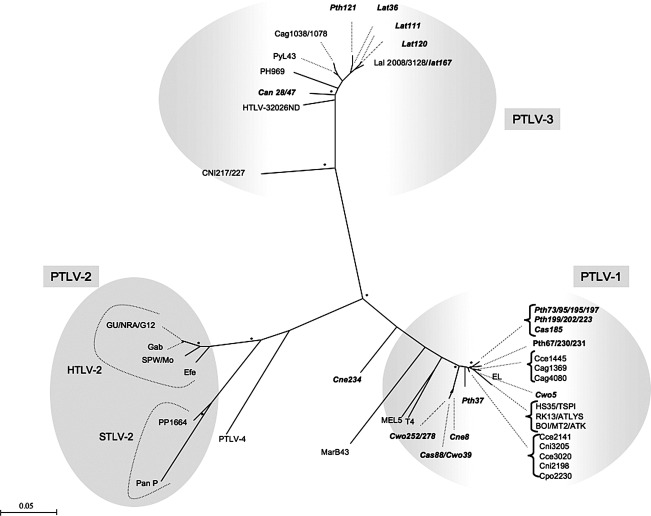
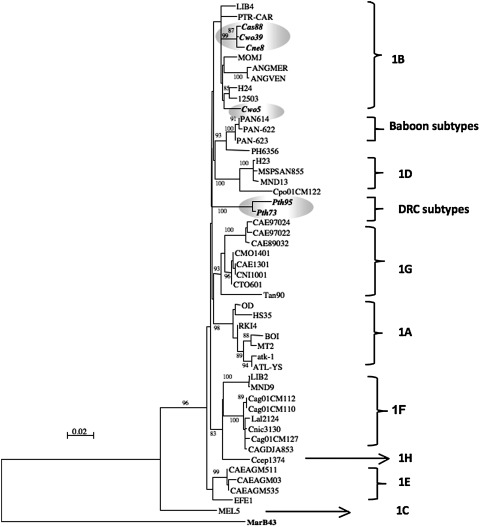
To detect STLV infection, polymerase chain reaction (PCR) analyses were performed on all DBS samples using previously described diagnostic tax-rex PCR (220 bp) allowing detection of all PTLVs.14 STLV infection was identified in 26 (7.9%) of the 330 DBS samples (Table 1). Except for Allan swamp's monkeys, for which only three samples were available, all other species were infected with STLV and prevalences ranged between 1.4% and 20% per species. STLV-positive animals were identified only in Kole; all samples collected in Malebo and Monkoto were negative by PCR screening. Newly derived STLV nucleotide sequences were aligned with reference sequences from the GenBank as well as STLVs previously characterized in the laboratory by using MEGA4 and Clustal X v.2 with minor manual adjustments when necessary.20 Nucleotide sites that could not be unambiguously aligned were excluded from the analyses. Appropriate models of evolution were selected for each data set using Topali v2.5 software21 and maximum likelihood phylogenies were reconstructed using PHYML.22 The analyses were performed using a discrete gamma distribution to account for variable substitution rates among sites with four rate categories and the TN93 model. Nucleotide frequencies, nucleotide changes rate, and gamma distribution shape parameters were estimated from the data. The starting tree was obtained by using PHYML. One hundred bootstrap replications were performed to assess confidence in the topology. Sequence and phylogenetic analyses of the tax fragment identified 17 STLV-1 and seven STLV-3, and one STLV sequence, derived from a De Brazza monkey (Cercopithecus neglectus, Cne234), could not be clearly classified in a known PTLV lineage (Fig. 2).
Table 1.
