Abstract
Background
Cardioplegia and cardiopulmonary bypass(CP/CPB) subjects myocardium to complex injurious stimuli that can result in cardiomyocyte and vascular contractile abnormalities. Rottlerin, originally identified as a PKCδ inhibitor has a number of known additional effects that may be beneficial in the setting of CP/CPB. We tested the hypothesis that rottlerin would mitigate deleterious effects associated with CP/CPB.
Methods and Results
Langendorff-perfused isolated rat hearts were subjected to 2 hours intermittent cold (10 deg C) cardioplegia (St Thomas II) followed by 30 min normothermic reperfusion. Cardioplegia was delivered every 30 min, for 1 min. Hearts were treated with (CP+R, n=7) or without (CP, n=9) the PKCδ inhibitor, rottlerin (1μM) and/or the BKCa++ channel inhibitor Paxilline (100 nM) supplied in the cardioplegia. Hearts constantly perfused with Krebs-Heinslet buffer served as controls (n=6). Baseline parameters of cardiac function were similar between groups. CP resulted in reduced cardiac function (LVDP:39±3.8%,±dP/dt: 32±4.4%,-41±5.1% decrease compared to baseline). Treatment with 1uM Rottlerin significantly improved CP-induced cardiac function (LVDP: 20±5.9%, ±dP/dt: 5.2 ±4.5%, -11.6 ± 4.7% decrease versus baseline, (p < .05 CP+R vs CP)). Rottlerin also caused a significant increase in coronary flow post reperfusion (CP 34±4.2% decrease from baseline, vs CP+R 26±9.6% increase over baseline, p=.01). Independent of vascular effects, CP significantly decreased isolated myocyte contraction which was restored by rottlerin treatment. The BKCa++ channel inhibitor greatly reduced the majority of beneficial effects associated with Rottlerin.
Conclusions
Rottlerin significantly improves cardiac performance following cardioplegic arrest via improved cardiomyocyte contraction and coronary perfusion.
Keywords: Cardioplegia, potassium channel, PKC, rottlerin, Akt, ischemia
Introduction
Cardiac surgery using cardioplegia and cardiopulmonary bypass (CP/CPB) subjects myocardium to hypothermic reversible ischemic injury that can impair cardiac function (a.k.a. myocardial stunning). The main protective benefits of CP are mediated through myocardial hypothermia, and diastolic arrest which preserves myocardial energy reserves. The ischemic insults associated with cardioplegic arrest during surgery include myocyte hypoxia, acidosis, oxidant dependent damage, metabolic and structural alterations, and reduced cardiac function(1,2,3,4). In addition to direct effects on cardiomyocytes, cardioplegia can result in marked coronary vascular complications including impaired vasodilation, propensity for spasm, and overall decreased perfusion(5). Although contractile impairment in the majority of patients resolves quickly, ~10% can develop a cardiac low-output syndrome attributable in part to depressed left ventricular or atrial contractile function. Consequently, low-output syndrome prolongs recovery times and significantly elevates risk of mortality(6).Furthermore, the need for enhanced cardioprotection is required for specific high-risk patient populations (i.e. Prolonged surgical times, low EF, elderly, etc...).
Rottlerin has been reported as a PKCδ inhibitor. PKCδ has been implicated in depressed cardiac function and cell death post-I/R injury, as well as promoting vascular smooth muscle contraction(7,8,9). However, rottlerin as a true inhibitor of PKCδ has been called into question and generated considerable controversy(10,11). Other PKCδ independent effects of rottlerin have recently been recognized. Rottlerin has been reported as a potent large conductance potassium channel (BKCa++) opener(12). Opening of BKCa++ channels is beneficial for post-ischemic alterations in vasomotor activity (13). In addition, other BKCa++ channel openers are reported to limit ischemia related mitochondrial Ca++ overload. (14,15). Finally, rottlerin is also capable of reducing oxygen-radical formation(10). All of these mechanisms of injury occur during cardioplegic arrest and reperfusion. Therefore, rottlerin, through a combination of targets, may block many of the deleterious side effects associated with cardioplegic arrest that limit both vascular and cardiomyocyte function.
