Abstract
Background
Current American Thyroid Association (ATA) guidelines recommend routine cervical ultrasound (US) in thyroid nodule evaluation. Specific US characteristics can help diagnose papillary thyroid carcinoma (PTC). The aim of this blinded cohort study was to determine whether these specific US characteristics can also reliably detect the more aggressive variants of PTC that are often associated with the BRAFV600E mutation.
Methods
After Institutional Review Board approval, we identified a cohort of patients from January 2007 to December 2009 with histologic PTC≥1 cm who had cervical US, initial thyroid surgery, and molecular testing for BRAFV600E on fine-needle aspiration biopsy or histology. Preoperative US images were evaluated by a single radiologist, who was blinded to BRAF status, for nodule size and the presence or absence of the following suspicious US features: taller-than-wide shape, ill-defined margins, hypoechogenicity, calcifications, noncystic composition, and absent halo.
Results
BRAF-positivity was associated with most known suspicious US findings, including taller-than-wide shape (47% vs. 7%, p<0.001), ill-defined margins (42% vs. 9%, p<0.001), hypoechogenicity (83% vs. 36%, p<0.001), micro/macrocalcifications (87% vs. 24%, p<0.001), and absent halo (85% vs. 27%, p<0.001) but was not associated with noncystic composition. When ≥3 suspicious US features were present, BRAF-positivity was predicted with a positive predictive value of 82%. The absence of suspicious US features together with negative BRAF testing predicted PTC without extrathyroidal extension or lymph node metastasis (negative predictive value 88%).
Conclusions
With routine preoperative cervical US and molecular testing, a trained radiologist or surgeon can improve the preoperative characterization of PTC, potentially impacting risk stratification and initial surgical management.
Introduction
Thyroid nodules are common, and the incidence of papillary thyroid carcinoma (PTC) is rising (1,2). The systematic evaluation of thyroid nodules is, thus, important and can be guided by evidence-based consensus recommendations such as those from the American Thyroid Association (ATA) (3) and the American Association of Clinical Endocrinologists (AACE) (4). Standard care relies on a thorough history and physical, in addition to radiographic characterization by cervical ultrasound (US).
US has been a valuable tool in characterizing and diagnosing thyroid pathology for over fifty years. Initially, crude US images were only able to differentiate cystic from solid lesions, but the field has undergone rapid technological evolution and now includes color Doppler, B-mode imaging, three-dimensional imaging, and elastography, allowing physicians to provide a much more detailed assessment. Improved discrimination between benign and malignant nodules can help determine which nodules should undergo fine-needle aspiration biopsy (FNA), and numerous malignant sonographic features have been described, including microcalcifications, hypoechogenicity, an irregular margin, loss of the halo, noncystic composition, intratumoral hypervascularity, and taller-than-wide shape (5–8). In a large, multicenter, and retrospective study of thyroid nodule US features, Moon et al. (9) reported that several of these features predict thyroid malignancy with varying sensitivity and specificity.
In addition to predicting malignancy, preoperative US ideally would also provide prognostic information to help determine extent of initial thyroidectomy and lymphadenectomy. Nodule size and the presence of suspicious central and lateral compartment lymphadenopathy are two features that are routinely assessed on US (10). Extrathyroidal extension (ETT) and invasion into surrounding structures can sometimes be assessed, although with low sensitivity. BRAFV600E mutation is a somatic alteration that results in oncogenic activation of the MAPK signaling pathway and has been associated with PTC that has aggressive histopathologic features such as ETT and lymph node metastasis (LNM) (11–13). Preoperative detection of BRAFV600E on FNA specimens can potentially alter the initial operative approach (13–15).
Several groups have also tried to correlate BRAF status with US characteristics with varying results. Kwak et al. (16) reported a correlation between BRAFV600E and small tumor size, high TNM stage, and extracapsular invasion, but found no significant correlation between suspicious US features and BRAF positivity. Another study by Hwang et al. (17) also demonstrated no significant associations between BRAF status and suspicious US features. However, both these studies predominantly evaluated small papillary thyroid microcarcinomas, with mean tumor sizes of 6 and 9 mm, respectively. The small size was likely a significant confounding factor in accurately characterizing these lesions by preoperative US.
