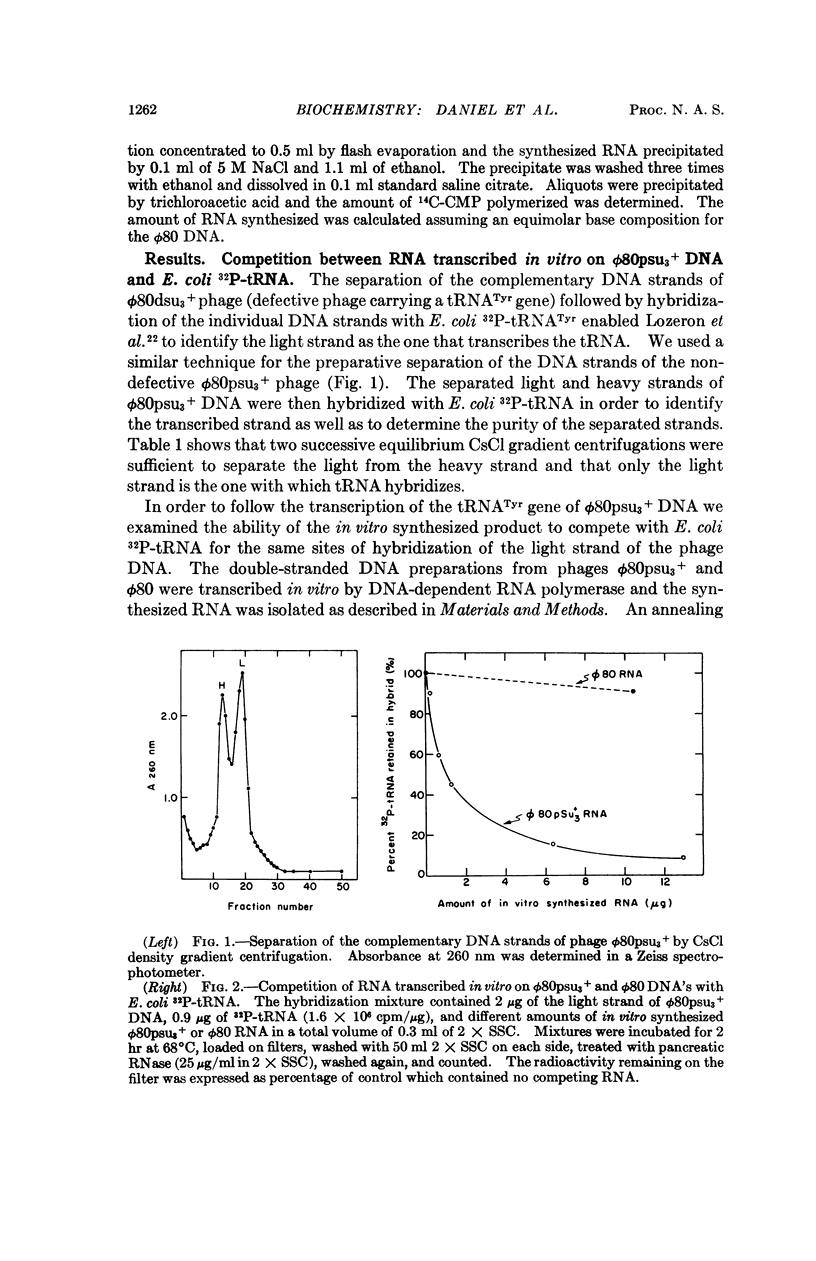
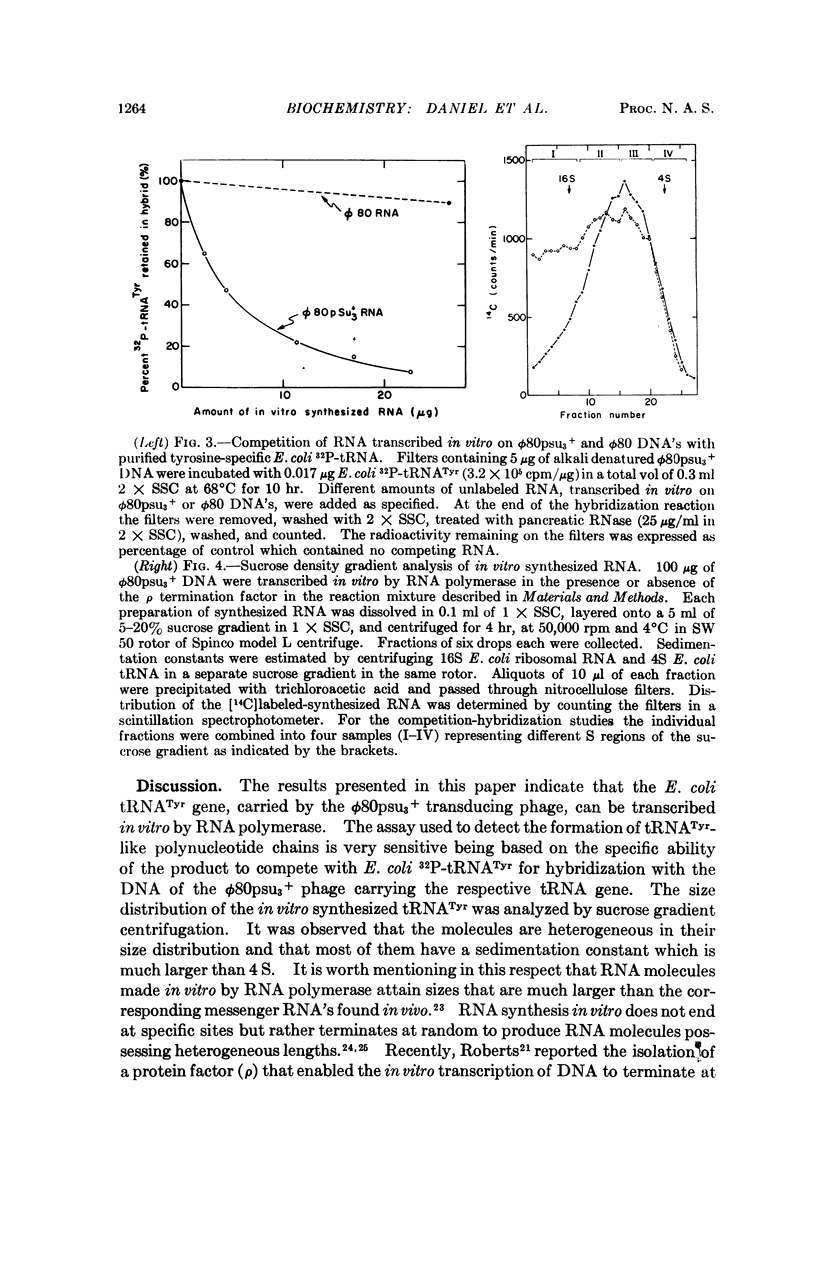
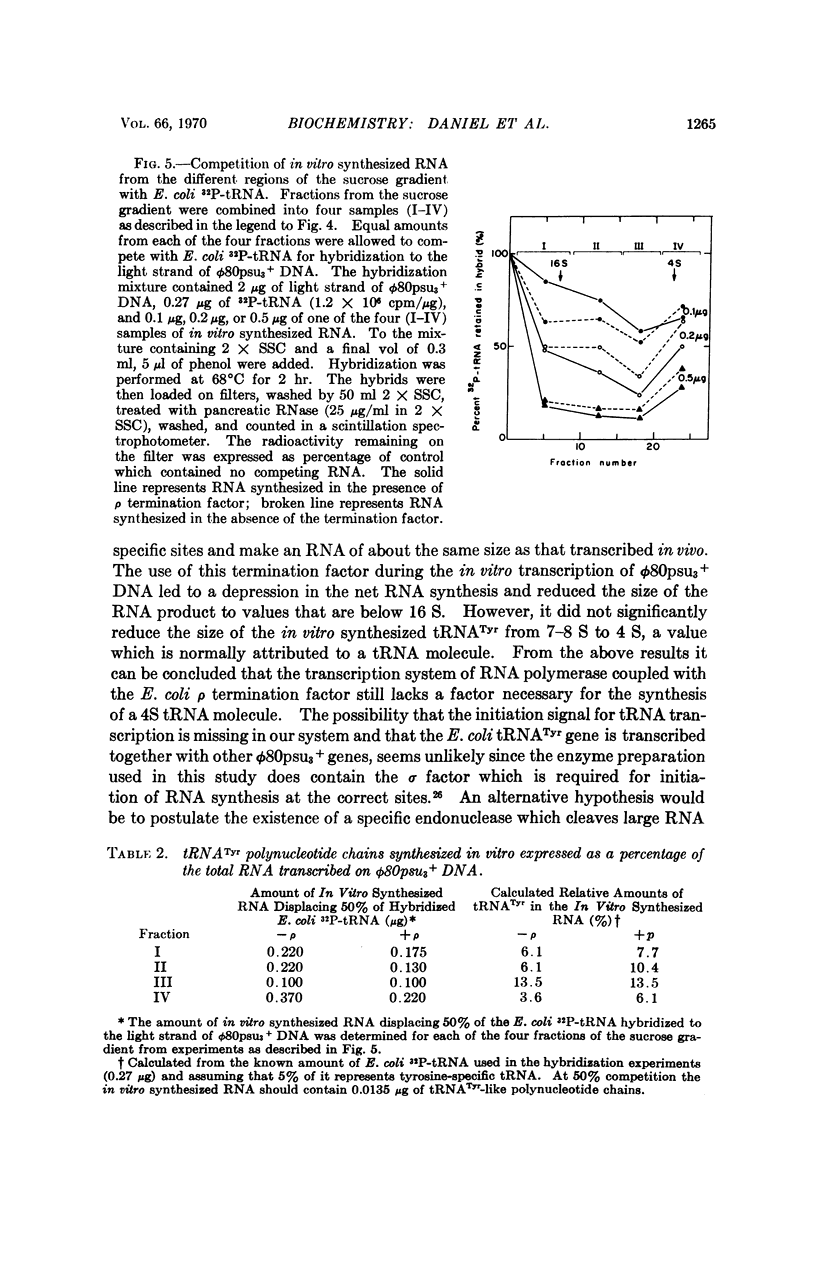
Abstract
The Escherichia coli tRNATyr gene carried by the ϕ80psu3 + transducing phage was transcribed in vitro by DNA-dependent RNA polymerase. The enzymatically synthesized tRNATyr-like polynucleotide chains were detected by competition with purified E. coli32P-tRNATyr for specific hybridization sites on ϕ80psu3 + DNA. Analysis by sucrose gradient centrifugation showed the tRNATyr-like chains to possess a heterogeneous distribution with respect to sedimentation coefficients, with a broad peak around 8 S. The presence of the termination factor ρ during the transcription did not significantly reduce the average size of the in vitro synthesized tRNATyr-like chains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh T., Ozeki H. Suppressor gene Su3+ of E. coli, a structural gene for tyrosine TRNA. Proc Natl Acad Sci U S A. 1968 Mar;59(3):792–799. doi: 10.1073/pnas.59.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREMER H., KONRAD M. W. A COMPLEX OF ENZYMATICALLY SYNTHESIZED RNA AND TEMPLATE DNA. Proc Natl Acad Sci U S A. 1964 May;51:801–808. doi: 10.1073/pnas.51.