Methods
Isolated Langendorff perfused model of cardioplegic arrest
Male Sprague-Dawley rats (Charles River, MA) were anesthetized i.p. with 80 mg/kg ketamine and 5 mg/kg xylazine, anticoagulated with heparin (2,000 U/kg, iv), and the heart rapidly exposed. The aorta was immediately cannulated and retrograde perfused in Langendorff mode with a water jacketed organ chamber and perfusion system (IH-SR, Harvard Apparatus, Boston, MA). Following cannulation the heart was cleaned of excess tissue and vessels, the left atrium removed, and a balloon placed in the left ventricle. Left ventricular end diastolic pressure (LVEDP) was set to ~8 mmHg at the beginning of the experiment. A temperature probe placed in the pulmonary artery monitored myocardial temperature. The hearts were perfused in constant pressure mode (~70 mmHg) with a modified KHB (118 NaCl, 4.7 KCl, 1.25 CaCl2, 1.66 MgSO4, 24.88 NaHCO3, 1.18 KH2PO4, 2.0 Napyruvate in mM) for 30 min to stabilize and record baseline measurements. During baseline measurements myocardial temperature was maintained at 37 °C. Groups subjected to cold crystalloid cardioplegia solution were perfused with St Thomas II solution (110 NaCl, 16 KCl , 16 MgCl2, 1.5 CaCl2 1, and 10 NaHCO3 in mM). Myocardial cooling during CP was initiated at the onset of cardioplegia infusion via rapidly switching the Langendorff organ chamber and perfusate to a refrigerated circulator. Myocardial temperature was maintained at 10°C for the duration of CP. Cardioplegia groups were perfused initially for 2 min, followed by a 1 minute infusion at 30, 60, and 90 min arrest, respectively. Following 120 min, the organ chamber and perfusate were switched back to a heating circulator and the heart perfused at 70 mmHg with modified KHB. Myocardial temperature was subsequently maintained at 37 °C. Indices of ventricular function, perfusion pressure, myocardial temperature, and organ chamber temperature were measured continuously throughout the experiment using a LDS-Ponemah data acquisition system. At the conclusion of the experiment the heart was removed from the perfusion apparatus and a small mid transverse slice was removed and immediately placed in 10% formalin for confocal microscopy. A second section was taken for heart wet/dry weight ratio. Samples were weighed directly after procurement and following desiccation for 24 hrs at 50 C. The remainder of the tissue was immediately placed in liquid N2.
SDS-PAGE and immunoblot
were performed using standard methodology as previously described(16). Antibodies for immunoblot were as follows: phospho-specific and/or total PKCδ, cTnI, Akt, PDK1, PTEN, and ERK were from Cell Signaling (Beverly, MA), Phospholamban (Millipore), and phospho- and total HSP27 and cryAB antibodies from Stressgen (Vancouver, B.C.).
Culture and purification of adult rat cardiomyocytes
Adult rat cardiomyocyte cultures were obtained from hearts according to a previously published protocol with modifications(17). Briefly, hearts were excised from anesthetized adult rats, the aorta cannulated, and hearts perfused with 0.3% collagenase solution in perfusion buffer consisting of MEM (Joklik modification) supplemented with creatine, BDM, taurine, and insulin for 45 min. After perfusion, ventricles were removed and minced in the Ca2+-free collagenase solution for 3-5 min. Chunks were then incubated in 10 ml perfusion buffer supplemented with BSA and 0.3 mM CaCl2. Cells were washed in collagenase free perfusion buffer 3 times with centrifugation. Myocytes were resuspended in DMEM culture medium supplemented with creatine, carnitine, taurine, penicillin /streptomycin, and insulin. Myocytes were plated at a density of 2 × 104 cells/cm2 on 10 ug/ml laminin coated dishes for length measurements the following day.
In vitro Cardioplegia and myocyte length recordings
Cultured rat myocytes were switched to crystalloid cardioplegia solution bubbled with 5%CO2 / 95% N2 under anoxic conditions. Cells were then placed in a hypoxia chamber evacuated with 5%CO2 / 95% N2 for the indicated times and retained at 4 C. For simulated reperfusion, cardioplegia treated cells were removed from the hypoxic chamber and returned to the cell culture incubator and cardioplegia solution was replaced with a modified Krebs Heinseleit Buffer (KHB) containing in mM: 118 NaCl, 4.7 KCl, 1.25 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES and 10 glucose. Control cells were maintained in a standard cell culture incubator for the duration of CP, and switched to 37C KHB at the time of simulated reperfusion. For length measurement cells were moved to a heated stage, with temperature controlled perfusion, and field stimulation capable of accepting 35 mm culture dishes (Harvard Apparatus, Cambridge, MA). The cells were imaged via a CCD camera equipped inverted microscope attached to a raster line video edge detector (Living Systems Instrumentation, Burlington, Vt). Voltage signals were captured via a data acquisition unit attached to a computer running Ponemah physiology platform software. The recorded length data can be visualized in real time at 60 Hz via a computer monitor and stored for subsequent analysis of % length change and +/- dL/dt. The video edge detector and data capture software were calibrated using voltage signals from a slide micrometer before each experiment. Only rod shaped viable cells that responded appropriately to field stimulation were used.