The Number and Percentages of Simian T Cell Lymphotropic Virus-Positive Samples per Species as Detected by Polymerase Chain Reaction Followed by Sequence and Phylogenetic Tree Analysis as Described
| Species | Common name | STLV-1 N/N tested (%) | STLV-3 N/N tested (%) | Other STLV N/N tested (%) | Total N/N tested (%) |
|---|---|---|---|---|---|
| Allenopithecus negroviridis | Allan swamp monkey | 0/3 (0.0%) | 0/3 (0.0%) | 0/3 (0.0%) | 0/3 (0.0%) |
| Colobus angolensis | Angola pied colobus | 0/25 (0.0%) | 2/25 (8.0%) | 0/25 (0.0%) | 2/25 (8.0%) |
| Cercopithecus neglectus | De Brazza monkey | 1/10 (10.0%) | 0/10 (0.0%) | 1/10 (10.0%) | 2/10 (20.0%) |
| Cercopithecus ascanius | Red tailed monkey | 2/147 (1.4%) | 0/147 (0.0%) | 0/147 (0.0%) | 2/147 (1.4%) |
| Cercopithecus wolfi | Wolf's monkey | 4/33 (9.1%) | 0/33 (0.0%) | 0/33 (0.0%) | 4/33 (12.1%) |
| Lophocebus aterrimus | Black mangabey | 0/33 (0.0%) | 4/33 (9.1%) | 0/33 (0.0%) | 4/33 (12.1%) |
| Piliocolobus tholloni | Thsuapa red colobus | 11/79 (13.9%) | 1/79 (1.3%) | 0/79 (0.0%) | 12/79 (15.1%) |
| Total | 18/330 (5.4%) | 7/330 (2.1%) | 1/330 (0.3%) | 26/330 (7.9%) |
STLV, simian T cell lymphotropic virus.
FIG. 2.
Primate T cell lymphotropic virus (PTLV) phylogeny inferred using 195-bp tax-rex sequences. Numbers correspond to internal branch support derived from 100 bootstrap replicates (only values above 80 are shown). Scale bar represents the number of nucleotide substitutions per site. Groves' primate taxonomy nomenclature is used.18 Nonhuman primates are coded using the first letter of the genus followed by the first two letters of the species name: Cwo, Cercopithecus wolfi; Cas, Cercopithecus ascanius; Cne, Cercopithecus neglectus; Can, Colobus angolensis; Pth, Piliocolobus tholloni; and Lat, Lophocebus aterrimus. New sequences from this study are highlighted in italic and bold.
STLV-1 infection was identified in four species: Cercopithecus ascanius (n=2), Cercopithecus wolfi (n=5), Piliocolobus tholloni (n=11), and C. neglectus (n=1). Three species, Colobus angolensis (n=2), Piliocolobus tholloni (n=1), and Lophocebus aterrimus (n=4), were infected with STLV-3. Finally, STLV-1 and STLV-3 cocirculate in Piliocolobus tholloni but we did not observe coinfected animals. Although the genomic region for the diagnostic PCR is small and phylogenetic signals are weak, phylogenetic analysis of the new 195-bp tax-rex STLV sequences from this study revealed a high genetic diversity in primates around Kole. For STLV-1, two major groups were observed; one group was composed of eight strains amplified from Piliocolobus tholloni and one strain from Cercopithecus ascanius (Cas185), and a second group contained strains from Cercopithecus wolfi (Cwo252, 39, 278), Cercopithecus neglectus (Cne8), and Cercopithecus ascanius (Cas88). Different lineages were also seen in the STLV-3 radiation.
To further characterize the new STLVs from this study, we amplified a fragment of LTR for at least one STLV-1 or STLV-3-positive sample per monkey species. For STLV-1, seminested PCRs were performed using 8255not and LTRU5E or Enh280 and 5PLTR as primers for the first round and 8255not and 420LTR or tatabox and 5PLTR as primers for the second round, as previously described, to obtain fragments of 418, 450, or 780 bp.13,14 For STLV-3, a 678- to 900-bp fragment in the LTR region was amplified by the combination of previously described and newly designed primers. Seminested PCRs were performed using AV45 or AV51 and px-LTRas as primers in the first round and AV42 or px-LTRs and px-LTRas as primers for the second round as previously described.14 Newly designed primers were P3MPLF1 (5′-CVACCACTGCTACRACCCCCAAG-3′) and P3MPLR1 (5′- CRGATGATTCAGCTATTTGTCCTC-3′) primers as first round and P3MPLF2 (5′- AAGAYACWCCCCCTTCCGAAAC-3′) and P3MPLR2 (5′- CCGTCTCGRGGYTCATCATC -3′) as second round. PCR for both rounds were performed using the expand High Fidelity PCR kit (Roche Molecular Biochemicals, Mannheim, Germany) and included a hot start (94°C for 3 min) with the following cycle conditions: 14 cycles of denaturation at 95°C for 20 s, annealing at 56°C for 30 s (with temperature decreasing 0.5°C per cycle), and extension at 72°C for 1 min followed by 30 cycles of denaturation at 95°C for 20 s, annealing at 52°C for 30 s, and extension for 1 min. PCR products were purified on 1% agarose gel with a GENECLEAN Turbo kit (Q.BIOgene, MP Biochemichals) and direct sequencing of both strands with ABI PRISM Big Dye Terminator Cycle sequencing Ready Reaction kit with amplitaq FS DNA polymerase on an automated sequencer (ABI 3130XL, applied Biosystems, Courtaboeuf, France). Sequences were then assembled using the software package Lasergene (DNASTAR, Inc., Madison, WI).