In this study, we examined a series of PTC ≥1 cm to determine whether specific US characteristics can reliably predict the BRAFV600E mutation as well as detect the more aggressive variants of PTC that are usually associated with the BRAFV600E mutation.
Materials and Methods
After institutional board review approval (Protocol No. 10020508), the clinical, pathologic, and radiologic parameters of 1007 consecutive patients with a diagnosis of thyroid cancer (ICD-9 193.0) from 1/07 to 12/09 were reviewed for the following study inclusion criteria: US performed <120 days before surgery, histologic PTC measuring ≥1 cm after thyroidectomy, and molecular testing for BRAFV600E on either the FNA or pathology specimen. BRAFV600E mutation was detected using real-time polymerase chain reaction, and fluorescence melting curve analysis as previously described and was performed as a part of the routine clinical care during the study period on (i) all FNA results in the follicular lesion of undetermined significance, follicular or oncocytic neoplasm, suspicious, and positive for malignancy categories and (ii) all PTC >3 mm that did not already have positive molecular testing results on FNA (18).
A series of 106 patients who met inclusion criteria were further studied, among whom 55 patients had BRAF-positive PTC and 51 patients had BRAF-negative PTC. Nine patients had more than one PTC on final pathology. After the pathology reports and US studies were reviewed to ensure that the diagnosed PTC correlated to the same thyroid nodule that had been initially evaluated on US and was biopsied preoperatively, each PTC was considered separately and included in the study. Any PTC that was incidental and not biopsied before surgery was excluded. Finally, each final pathology report was examined for lymph node involvement or ETT. A central compartment lymph node dissection was performed for appropriate clinical factors that included a preoperative FNA which was positive for PTC, abnormal central compartment lymph nodes on preoperative US, or suspicious intraoperative findings. Lateral compartment lymph node dissection was performed if suspicious lateral lymphadenopathy was seen on neck US and metastatic disease was confirmed on FNA.
For this study, a single radiologist (M.E.T.), who was blinded to the BRAF status, independently reviewed all existing preoperative US examinations and recorded the sonographic characteristics for each PTC (n=115). Each US examination under review was performed by a trained US technician using standardized institutional protocols that routinely use transverse and sagittal cine loops as well as additional static and real-time imaging of suspicious nodules and lymph nodes. At the time of the examination, repeat sonography was performed by an US radiologist, as needed. A standardized data collection tool was used by the reviewer to categorize size (cm) and six US features: shape (taller-than-wide, round-to-oval), margin (ill-defined, circumscribed), echogenicity (hypoechoic, isoechoic, hyperechoic), halo (presence, absence), calcifications (microcalcification, macrocalcifications, peripheral), and cystic composition (graded as 0, 25%, 50%, 75%, or 100%). Of these, based on previously described criteria associated with PTC, we classified the following six US features as suspicious: taller-than-wide shape, ill-defined margin, absence of halo, hypoechogenicity, noncystic composition (graded as 0), and presence of microcalcifications (5–9). Intranodular hypervascularity has been usually considered as being a suspicious US feature; however vascularity on static images was difficult to assess objectively even with color Doppler, and was not evaluated.
Statistical analysis was performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL). The Student's t-test was used for testing continuous variables, and Fisher's exact test was used for comparing categorical variables. Sensitivity, specificity, and positive predictive values for predicting BRAF status using the index of the number of positive US features were calculated. The area under the receiver operating characteristic (ROC) curve was estimated. A p<0.05 was considered statistically significant. Multivariate analysis was performed using linear regression, and the results were tested by chi-square analysis.