Statistical Analysis
All statistical analyses were performed with Sigma Stat software (Systat, Chicago, Ill). For analysis of CP and CP+rottlerin functional time courses, Two Way RM ANOVA with student Newman-Keuls post-hoc analysis was performed. A Two Way ANOVA, Student Newman-Keuls was performed for isolated myocyte experiments. All other statistical tests were One Way ANOVA, Student Newman-Keuls. P<.05 determined significance.
Results
Rottlerin alleviates CP-induced myocardial stunning in the isolated heart
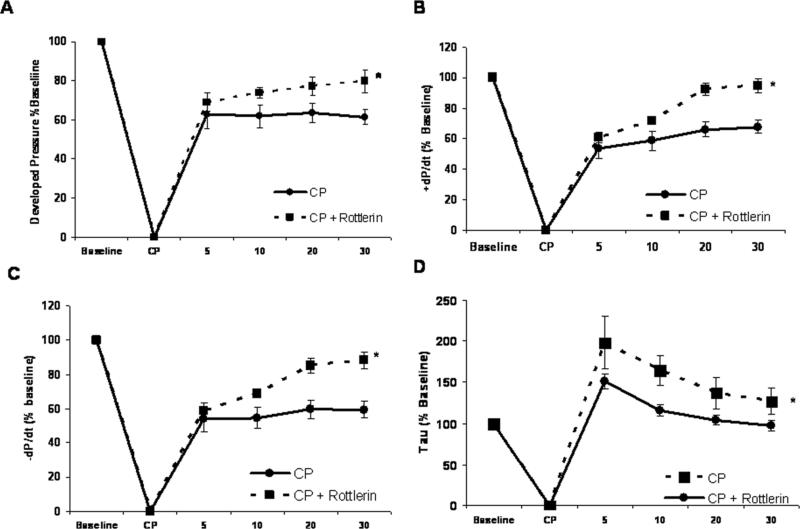
Cold crystalloid cardioplegia (CP) for two hours in the isolated rat hearts causes depressed cardiac contractile function upon reperfusion. During cardioplegia myocardial temperature was maintained at ~10 °C, followed by rewarming to 37°C during 30 minutes reperfusion as described previously (18). A representative experiment is presented in supplemental figure 1. Upon reperfusion there were significant reductions in systolic pressure, developed pressure, and positive and negative first derivatives of LVP compared to baseline (Figure 1A-D). Inclusion of 1 uM Rottlerin in the cardioplegic solution significantly improved indices of cardiac function including developed pressure (Figure 1A), +/- dP/dt (Figures 1B and C), and Tau (Figure 1D). There were no significant changes in heart rate between groups (Figure 1E).
Figure 1. Rottlerin rescues cold cardioplegia-induced depressed contractile function.
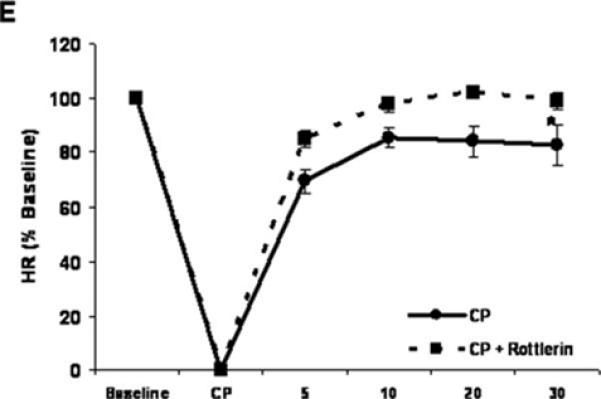
Two hours intermittent CP depresses isolated heart contractile function. Inclusion of Rottlerin (1 μM) enhances cardiac function following reperfusion. A) Developed Pressure, B) +dP/dt, C) –dP/dt, D) Tau, and E) Heart rate. X-axis time : Baseline – pre CP function, CP – Cardioplegia, 5 - 30 – min reperfusion. n = minimum of 7 per group. * indicates p < .05 , Two way RM ANOVA.