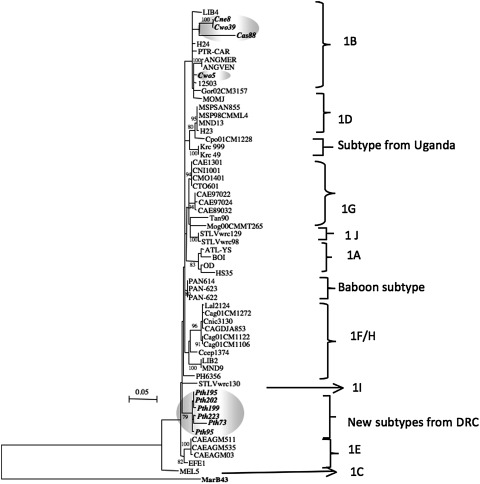
A total of 12 new partial STLV-1 LTR sequences were obtained; a 450-bp sequence (3′ end of LTR) was amplified and sequenced for 10 samples, for eight samples a 418-bp (5′ end of LTR) fragment was obtained, and for six samples the 780 bp was sequenced. Figure 3 shows the corresponding phylogenetic trees, obtained by methods described above, and illustrates the presence of two different STLV-1 subtypes. The majority of the STLV-1 strains obtained from P. tholloni formed a separate cluster, probably representing a new subtype. The remaining strains obtained from C. wolfi, C. neglectus, and C. ascanius together with two strains from P. tholloni fall within subtype B, which is widespread among different primate species in Central Africa.
FIG. 3.
Inference of PTLV-1 phylogeny using 418 bp (3′ end LTR) (a), 401bp (5′ end LTR) (b), and 750 bp (c) LTR sequences. Numbers correspond to internal branch support derived from 100 bootstrap replicates (only values above 75 are shown). Scale bar represents the number of nucleotide substitution per site. Groves' primate taxonomy nomenclature is used (Groves, 2001). Nonhuman primates are coded using the first letter of the genus followed by the first two letters of the species name: Cwo, Cercopithecus wolfi; Cas, Cercopithecus ascanius; Cne, Cercopithecus neglectus; Can, Colobus angolensis; Pth, Piliocolobus tholloni; and Lat, Lophocebus aterrimus. Sequences from this study are highlighted in italic and bold.