Results
There were no differences between the BRAF-negative (n=51) and BRAF-positive (n=55) PTC patients in mean age at presentation (47.8 years vs. 48.2 years, p=0.98) or gender distribution (percentage men, 21.6% vs. 21.8%, p=0.98). Compared with the BRAF-negative PTC patients, the BRAF-positive PTC patients were more likely to present at a higher (III/IV) TNM stage (28% vs. 11%, 0.006). Altogether, among the 106 patients, there were 115 PTC, as 9 patients had 2 PTC. On univariate analysis (Table 1), 5/6 suspicious US characteristics were more likely to be seen on preoperative US of BRAF-positive PTC than BRAF-negative PTC: taller-than-wide shape (47% vs. 7%, p<0.001), ill-defined margin (42% vs. 9%, p<0.001), hypoechogenicity (83% vs. 36%, p<0.001), micro/macrocalcifications (87% vs. 24%, p<0.001), and absent halo (85% vs. 27%, p<0.001). Of the six examined US features, noncystic composition was the only US characteristic that did not appear to determine BRAF status (38% vs. 49%, p=0.25). No suspicious US features were identified in 16/51 (31%) of the BRAF-negative PTC and 3/55 (5%) of the BRAF-positive PTC.
Table 1.
Correlation Between Malignant Ultrasound Features and Size with BRAFV600E Status Using Univariate Analysis
| BRAF-positive | BRAF-negative | p-Value | |
|---|---|---|---|
| Nodules (n) | 60 | 55 | |
| Size, mean±SD (cm) | 2.0±0.16 | 2.4±0.14 | 0.031 |
| Taller-than-wide shape | 47% | 7% | <0.001 |
| Ill-defined margin | 42% | 9% | <0.001 |
| Hypoechoic | 83% | 36% | <0.001 |
| Halo absent | 85% | 27% | <0.001 |
| Micro/macrocalcifications | 87% | 24% | <0.001 |
| Noncystic | 38% | 49% | 0.245 |
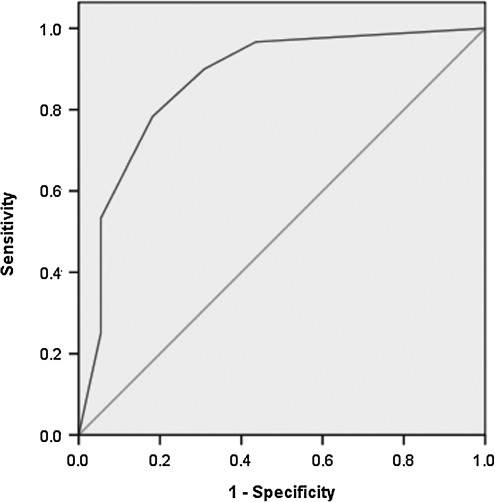
After evaluating the five suspicious US features associated with BRAF for correlation by chi-square analysis, they were all found to be significantly associated with each other, and there was no predominance observed in multivariate analysis. We, thus, devised a scoring system (0–5) based on the additive number of suspicious preoperative US characteristics seen for each PTC. We found that BRAF-positive PTC were associated with more suspicious features than BRAF-negative PTC (median, 4 vs. 0, p<0.001), and the risk of having a BRAF-positive PTC increased with the presence of even one of the five examined US features (OR 9, p=0.02). As the number of US features increased, the risk of BRAF positivity also increased (Table 2). The presence of ≥4 features had a very high likelihood of associated BRAF positivity (OR 165, p<0.001). PTC with three or more evaluated features predicted BRAF positivity with a sensitivity of 78%, specificity of 82%, and a positive predictive value of 82%. An ROC curve was calculated to verify the relationship between the number of malignant US features and BRAF positivity (Fig. 1). The area under the curve was 0.871 (p<0.001), indicating that the accuracy of the test was good with a value of 1.0 representing a perfect test.
Table 2.
Number of Malignant Ultrasound Features as Predictors of BRAFV600E Status
| Sensitivity (%) | Specificity (%) | PPV (%) | Odds ratio (p-value) | |
|---|---|---|---|---|
| ≥1 features | 97 | 56 | 71 | 9 (0.023) |
| ≥2 features | 90 | 69 | 76 | 16 (0.002) |
| ≥3 features | 78 | 82 | 82 | 33 (<0.001) |
| ≥4 featuresa | 53 | 95 | 91 | 165 (<0.001) |
Four or five features.
FIG. 1.
Receiver operating characteristic (ROC) curve for relationship between malignant ultrasound features and BRAFV600E-positive papillary thyroid carcinoma.