Rottlerin improves CP-induced reductions in coronary perfusion
Cardioplegic arrest and 30 min reperfusion reduces coronary flow compared to baseline. Hearts treated with 1 uM rottlerin in the cardioplegic solution show significant improvements over CP alone (Figure 2A and B). At 30 min of reperfusion, CP+Rott group displays a significant increase in flow over baseline, while CP and sham hearts display overall reductions in flow (Figure 2B) There were no significant increases in wet/dry tissue weight ratios between groups indicating no deleterious increases in tissue edema (Supplemental Figure 2).
Figure 2. Rottlerin increases CP-induced decreases in coronary flow.
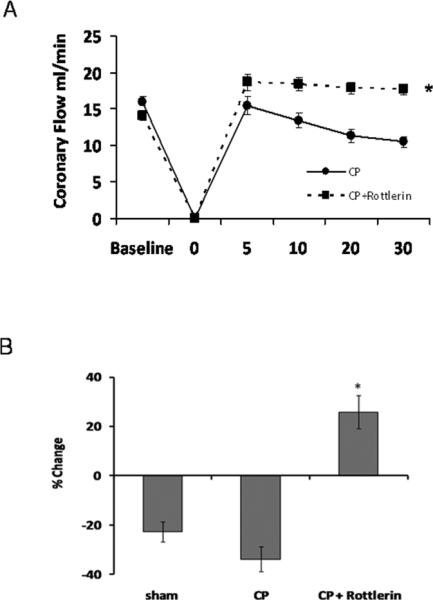
A) Rottlerin alleviates CP/reperfusion induced decreases in coronary flow. * p<.05 using two RM ANOVA. B) % change over baseline at 30 min post-reperfusion. CP with rottlerin increases coronary flow when compared to baseline. p < .05 using one way Anova , Student Newman-Keuls.
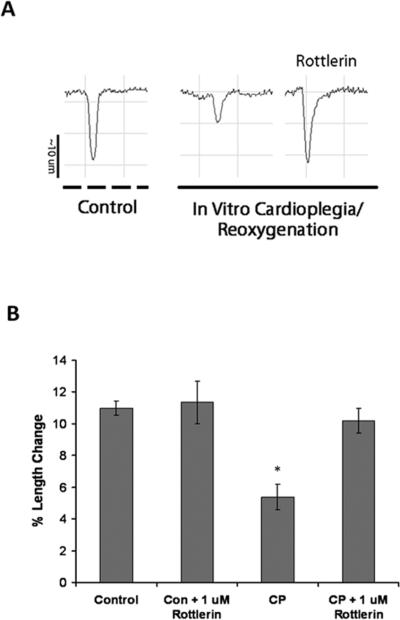
Rottlerin blocks CP-impaired myocyte contractility in vitro
To explore whether rottlerin alleviates CP-induced depressed contractile function solely through vascular effects, isolated rat cardiac myocytes were subjected to in vitro CP and simulated reperfusion with reoxygenation and rewarming. In vitro CP resulted in depressed myocyte contraction as determined by decreased myocyte length shortening (Figure 3A and B). Inclusion of 1 uM rottlerin completely rescued CP-induced changes in myocyte length shortening (Figure 4A and B), indicating direct effects on cardiomyocytes.
Figure 3. Rottlerin rescues cardioplegia-induced myocyte contractile defecits invitro.
A). Representative tracings of Isolated rat myocyte length changes following 3 hours hypoxic hypothermic crystalloid cardioplegia and normothermic reoxygenation. Isolated myocytes were treated with or without 1 uM Rottlerin in the cardioplegia or left in normoxic cell culture medium (Control). B Myocytes subjected to in vitro hypoxic, hypothermic, cardipolegia and rewarming/oxygenation display reduced contractile activity that is rescued by Rottlerin. Minimum n=12 myocytes per group from at least 3 independent isolations, * denotes p<.05 using Two Way ANOVA.
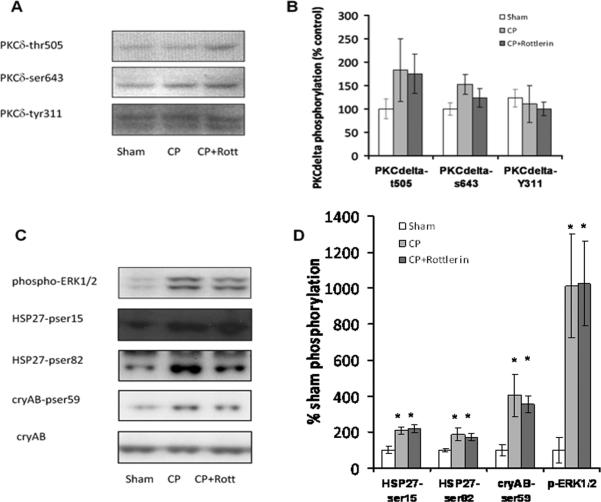
Figure 4. Rottlerin does not alter phosphorylation of PKCdelta.
