Abstract
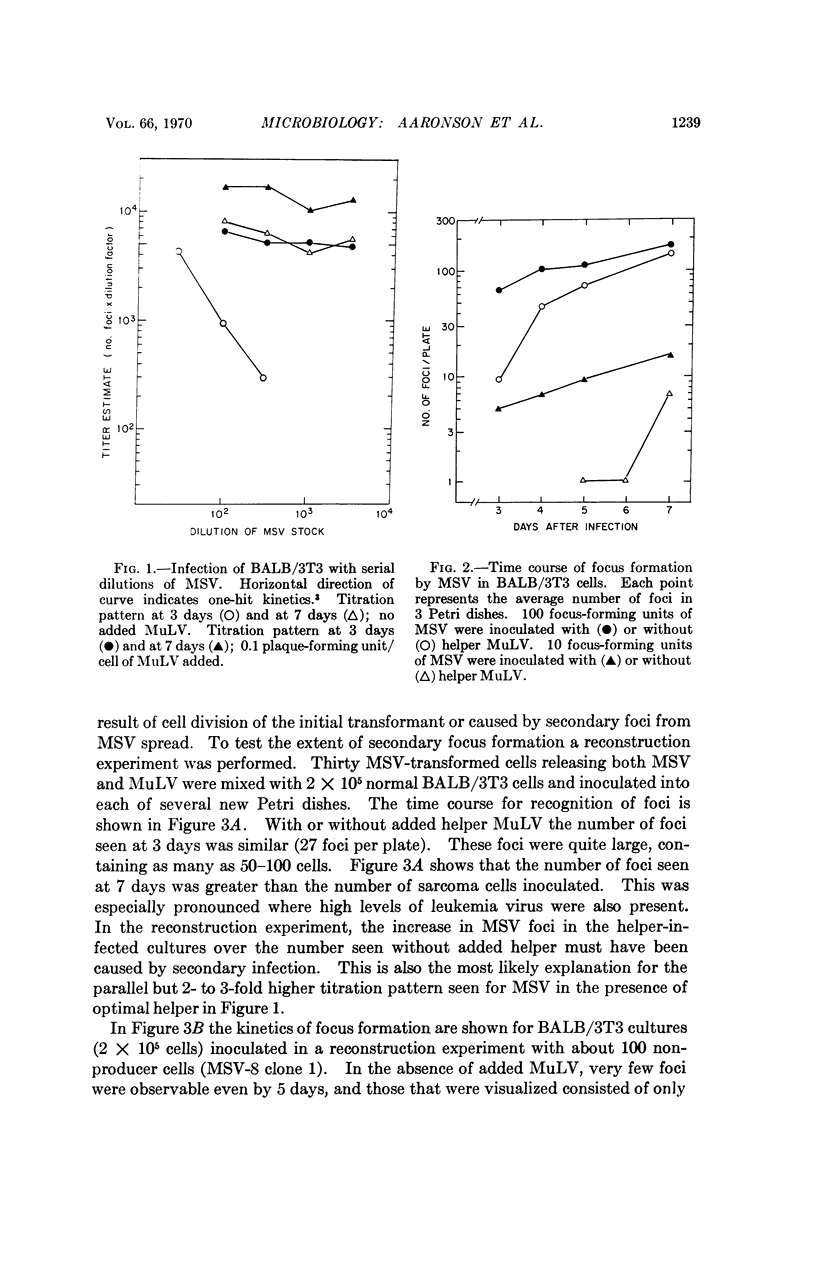
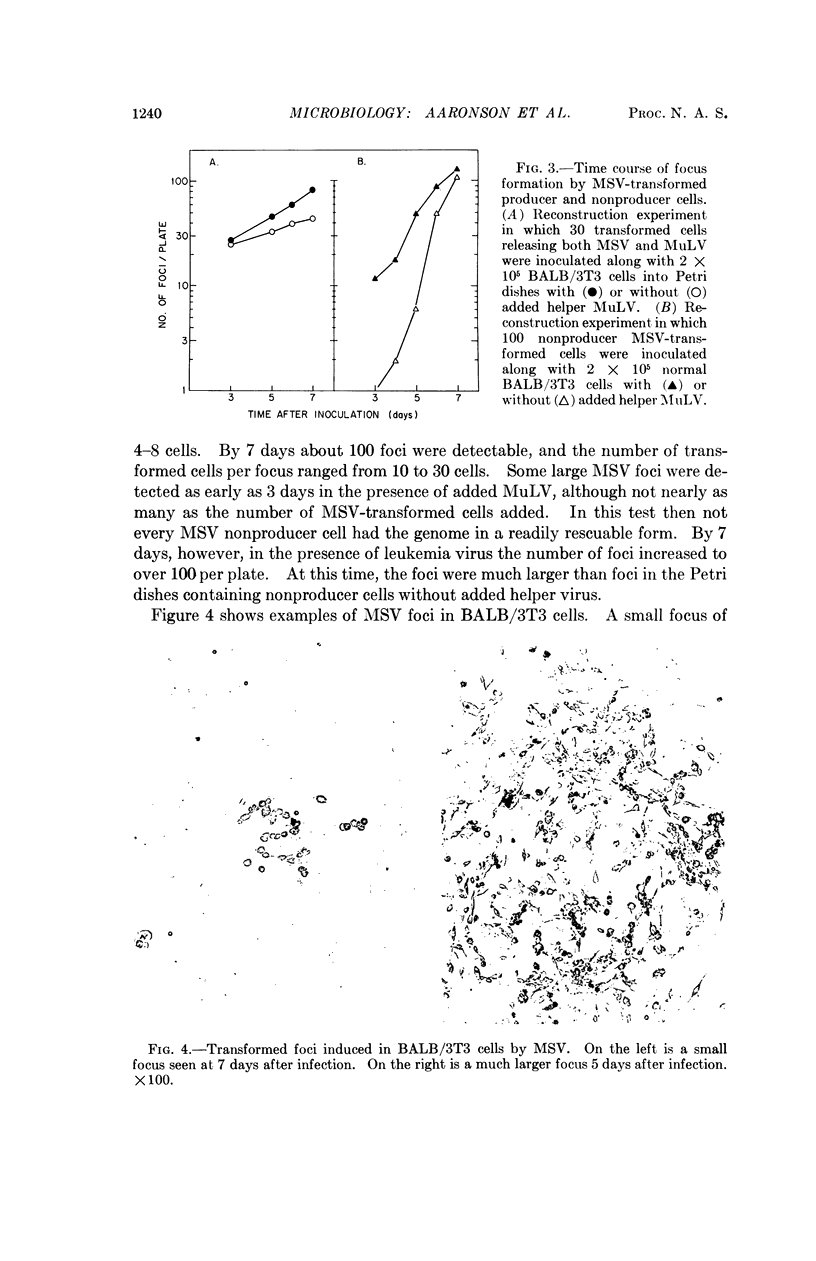
Murine sarcoma virus induces foci of morphologically altered cells in BALB/3T3 cultures. Focus formation in mouse cells has been thought to require the presence of a helper, murine leukemia virus, which is present in murine sarcoma virus stocks but by itself does not induce any morphological transformation of mouse cells. The present studies show that early after infection, the titration pattern for murine sarcoma virus in BALB/3T3 cells is “two-hit” since only foci produced by virus spread can be detected. Such foci require the presence of both viruses in the initially infected cell. By seven days the titration pattern is “one-hit” under culture conditions which allow the growth and detection of small foci of transformed cells induced by murine sarcoma virus alone. The “two-hit” titration pattern results from the inability to detect these foci. We conclude that murine sarcoma virus is able to transform mouse cells without requiring murine leukemia virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Cell transformation by viruses. Science. 1969 Nov 21;166(3908):962–968. doi: 10.1126/science.166.3908.962. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Wolford R., Huebner R. J. Adenovirus type 12-rat embryo transformation system. J Virol. 1967 Apr;1(2):362–367. doi: 10.1128/jvi.1.2.362-367.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Complement fixation and tissue culture assays for mouse leukemia viruses. Proc Natl Acad Sci U S A. 1965 May;53(5):931–938. doi: 10.1073/pnas.53.