We also evaluated the histopathologic characteristics of PTC to determine whether BRAF testing and/or US can eventually predict aggressive features such as ETT and LNM. Confirming our previous observations, BRAF-positive PTC (n=60) were smaller than BRAF-negative PTC (n=55, mean PTC size 2.0 cm vs. 2.4 cm, p=0.03) and were more likely to be associated with aggressive histopathologic characteristics such as ETT (52% vs. 24%, p=0.002) and tall cell variant (33% vs. 10%, p=0.006) (15). Of the 44 PTC with ETT, 31 PTC were BRAF-positive and 41 had ≥1 suspicious US feature. BRAF positivity predicted ETT with a positive predictive value (PPV) and an negative predictive value (NPV) of 52% and 76%, respectively, while the presence of ≥1 suspicious US feature predicted ETT with a PPV and an NPV of 43% and 84%, respectively. The absence of both BRAF and any suspicious US feature was associated with the highest NPV (88%) for ETT, while the presence of either BRAF or ≥1 of the US feature had a PPV for ETT of only 43%.
Central compartment LNM were present in 39/94 PTC with resected central compartment lymph nodes and were more common with BRAF-positive PTC (52% vs. 28%, p=0.02). BRAF positivity predicted central compartment LNM with a PPV of 52% and an NPV of 76%, while the presence of ≥1 suspicious US feature had a PPV and an NPV of 46% and 92%. Similar to predicting ETT, the absence of both BRAF and any suspicious US feature had the highest NPV for central compartment LNM of 100%, but the presence of either BRAF or ≥1 suspicious US feature predicted central compartment LNM with a PPV of 45%.
Discussion
Cervical US is an accurate and noninvasive modality for thyroid nodule characterization, and there are a number of US characteristics that have been associated with malignancy (5–9). The presence of these features can help determine which nodules are to be evaluated further with FNA and in the 2009 ATA Management Guidelines for patients with thyroid nodules and differentiated thyroid cancer, the presence of these suspicious US characteristics should prompt FNA in nodules that are as small as 5 mm (3).
Besides identifying nodules that merit biopsy or surgical therapy, US is also being used to determine the extent of thyroidectomy as well as the need for and extent of cervical lymphadenectomy. Mendez et al. (19) determined that microcalcifications, irregular borders, hypoechogenicity, and taller-than-wide shape were associated with malignancy in nodules with indeterminate (follicular or Hurthle cell neoplasm, and suspicious for malignancy) FNA results, and found that the presence of ≤1 of the 4 suspicious US features was associated with a ≤35% risk of malignancy. Conversely, if the indeterminate nodule had ≥2 of the 4 US features present, it was observed that the risk of malignancy was high (>70%), and an upfront total thyroidectomy in such patients could be considered (19). Regarding cervical lymphadenopathy, nonpalpable LNM are often detected by US (in up to 30% of patients), and routine preoperative US potentially prevents early reoperations for missed nodal metastasis (10,20). However, after negative preoperative US, up to 40% of patients with PTC <2 cm are found at surgery to have unexpected metastatic central compartment lymph node disease (21). We and others believe that preoperative US is an excellent noninvasive method of staging and should be routinely performed, but there is great interest in identifying other preoperative features that are predictive of aggressive PTC which may prompt more extensive initial surgery, such as central compartment lymph node dissection.
BRAFV600E is a molecular marker of PTC and has been shown to correlate with PTC that have aggressive histopathologic features. Our data agree with other series which demonstrate that BRAF-positive PTC were more likely than BRAF-negative PTC to be tall cell variants, have ETT, and have an increased rate of LNM (11–13). Patients with BRAF-positive PTC may also have an increased risk of recurrence and cancer-specific mortality (13,22). Preoperative knowledge of BRAF status could change the extent of initial thyroidectomy and extent of initial lymphadenectomy, although whether this translates into improved disease-related outcomes needs further study (13–15).