A Immunoblot analysis of isolated heart LV tissue probed with phospho-specific antibodies to PKCδ B) Graph displays normalized % increase in PKCδ phosphorylation over sham. C) Phosphorylation of ERK1/2, HSP27, and αB-crystallin (reported indirect downstream targets of PKC δ). D) Graph displays normalized % increase in phosphorylation over sham. Minimum n=6 / group. * indicates statistical significance vs. sham, p <.05, One Way Anova, Student Newman-Keuls.
Rottlerin's cardioprotective effects are independent of PKCδ
PKCδ phosphorylation is known to correlate with increased PKCδ activity(19). However, CP did not increase the phosphorylation of PKCδ on Y311, T505, or S643. There was an insignificant trend for CP-induced phosphorylation of PKCδ-T505. However, treatment with 1 uM rottlerin did not attenuate this trend or decrease basal phosphorylation on any of the residues examined (Figure 4A and B). Phosphorylation of ERK and p38-MAPK/HSP27 have been implicated as downstream of PKCδ activation(20,16,21). HSP27 is a downstream target of p38-MAPK that may also regulate contractile deficits associated with cardioplegia-induced stunning (22,18). Although CP-induces phosphorylation of ERK and HSP27, these effects were independent of rottlerin (figure 4C and D).
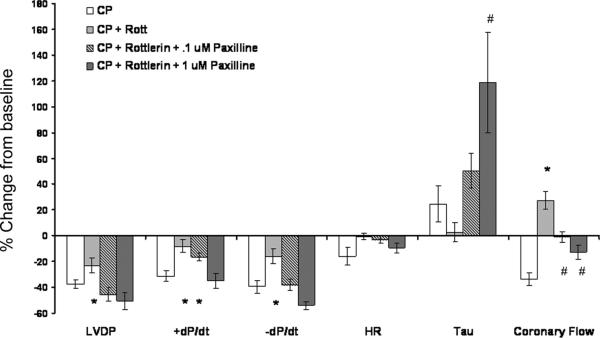
BKCa++ channels mediate Rottlerin-induced improvements in post-CP cardiac and vascular function
To determine if rottlerin mediates its effects via opening of BKCa++ channels, hearts were treated with rottlerin (1uM) and the BKCa++ channel blocker Paxilline (100 nM and 1 uM) both supplied in the cardioplegia. Paxilline blocked the majority of rottlerin's benefical effects at 100 nM and all the measured beneficial effects at 1 uM (figure 5). However, treatment with rottlerin and 1 uM Paxilline caused significant increases in LVEDP and Tau compared to CP treatment alone (Supplemental figure 3). Full time courses of paxilline treated groups are presented in supplemental figure 3.
Figure 5. Beneficial effects of rottlerin are mediated through BKCa++ channels.
Cotreatment with rottlerin (1 uM) and paxilline (100 nM and 1 uM) in cardioplegia blocks the protective effects of rottlerin alone. A graph displays experimental data as in figure 1, with the % change from baseline of the 30 minute reperfusion timepoint. Minimum n=6, except CP + Rott + Paxilline 1 uM , n=4. * indicates different from CP, # indicates different from both CP and CP + rottlerin groups, p < .05, One Way ANOVA, Student Newman Keuls.
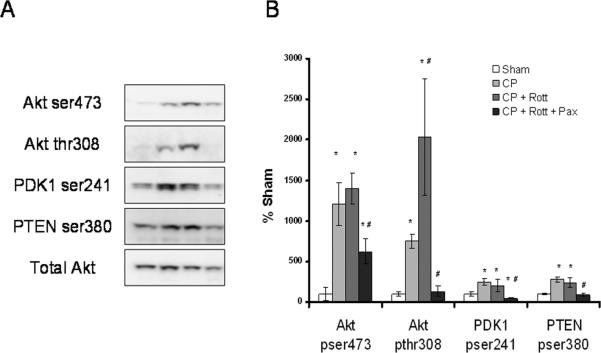
Rottlerin enhances CP-induced Akt phosphorylation via BKCa++ channels
CP caused significant increases in members of the Akt signaling cascade including Akt ser473, Akt thr308, ser241 PDK1 and, ser380 PTEN (Figure 6). Compared to CP alone, Rottlerin significantly increased the phosphorylation of Akt on thr308. All targets of the Akt pathway measured were significantly reduced in the rottlerin groups co-treated with Paxilline (100 nM) (figure 6) indicating this pathway is controlled via BKCa++ channel modulation.