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J. The murine leukemia-sarcoma virus complex. Proc Natl Acad Sci U S A. 1967 Sep;58(3):835–842. doi: 10.1073/pnas.58.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- O'Connor T. E., Fischinger P. J. Titration patterns of a murine sarcoma-leukemia virus complex: evidence for existence of competent sarcoma virions. Science. 1968 Jan 19;159(3812):325–329. doi: 10.1126/science.159.3812.325. [DOI] [PubMed] [Google Scholar]
- Parkman R., Levy J. A., Ting R. C. Murine sarcoma virus: the question of defectiveness. Science. 1970 Apr 17;168(3929):387–389. doi: 10.1126/science.168.3929.387. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958 Dec;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Aaronson S. A. Properties of clonal lines of murine sarcoma virus transformed Balb-3T3 cells. Virology. 1969 May;38(1):174–179. doi: 10.1016/0042-6822(69)90140-8. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Green H. Cell growth and the initiation of transformation by SV40. Proc Natl Acad Sci U S A. 1966 Feb;55(2):302–308. doi: 10.1073/pnas.55.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K. DEAE-dextran: enhancement of cellular transformation induced by avian sarcoma viruses. Virology. 1967 Sep;33(1):175–177. doi: 10.1016/0042-6822(67)90109-2. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y., Ikawa Y., Sugano H. Transformation of a mouse cell line by murine sarcoma virus (Moloney). Int J Cancer. 1968 Nov 15;3(6):743–750. doi: 10.1002/ijc.2910030607. [DOI] [PubMed] [Google Scholar]