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt D., Darnell J. E., Jr tRNA synthesis in HeLa cells: a precursor to tRNA and the effects of methionine starvation on tRNA synthesis. J Mol Biol. 1969 May 28;42(1):43–56. doi: 10.1016/0022-2836(69)90485-9. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V., Lavi S., Littauer U. Z. Isolation of biologically active transfer RNA from transfer RNA-DNA hybrids. Biochim Biophys Acta. 1969 May 20;182(1):76–84. doi: 10.1016/0005-2787(69)90522-x. [DOI] [PubMed] [Google Scholar]
- FLEISSNER E., BOREK E. A new enzyme of RNA synthesis: RNA methylase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1199–1203. doi: 10.1073/pnas.48.7.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN H. M., RICH A. Formation of a DNA-soluble RNA hybrid and its relation to the origin, evolution, and degeneracy of soluble RNA. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2101–2109. doi: 10.1073/pnas.48.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HEINRIKSON R. L., GOLDWASSER E. STUDIES ON THE BIOSYNTHESIS OF 5-RIBOSYLURACIL 5'-MONOPHOSPHATE IN TETRAHYMENA PYRIFORMIS. J Biol Chem. 1964 Apr;239:1177–1187. [PubMed] [Google Scholar]
- Hayward R. S., Weiss S. B. RNA thiolase: the enzymatic transfer of sulfur from cysteine to sRNA in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1966 May;55(5):1161–1168. doi: 10.1073/pnas.55.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S., Stretton A. O., Brenner S. Amber suppressors: efficiency of chain propagation and suppressor specific amino acids. J Mol Biol. 1965 Dec;14(2):528–533. doi: 10.1016/s0022-2836(65)80202-9. [DOI] [PubMed] [Google Scholar]
- Landy A., Abelson J., Goodman H. M., Smith J. D. Specific hybridization of tyrosine transfer ribonucleic acids with DNA from a transducing bacteriophage phi-80 carrying the amber suppressor gene su 3. J Mol Biol. 1967 Nov 14;29(3):457–471. doi: 10.1016/0022-2836(67)90112-x. [DOI] [PubMed] [Google Scholar]
- Lozeron H. A., Szybalski W., Landy A., Abelson J., Smith J. D. Orientation of transcription for the amber suppressor gene su 3 as determined by hybridization between tyrosine tRNA and the separated DNA strands of transducing coliphage phi80d su3. J Mol Biol. 1969 Jan 14;39(1):239–243. doi: 10.1016/0022-2836(69)90345-3. [DOI] [PubMed] [Google Scholar]