Figure 6. Rottlerin enhances CP-induced Akt pathway activation via BKCa++channels.
A Immunoblot analysis of isolated heart LV tissue probed with phospho-specific antibodies to PDK1, PTEN, and phsopho and total Akt. B) Graph displays normalized (total Akt) % increase in phosphorylation over sham. n=6/group. * indicates different from sham, # indicates different from CP, p < .05, One way Anova, Student Newman-Keuls.
Rottlerin does not reduce protein or lipid oxidation
To determine if Rottlerin mediates its effects through anti-oxidant mechanisms, tissue protein and lipid oxidation were indirectly measured by carbonyl and MDA content, respectively. Neither CP nor addition of Rottlerin significantly altered protein (Supplemental figure 4A) or lipid oxidation (Supplemental figure 4B) as compared to sham treatment.
Rottlerin does not alter phospholamban (PLN) or cTnI phosphorylation
Phosphorylation of PLN and cTnI are implicated in regulation of myocardial contractility. Neither CP nor CP + 1 μM rottlerin changed basal levels of cTnI or PLN phosphorylation (Supplemental Figure 5).
Discussion
The principle findings of the current study indicate that rottlerin improves functional recovery of isolated hearts following cold cardioplegic arrest. As an additive to cardioplegia, rottlerin: 1) increased isolated heart contractile performance, 2) significantly enhanced myocardial perfusion, and 3) directly increased contractile performance of cardiac myocytes independent of vascular effects. All of these beneficial effects appeared to be independent of PKCδ activation as measured by phosphorylation status and screening of potential downstream targets. In contrast, rottlerin's beneficial effects were reduced by paxilline, implicating an important role for BKCa++ channels.
Cardioplegic arrest can result in multiple complications following surgery including enhanced coronary artery tone and decreased myocardial perfusion, as well as perfusion independent effects that cause myocardial stunning and contractile deficits. Rottlerin may mediate improvements in cardiomyocyte and smooth muscle function through known mechanisms of action that are independent of PKCδ.
First, rottlerin has been identified as an extremely potent large conductance K+ channel activator (BKCa++)(12) . Activation of BKCa++ channels results in coronary smooth muscle vasodilation(23,13) . Furthermore, activation of BKCa++ channels with NS1619 (another BK channel activator) demonstrated improved vasodilation in vessels contracted with high K+ cardioplegia(13). Inhibition of the BKCa++ channels with paxilline greatly reduced the vasodilatory effect of rottlerin, indicating rottlerin also works through smooth muscle BKCa++ channels to improve coronary flow post-CP. Second, rottlerin-induced activation of BKCa++ channels may directly alleviate stunning of cardiac myocytes. Classical mechanisms of myocardial stunning associated with CP/CPB include the oxidant radical and Ca++ overload hypothesis(6,2,24,3,4). The non-exclusive views propose: 1) oxidant dependent damage/oxidant-dependent activation of signaling which negatively modulates the contractile apparatus and 2) alterations in Ca++ homeostasis which promotes contractile abnormalities and metabolic alterations via mitochondrial damage. BKCa++ channels reside in the cardiomyocyte inner mitochondrial membrane(25). BKCa++ channel activation is proposed to increase mitochondrial K+ accumulation which in turn electrochemically limits mitochondrial Ca++ influx and reduces mitochondrial depolarization and permeability transition pore (MPTP) opening(14,26). Known cardioprotective effects associated with the BKCa++ channel openers NS1619, NS11021 and DiCl-DHAA following ischemia reperfusion injury include reductions in mitochondrial Ca++ overload, mitochondrial membrane depolarization, increased cell viability, and improved function in whole hearts(25,27,25) (15). Indeed, adaptation to hypoxia alone can partially activate the mitochondrial BKCa++ channels which is recognized as a protective response(28,29).