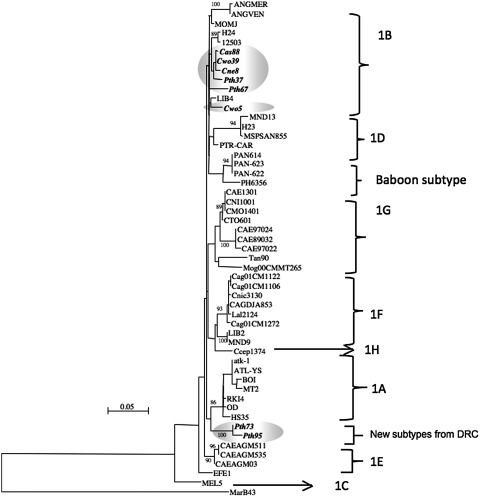
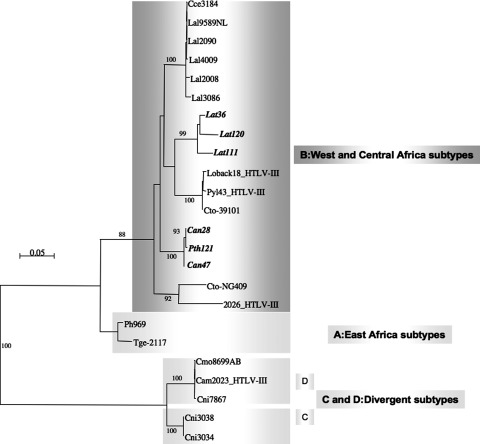
Six new STLV-3 sequences were obtained in LTR; for three samples a 900-bp fragment could be amplified (Can28, Can47, and Lat36) and sequenced and for three only a 680-bp fragment was obtained (Lat111, Lat120, and Pth121). The six new LTR sequences formed two separate clusters in the STLV-3 central African subtype B radiation; one cluster consisted of the two strains derived from Colobus angolensis and the single strain derived from Piliocolobus tholloni, whereas the other cluster was formed by the three strains derived from Lophocebus atterrimus (Fig. 4). Unfortunately, all attempts to amplify fragments of the LTR region were unsuccessfull for the divergent strain derived from C. neglectus.
FIG. 4.
Inference of PTLV-3 phylogeny using 589 bp tax-pX-LTR sequences. Numbers correspond to internal branch support derived from 100 bootstrap replications (only values above 85 are shown). Scale bar represents the number of nucleotide substitutions per site. Primate taxonomy nomenclature is according to Groves (Groves, 2001). Nonhuman primates are coded using the first letter of the genus followed by the first two letters of the species name: Lal, Lophocebus albigena; Cag, Cercocebus agilis; Cce, Cercopithecus cephus. Sequences in italic and bold text are from the current study.25
In this study we analyzed the prevalence and genetic diversity of STLVs on 330 DBS samples derived from primate bushmeat in DRC. No extensive studies have yet been conducted on STLV infection in monkeys from DRC, which mainly harbors endemic species because of the geographic barriers constituted by the Congo, Ubangui, and Kasai rivers.18 Overall, we showed that 7.9% of primate bushmeat in our study is infected with STLV, 70% with STLV-1, and the remaining part with STLV-3. Similarly, as observed in previous studies, prevalences varied per species, ranging here from 1.4% to 20%.14,23,24 Although STLV-2 infections have today been described only in bonobos (Pan paniscus), a species endemic to DRC, we did not observe STLV-2 infections in other primate species from DRC and no simian counterpart for HTLV-4 was identified. However, we show for the first time STLV infection in C. mona wolfi, C. ascanius whitesidei, L. aterrimus aterrimus, C. angolensis, and P. tholloni, which are all species or subspecies that are endemic in DRC or present only in DRC and neighboring countries. For the first time we also identified STLV infection in De Brazza monkeys (C. neglectus), because samples from this species in previous studies conducted in Cameroon were negative.14
Although our sample size was small and covers only a small geographic area of DRC, we observed a high variety of STLV variants, including most likely a new STLV-1 subtype and new lineages in the STLV-3 subtype B from west and central Africa. We also documented a new STLV strain in C. neglectus, which could not be classified into a known PTLV type; unfortunately we obtained only a small fragment in tax and were not able to amplify a larger fragment in LTR to better characterize this new variant. Three different STLV variants cocirculate among P. tholloni (Tshuapa red colobus) in which STLV-1 subtype B, a new STLV-1 subtype, and STLV-3 have been identified. These results are consistent with the occurrence of multiple cross-species transmissions between different NHP species as well as between primates and humans.3,6,9,13,14
Exposure to blood, secretions, or tissues from infected primates, through hunting and butchering of bushmeat, represents the most plausible source for human infection with STLV. As illustrated in this and other studies, humans are still hunting and butchering a wide diversity of primate species today and the possibility of ongoing cross-species transfers has to be considered.16,17 Our observation of 7.9% prevalence of STLV infection among primate bushmeat is comparable to the 8 –11% prevalences recently found in primate bushmeat in Cameroon.14,23