- MATSUSHIRO A., SATO K., KIDA S. CHARACTERISTICS OF THE TRANSDUCING ELEMENTS OF BACTERIOPHAGE PHI-80. Virology. 1964 Jul;23:299–306. doi: 10.1016/0042-6822(64)90251-x. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Wimmer E., Tener G. M. The isolation of yeast tyrosine and tryptophan transfer ribonucleic acids. Biochemistry. 1968 Jul;7(7):2629–2634. doi: 10.1021/bi00847a027. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Enzymic synthesis of RNA from T7 DNA. J Mol Biol. 1966 Oct 28;21(1):115–127. doi: 10.1016/0022-2836(66)90083-0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Weigert M. G., Lanka E., Garen A. Amino acid substitutions resulting from suppression of nonsense mutations. II. Glutamine insertion by the Su-2 gene; tyrosine insertion by the Su-3 gene. J Mol Biol. 1965 Dec;14(2):522–527. doi: 10.1016/s0022-2836(65)80201-7. [DOI] [PubMed] [Google Scholar]
- Weiss S. B., Legault-Demare J. Pseudouridine Formation: Evidence for RNA as an Intermediate. Science. 1965 Jul 23;149(3682):429–431. doi: 10.1126/science.149.3682.429. [DOI] [PubMed] [Google Scholar]
- Yura T., Imai M., Okamoto T., Hiraga S. Transcription of the tryptophan operon of Escherichia coli in vitro. I. Detection and quantitative determination of specific RNA. Biochim Biophys Acta. 1968 Dec 17;169(2):494–510. doi: 10.1016/0005-2787(68)90058-0. [DOI] [PubMed] [Google Scholar]