Although other groups have studied the correlation of BRAF status with US characteristics, the results have been variable and very likely confounded by the small mean size (<1 cm) of the studied PTCs. In this study, we examined a series of PTC ≥1 cm. Using a standardized data collection tool, an ultrasonographer blinded to BRAF status re-evaluated all preoperative US for six previously described suspicious US features for PTC. On univariate analysis, most (5/6) of the suspicious US characteristics, with the exception of noncystic composition, were highly associated with BRAF positivity, including taller-than-wide shape, ill-defined margin, hypoechogenicity, absent halo, and micro/macrocalcifications. The presence of even one of the five features was associated with a ninefold increase in the risk of BRAFV600E (p=0.023). We found that the likelihood of BRAF positivity significantly increased when more than one suspicious feature was seen, and when three or more features were identified on preoperative US; the sensitivity, specificity, and PPV of predicting BRAF status was 78%, 82%, and 82%, respectively.
Ultimately, the importance of accurate preoperative risk stratification is to identify those PTC that are more likely to be associated with aggressive histopathology so as to inform the extent of initial surgery. Microscopic and even macroscopic ETT and central compartment LNM are two histopathologic features that are difficult to be evaluated on preoperative US, but have important staging implications for PTC patients. We evaluated whether BRAF status and/or the presence of at least one of the suspicious US characteristics predicted ETT or central compartment LNM. Although BRAF positivity and the presence of any one of the five correlative US features were more likely to be associated with ETT and/or central compartment LNM, not all cases had aggressive features on final histopathology, thus accounting for the low positive predictive value (40%–50%). However, we observed that the absence of both BRAFV600E positivity and any suspicious US features on preoperative US predicted PTC without ETT or central compartment LNM with an NPV of 88% and 100%, respectively. Thus, the presence of either BRAF-positive status or any suspicious US features should raise concern for ETT and/or central compartment LNM, but perhaps equally informative to the surgeon at the time of initial thyroidectomy is that the absence of any risk factor is highly predictive of PTC without aggressive histopathologic characteristics.
There are several limitations to our study. One limitation is that cervical US is highly operator dependent. All the US images were reviewed by a single, experienced radiologist, but the studies were performed by different US technologists. Despite utilizing standardized imaging protocols, interobserver variability for US imaging remained an issue, and ongoing prospective validation studies with a review by two independent radiologists will help address this limitation. Another bias inherent to the study design is that the radiologist who performed the blinded review was aware that all the nodules were PTC. Therefore, whether the current conclusions can be applied to patients who present with a thyroid nodule of unknown histology is not clear.
In summary, we investigated the suspicious US characteristics previously known to be predictive of PTC and found that five of the six are highly associated with BRAF-positive PTC. The presence of either BRAF positivity and/or ≥1 suspicious US characteristic was able to identify the PTC associated with ETT and central compartment LNM in almost all patients. Conversely, the absence of BRAFV600E and all five suspicious US features is highly predictive of PTC without aggressive histopathology, and these patients may not benefit from surgical adjuncts such as prophylactic central compartment lymph node dissection. Even without available preoperative BRAF testing, absence of the five suspicious US features had a high NPV for PTC with ETT or LNM. The current findings suggest that since an operative strategy during thyroidectomy for PTC requires adequate surgical margins and an assessment for central lymphadenopathy, patients with PTC who are sonographically suspicious for BRAF positivity may not be the best candidates for minimally invasive thyroidectomy techniques. Our data on the preoperative US appearance of poor-prognosis PTC further contribute to the evolving management of PTC and provide additional confirmation that routine cervical US and routine molecular testing can contribute to improving risk stratification and impact initial surgical management for PTC patients.
Acknowledgment
This study received statistical support from the University of Pittsburgh Clinical Translational Sciences Institute (NIH/NCRR/CTSA Grant UL1 RR024153).
Disclosure Statement
No competing financial interests exist.