In addition, we found that rottlerin treatment greatly enhanced the CP-induced phosphorylation of Akt on the required activation residue thr308. Akt activation is reported to be an upstream mediator of mitochondrial K+ channel function as well as a modulator of the MPTP (30,31). However our data indicates that Akt functions downstream of rottlerin dependent activation of BKCa++ channels, as BKCa++ channel inhibition with paxilline blocked the CP and CP + rottlerin-induced increases in Akt pathway activation (figure 6). Although activation of Akt signaling is considered beneficial and pro-survival post-I/R injury, it is unclear what specific role (if any) Akt may play in modulating the acute increases in myocardial function following CP + rottlerin treatment. Further studies will need to address if this is a necessary aspect of improved function associated with rottlerin.
Rottlerin has demonstrated antioxidant properties, although it is unclear if these effects are due to BKCa++ opening or other additional mechanisms(32,10). However, we did not find any evidence that rottlerin directly influences oxidant dependent damage as measured by total lysate protein and lipid oxidation. Specific investigation of these effects may be required in isolated mitochondria and/or using more sensitve methods. Finally, it is highly likely that the dose of rottlerin used in these studies did not inhibit PKCδ. Previous studies demonstrated that PKCδ inhibitory doses are significantly higher than 1 μM. Numerous reports indicate that rottlerin may indirectly alter PKCδ activation, but at concentrations (i.e. > 10 uM) that lead to mitochondrial uncoupling and global reductions in ATP and subsequent kinase activity(10,11,33). However, the dose of rottlerin used in the current study is considerably less than any dose that was previously shown to alter cellular ATP levels and lead to activation of AMPK(33). In addition, we found no activation of AMPK with or without rottlerin in the CP-treated groups, indicating preservation of myocardial energy reserves (data not shown). We also found no phosphorylation of PKCδ or potential downstream targets. Conversely, 0.5 μM rottlerin significantly increases BKCa++ channels opening in cell and cell free systems (12).
In conclusion we demonstrate that rottlerin, as an additive to cold crystalloid cardioplegia improves CP-induced myocardial stunning and vasomotor regulation likely via direct activation of BKCa++ channels and not PKCd dependent effects.. To our knowledge this is the first use of rottlerin and/or a BKCa++ activator in a whole heart model of cardioplegic arrest and we demonstrate improved function of both cardiomyocyte and vascular function following reperfusion. These results indicate that rottlerin as a cardioplegia additive, with no requisite pre and/or post treatment, may provide significant therapeutic benefit for cardiac surgery patients.
Limitations
While our model seeks to faithfully reproduce injury associated with cardioplegic arrest in patients, a number of important shortcomings exist. We did not address injury associated with extracorporeal circulation and reperfusion injury associated with blood components Second, the most common form of cardioplegia currently in use is cold blood crystalloid cardioplegia, it is unclear the effect blood as a cardioplegia additive would have on our results. Future studies will need to address the potential benefit of rottlerin with a model that incorporates cardioplegic arrest and reperfusion post-cardiopulmonary bypass.
Supplementary Material
Acknowledgments
Funding Sources: Supported by NIH grants: R00-HL093352 (R.T.C.) and R01-HL046716 (F.W.S)
Footnotes
[39] CV surgery: other [36] CV surgery: coronary artery disease [138] Cell signalling/signal transduction [105] Contractile function [151] Ischemic biology - basic studies
Conflict of interest disclosure:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolli R. Basic and clinical aspects of myocardial stunning. Prog.Cardiovasc.Dis. 1998;40:477–516. doi: 10.1016/s0033-0620(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 3.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–3167. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 4.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 5.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur.J.Cardiothorac.Surg. 2004;26:1002–1014. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Weisel RD. Myocardial stunning after coronary bypass surgery. J.Card Surg. 1993;8:242–244. doi: 10.1111/j.1540-8191.1993.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki K, Hahn HS, Dorn GW, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108:869–875. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 9.Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S, Takeshita A. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler.Thromb.Vasc.Biol. 2003;23:2209–2214. doi: 10.1161/01.ATV.0000104010.87348.26. [DOI] [PubMed] [Google Scholar]