In summary, we found broad diversity of STLV in NHPs from DRC and identified a potential new PTLV lineage. Our results provide increasing evidence that the diversity and geographic distribution of PTLVs are much greater than previously thought. Nonetheless, screening of larger numbers of NHPs in DRC is required to understand the STLV diversity and evolution in this part of Africa, which harbors a large number of endemic primate species. This high degree of PTLV divergence observed in Central Africa provides further evidence that the ancestor of these different types of viruses must be of African origin. Given the ongoing contacts between infected NHP and African populations through hunting and butchering, it is likely that STLV cross-species transmissions are still occurring. Given the enormous country size, absence of road infrastructure, and difficult communication, people in DRC rely on bushmeat for subsistence in many areas.25 Moreover, 60% of the approximately 70 million inhabitants live in rural areas, suggesting high levels of exposure among people living in DRC. The increasing genetic diversity of STLVs can also be a challenge for HTLV diagnosis, especially for PTLV-3, because such antigens are not used in commercially available screening and confirmation assays. Today, no or few data are available for HTLV infections and their genetic diversity in humans in DRC, but our findings of divergent STLV-3 in hunted primate species suggest that HTLV-3 infections could also be present in this country.
Accession Numbers
The sequences described in this paper were submitted to the GenBank Nucleotide Sequence Database under accession numbers JN210930 to JN210967.
Acknowledgments
This work was supported in part by grants from the National Institute of Health (RO1 AI 50529) and the Agence Nationale de Recherches sur le SIDA (ANRS 12125/12182). Steve Ahuka-Mundeke is supported by a grant from Infectiopole Sud, France. We thank the Ministries of Health and Environment and the National Ethic committee from the DRC for permission to perform this study and all the field staff from DRC. We thank the staff of the World Wildlife Fund for Nature (WWF) in DRC and Dr. Didier Mazongo, Drs. Lisette and Gerry Makaya, Mrs. Bucard Besomba, and Mr. Vincent Ntshikala for their collaboration and participation in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Vandamme AM. Salemi M. Desmyter J. The simian origins of the pathogenic human T-cell lymphotropic virus type I. Trends Microbiol. 1998;6:477–483. doi: 10.1016/s0966-842x(98)01406-1. [DOI] [PubMed] [Google Scholar]
- 2.Mahieux R. Gessain A. The human HTLV-3 and HTLV-4 retroviruses: New members of the HTLV family. Pathol Biol (Paris) 2009;57:161–166. doi: 10.1016/j.patbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe ND. Heneine W. Carr JK. Garcia AD. Shanmugam V. Tamoufe U, et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc Natl Acad Sci USA. 2005;102:7994–7999. doi: 10.1073/pnas.0501734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proietti FA. Carneiro-Proietti AB. Catalan-Soares BC. Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 5.Switzer W. Pieniazek D. Swanson P. Samdal HH. Soriano V. Khabbaz RF, et al. Phylogenetic relationship and geographic distribution of multiple human T-lymphotropic virus type II subtypes. J Virol. 1995;69:621–632. doi: 10.1128/jvi.69.2.621-632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calattini S. Chevalier SA. Duprez R. Bassot S. Froment A. Mahieux R. Gessain A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology. 2005;9(2):30. doi: 10.1186/1742-4690-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H. Wolfe ND. Sintasath DM. Tamoufe U. Lebreton M. Djoko CF, et al. Emergence of a novel and highly divergent HTLV-3 in a primate hunter in Cameroon. Virology. 2010;401:137–145. doi: 10.1016/j.virol.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdonck K. Gonzalez E. Van Dooren S. Vandamme AM. Vanham G. Gotuzzo E. Human T-lymphotropic virus 1: Recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–281. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 9.Feuer G. Green PL. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene. 2005;24:5996–6004. doi: 10.1038/sj.onc.1208971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calattini S. Chevalier SA. Duprez R. Afonso P. Froment A. Gessain A. Mahieux R. Human T-cell lymphotropic virus type 3: Complete nucleotide sequence and characterization of the human tax3 protein. J Virol. 2006;80:9876–9888. doi: 10.1128/JVI.00799-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Switzer WM. Salemi M. Qari SH. Jia H. Gray RR. Katzourakis A, et al. Ancient, independent evolution and distinct molecular features of the novel human T-lymphotropic virus type 4. Retrovirology. 2009;6:9. doi: 10.1186/1742-4690-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Brussel M. Salemi M. Liu HF. Goubau P. Desmyter J. Vandamme AM. The discovery of two new divergent STLVs has implications for the evolution and epidemiology of HTLVs. Rev Med Virol. 1999;9:155–170. doi: 10.1002/(sici)1099-1654(199907/09)9:3<155::aid-rmv242>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Mahieux R. Ibrahim F. Mauclere P. Herve V. Michel P. Tekaia F, et al. Molecular epidemiology of 58 new African human T-cell leukemia virus type 1 (HTLV-1) strains: Identification of a new and distinct HTLV-1 molecular subtype in Central Africa and in Pygmies. J Virol. 1997;71:1317–1333. doi: 10.1128/jvi.71.2.1317-1333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liégeois F. Lafay B. Switzer WM. Locatelli S. Mpoudi-Ngolé E. Loul S, et al. Identification and molecular characterization of new STLV-1 and STLV-3 strains in wild-caught nonhuman primates in Cameroon. Virology. 2008;371:405–417. doi: 10.1016/j.virol.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Van Dooren S. Verschoor EJ. Fagrouch Z. Vandamme AM. Phylogeny of primate T lymphotropic virus type 1 (PTLV-1) including various new Asian and African non-human primate strains. Infect Genet Evol. 2007;7:374–381. doi: 10.1016/j.meegid.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe ND. Prosser TA. Carr JK. Tamoufe U. Mpoudi-Ngole E. Torimiro JN, et al. Exposure to nonhuman primates in rural Cameroon. Emerg Infect Dis. 2004;10:2094–2099. doi: 10.3201/eid1012.040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghokeng AF. Ayouba A. Mpoudi-Ngole E. Loul S. Liegeois F. Delaporte E. Peeters M. Extensive survey on the prevalence and genetic diversity of SIVs in primate bushmeat provides insights into risks for potential new cross-species transmissions. Infect Genet Evol. 2010;10:386–396. doi: 10.1016/j.meegid.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groves C. Primate Taxonomy. Smithsonian Series in Comparative Evolutionary Biology, Smithsonian Institution Press; 2001. [Google Scholar]
- 19.Monleau M. Butel C. Delaporte E. Boillot F. Peeters M. Effect of storage conditions of dried plasma and blood spots on HIV-1 RNA quantification and PCR amplification for drug resistance genotyping. J Antimicrob Chemother. 2010;65:1562–1566. doi: 10.1093/jac/dkq205. [DOI] [PubMed] [Google Scholar]
- 20.Larkin MA. Blackshields G. Brown NP. Chenna R. McGettigan PA. McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 21.Milne I. Lindner D. Bayer M. Husmeier D. McGuire G. Marshall DF, et al. TOPALi v2: a rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics. 2009;25:126–127. doi: 10.1093/bioinformatics/btn575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guindon S. Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 23.Sintasath DM. Wolfe ND. Lebreton M. Jia H. Garcia AD. Le Doux-Diffo J, et al. Simian T-lymphotropic virus diversity among nonhuman primates, Cameroon. Emerg Infect Dis. 2009;15:175–184. doi: 10.3201/eid1502.080584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leendertz SA. Junglen S. Hedemann C. Goffe A. Calvignac S. Boesch C. Leendertz FH. High prevalence, coinfection rate, and genetic diversity of retroviruses in wild red colobus monkeys (Piliocolobus badius badius) in Tai National Park, Cote d'Ivoire. J Virol. 2010;84:7427–7436. doi: 10.1128/JVI.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkie DS. Carpenter JF. Bushmeat hunting in the Congo Basin: An assessment of impacts and options for mitigation. Biodiversity Conservation. 1999;8:927–955. [Google Scholar]