References
- 1.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Kilfoy BA. Zheng T. Holford TR. Han X. Ward MH. Sjodin A. Zhang Y. Bai Y. Zhu C. Guo GL. Rothman N. Zhang Y. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Gharib H. Papini E. Paschke R. Duick DS. Valcavi R. Hegedüs L. Vitti P AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest. 2010;33:1–50. [PubMed] [Google Scholar]
- 5.Moon WJ. Baek JH. Jung SL. Kim DW. Kim EK. Kim JY. Kwak JY. Lee JH. Lee JH. Lee YH. Na DG. Park JS. Park SW Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papini E. Guglielmi R. Bianchini A. Crescenzi A. Taccogna S. Nardi F. Panunzi C. Rinaldi R. Toscano V. Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 7.Koike E. Noguchi S. Yamashita H. Murakami T. Ohshima A. Kawamoto H. Yamashita H. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg. 2001;136:334–337. doi: 10.1001/archsurg.136.3.334. [DOI] [PubMed] [Google Scholar]
- 8.Cappelli C. Pirola I. Cumetti D. Micheletti L. Tironi A. Gandossi E. Martino E. Cherubini L. Agosti B. Castellano M. Mattanza C. Rosei EA. Is the anteroposterior and transverse diameter ratio of nonpalpable thyroid nodules a sonographic criteria for recommending fine-needle aspiration cytology? Clin Endocrinol (Oxf) 2005;63:689–693. doi: 10.1111/j.1365-2265.2005.02406.x. [DOI] [PubMed] [Google Scholar]
- 9.Moon WJ. Jung SL. Lee JH. Na DG. Baek JH. Lee YH. Kim J. Kim HS. Byun JS. Lee DH Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology. Benign and malignant thyroid nodules: US differentiation—multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 10.Kouvaraki MA. Shapiro SE. Fornage BD. Edeiken-Monro BS. Sherman SI. Vassilopoulou-Sellin R. Lee JE. Evans DB. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–954. doi: 10.1016/s0039-6060(03)00424-0. discussion 954–955. [DOI] [PubMed] [Google Scholar]
- 11.Xing M. Clark D. Guan H. Ji M. Dackiw A. Carson KA. Kim M. Tufaro A. Ladenson P. Zeiger M. Tufano R. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27:2977–2982. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kebebew E. Weng J. Bauer J. Ranvier G. Clark OH. Duh QY. Shibru D. Bastian B. Griffin A. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–470. doi: 10.1097/SLA.0b013e318148563d. discussion 470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melck AL. Yip L. Carty SE. The utility of BRAF testing in the management of papillary thyroid cancer. Oncologist. 2010;15:1285–1293. doi: 10.1634/theoncologist.2010-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neill CJ. Bullock M. Chou A. Sidhu SB. Delbridge LW. Robinson BG. Gill AJ. Learoyd DL. Clifton-Bligh R. Sywak MS. BRAF(V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery. 2010;148:1139–1145. doi: 10.1016/j.surg.2010.09.005. discussion 1145–1146. [DOI] [PubMed] [Google Scholar]
- 15.Yip L. Nikiforova MN. Carty SE. Yim JH. Stang MT. Tublin MJ. Lebeau SO. Hodak SP. Ogilvie JB. Nikiforov YE. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009;146:1215–1223. doi: 10.1016/j.surg.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Kwak JY. Kim EK. Chung WY. Moon HJ. Kim MJ. Choi JR. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology. 2009;253:854–860. doi: 10.1148/radiol.2533090471. [DOI] [PubMed] [Google Scholar]
- 17.Hwang J. Shin JH. Han BK. Ko EY. Kang SS. Kim JW. Chung JH. Papillary thyroid carcinoma with BRAFV600E mutation: sonographic prediction. AJR Am J Roentgenol. 2010;194:W425–W430. doi: 10.2214/AJR.09.3512. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforova MN. Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009;19:1351–1361. doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 19.Méndez W. Rodgers SE. Lew JI. Montano R. Solórzano CC. Role of surgeon-performed ultrasound in predicting malignancy in patients with indeterminate thyroid nodules. Ann Surg Oncol. 2008;15:2487–2492. doi: 10.1245/s10434-008-0052-6. [DOI] [PubMed] [Google Scholar]
- 20.Stulak JM. Grant CS. Farley DR. Thompson GB. van Heerden JA. Hay ID. Reading CC. Charboneau JW. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–494. doi: 10.1001/archsurg.141.5.489. discussion 494–496. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet S. Hartl D. Leboulleux S. Baudin E. Lumbroso JD. Al Ghuzlan A. Chami L. Schlumberger M. Travagli JP. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009;94:1162–1167. doi: 10.1210/jc.2008-1931. [DOI] [PubMed] [Google Scholar]
- 22.Elisei R. Ugolini C. Viola D. Lupi C. Biagini A. Giannini R. Romei C. Miccoli P. Pinchera A. Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]