- 10.Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol.Sci. 2007;28:453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J.Biol.Chem. 2001;276:37986–37992. doi: 10.1074/jbc.M105073200. [DOI] [PubMed] [Google Scholar]
- 12.Zakharov SI, Morrow JP, Liu G, Yang L, Marx SO. Activation of the BK (SLO1) potassium channel by mallotoxin. J.Biol.Chem. 2005;280:30882–30887. doi: 10.1074/jbc.M505302200. [DOI] [PubMed] [Google Scholar]
- 13.Han JG, Yang Q, Yao XQ, Kwan YW, Shen B, He GW. Role of large-conductance calcium-activated potassium channels of coronary arteries in heart preservation. J.Heart Lung Transplant. 2009;28:1094–1101. doi: 10.1016/j.healun.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Kang SH, Park WS, Kim N, Youm JB, Warda M, Ko JH, Ko EA, Han J. Mitochondrial Ca2+-activated K+ channels more efficiently reduce mitochondrial Ca2+ overload in rat ventricular myocytes. Am.J.Physiol Heart Circ.Physiol. 2007;293:H307–H313. doi: 10.1152/ajpheart.00789.2006. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 16.Clements RT, Sodha NR, Feng J, Mieno S, Boodhwani M, Ramlawi B, Bianchi C, Sellke FW. Phosphorylation and translocation of heat shock protein 27 and alphaB-crystallin in human myocardium after cardioplegia and cardiopulmonary bypass. J.Thorac.Cardiovasc Surg. 2007;134:1461–1470. doi: 10.1016/j.jtcvs.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 18.Clements RT, Feng J, Cordeiro B, Bianchi C, Sellke FW. p38-MAPK-dependent Heat Shock Protein 27 (HSP27) and αB-crystallin (cryAB) Phosphorylation in Regulation of Myocardial Function Following Cardioplegic Arrest. Am.J.Physiol Heart Circ.Physiol. 2011 doi: 10.1152/ajpheart.00272.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem.J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginnan R, Guikema BJ, Singer HA, Jourd'heuil D. PKC-delta mediates activation of ERK1/2 and induction of iNOS by IL-1beta in vascular smooth muscle cells. Am.J.Physiol Cell Physiol. 2006;290:C1583–C1591. doi: 10.1152/ajpcell.00390.2005. [DOI] [PubMed] [Google Scholar]
- 21.Maizels ET, Peters CA, Kline M, Cutler RE, Jr., Shanmugam M, Hunzicker-Dunn M. Heat-shock protein-25/27 phosphorylation by the delta isoform of protein kinase C. Biochem.J. 1998;332(Pt 3):703–712. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Rajashree R, Liu Q, Hofmann P. Acute p38 MAPK activation decreases force development in ventricular myocytes. Am.J.Physiol Heart Circ.Physiol. 2003;285:H2578–H2586. doi: 10.1152/ajpheart.00365.2003. [DOI] [PubMed] [Google Scholar]
- 23.Feng J, Liu Y, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, Nishimura KK, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest. Circulation. 2008;118:S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolli R. Why myocardial stunning is clinically important. Basic Res Cardiol. 1998;93:169–172. doi: 10.1007/s003950050083. [DOI] [PubMed] [Google Scholar]
- 25.Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch. 2009;457:979–988. doi: 10.1007/s00424-008-0583-5. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto K, Ohya S, Muraki K, Imaizumi Y. A novel opener of large-conductance Ca2+ -activated K+ (BK) channel reduces ischemic injury in rat cardiac myocytes by activating mitochondrial K(Ca) channel. J.Pharmacol.Sci. 2008;108:135–139. doi: 10.1254/jphs.08150sc. [DOI] [PubMed] [Google Scholar]
- 28.Borchert GH, Yang CT, Kolar F. Mitochondrial BKCa channels contribute to protection of cardiomyocytes isolated from chronically hypoxic rats. Am.J.Physiol Heart Circ.Physiol. 2010 doi: 10.1152/ajpheart.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Y, Gu XQ, Bednarczyk P, Wiedemann FR, Haddad GG, Siemen D. Hypoxia increases activity of the BK-channel in the inner mitochondrial membrane and reduces activity of the permeability transition pore. Cell Physiol Biochem. 2008;22:127–136. doi: 10.1159/000149790. [DOI] [PubMed] [Google Scholar]
- 30.Miura T, Tanno M, Sato T. Mitochondrial kinase signalling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc Res. 2010;88:7–15. doi: 10.1093/cvr/cvq206. [DOI] [PubMed] [Google Scholar]
- 31.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim.Biophys.Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am.J.Physiol Heart Circ.Physiol. 2007;293:H1400–H1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 33.Kojima K, Motoshima H, Tsutsumi A, Igata M, Matsumura T, Kondo T, Kawashima J, Ichinose K, Furukawa N, Inukai K, Katayama S, Goldstein BJ, Nishikawa T, Tsuruzoe K, Araki E. Rottlerin activates AMPK possibly through LKB1 in vascular cells and tissues. Biochem.Biophys.Res Commun. 2008;376:434–438. doi: 10.1016/j.bbrc.2008.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.