Abstract
Endemic (nonvenereal) syphilis is relatively common in nonindustrialized regions of the world. We describe a case of local transmission in Canada and review tools available for confirming a diagnosis. Improved molecular tools and global clinical awareness are needed to recognize cases of endemic syphilis imported to areas where it is not normally seen.
Keywords: Treponema pallidum subsp. endemicum, bacteria, endemic treponematoses, RFLP, tpr genes, syphilis, transmission, Canada
Treponema pallidum subsp. endemicum is the causative agent of endemic syphilis, also called nonvenereal syphilis. Other diseases caused by nonvenereal treponematoses are yaws (T. pallidum subsp. pertenue) and pinta (T. carateum). The 3 diseases are a substantial cause of illness in the nonindustrialized world, but they are rarely encountered in industrialized areas. Endemic syphilis is encountered in dry, hot regions, including Sahelian areas of western Africa and parts of Botswana, Zimbabwe, and the Arabian Peninsula (1–4). The causative organism is transmitted by direct contact with secretions from lesions or on fomites. The clinical spectrum of these diseases involves various degrees of involvement of the skin, mucous membranes, and skeletal system, depending on the organism (1,2,5,6).
The Study
In February 2011, a 1-year-old girl was referred to our infectious diseases clinic for assessment of skin lesions. The patient had a history of several flesh-colored papules on her forehead at 2 months of age and on other areas of her body (hands, wrists, axilla, and anus) over the next several months. One week before our assessment, she began a 5-day course of azithromycin. Improvement in the lesions and resolution of an oral ulcer were reported by the parents at the assessment. The child appeared well. On examination in our clinic, flesh-colored papules were found on her right hand, wrist, and right axilla. A 1-cm condylomatous perianal lesion was also seen. The remainder of her examination findings were normal. Serologic screening for antibodies to T. pallidum was performed by using the Venereal Disease Research Laboratory (VDRL) test, which resulted in a 1:32 dilution, and the T. pallidum particle agglutination (TP-PA) assay of 4+ reactivity. Long bone radiographic results were normal.
The patient was born in Winnipeg, Canada, to a 39-year-old woman after a non-eventful pregnancy. The mother’s antenatal serologic results were negative for HIV and hepatitis B, and for syphilis by negative VDRL and TP-PA test results. The family lived in a refugee camp in the Republic of Senegal for 20 years before immigrating to Canada in November 2009. Review of laboratory records and discussions with public health services revealed that most of the family members had VDRL and TP-PA tests at a community clinic in early 2010 for unknown reasons, but they were lost to follow-up. The clinic was permanently closed and records were not available. The family had not traveled nor received visitors from overseas since moving to Canada.
We assessed all family members. Results of their examination and testing are described in Table 1. The patient’s 3-year-old brother had a 2-month history of progressive drooling and a hoarse voice. On examination, a 2 cm–diameter ulcer with raised edges and friable appearance was noted on the inside of his lower lip. He had a hoarse cry when agitated and drooled excessively, and he had palpable bilateral cervical lymphadenopathy. The remainder of his physical examination showed no abnormalities. His VDRL test result was positive at a 1:32 serum dilution, as was TP-PA with 4+ reactivity. PCR was performed on swab specimens from oral lesions by using previously described protocols (7) to detect the presence of 3 T. pallidum genes: bmp, polA, and tpp47. All 3 genes were detected.
Table 1. Serologic testing results for Treponema pallidum subspecies endemicum and clinical findings for a symptomatic patient and family members, Canada, 2011*.
| Family member† | Age, y/sex | VDRL result | TPPA result | Clinical findings | Molecular detection/PCR |
|---|---|---|---|---|---|
| Parent | 40/F | Negative | Negative | None | ND |
| Parent | 49/M | Negative | ND | None | ND |
| Child | 1/F | 1:32 | 4+‡ | Skin papules, perianal condylomata | –§ |
| Child | 3/M | 1:32 | 4+ | Oral ulcer, hoarse voice, drooling, adenopathy | +¶ |
| Child | 5/F | Weakly reactive | 4+ | None | ND |
| Child | 7/F | 1:2 | 4+ | None | ND |
| Child | 10/F | 1:8 | 4+ | None | ND |
| Child | 11/M | 1:4 | 4+ | None | ND |
| Child | 14/M | 1:16 | 4+ | None | ND |
| Child | 16/F | Weakly reactive | 4+ | None | ND |
| Child | 19/F | 1:16 | 4+ | None | ND |
*VDRL, Venereal Disease Research Laboratory test; TPPA, T. pallidum particle agglutination assay; ND, not done. †All family members received intramuscular benzathine penicillin (1.2 million U if >10 y old, 0.6 million U if <10 y old) after confirmatory PCR results for the 3-year-old child. ‡Positive reactions were measured on a scale of 1–4, with 4 as the highest value. §Oral swab specimen. Child had history of oral ulcer but had received a full course of azithromycin followed by oral lesion resolution before PCR was performed. She was born in Canada, had no travel history, and although the PCR result was negative, the test was performed after the patient received treatment. The patient had positive serologic test results for T. pallidum, a history of lesions characteristic of endemic syphilis, and no other plausible explanation for findings. ¶Oral ulcer swab specimen.
To determine whether the 3-year-old boy was infected with venereal syphilis or for nonvenereal T. pallidum, PCR was performed on a sample. The acidic repeat protein (arp) gene was amplified from the clinical specimen by using PCR primers and conditions as described (8). DNA sequencing of the purified PCR amplicons showed that the arp gene contained a central region of eight 60-bp repeats; all repeats contained identical nucleotide sequences (GenBank accession no. JN674561). The translated amino acid sequence, REVEDVPKVVEPASEREGGE, is characterized as a Type II repeat motif and has been described only in arp genes from nonvenereal T. pallidum subspecies (8,9).
Additional differentiation was accomplished by identification of subspecies-specific signature sequences in the tprI and tprC genes (10): the genes were amplified from the clinical specimen and from syphilis control DNA prepared from T. pallidum subsp. pallidum Nichols strain by using PCR. Because DNA for T. pallidum subsp. endemicum and T. pallidum subsp. pertenue was not commercially available, it was not included in this study. The subspecies-specific signatures were identified by restriction fragment length polymorphism (10) and DNA sequencing.
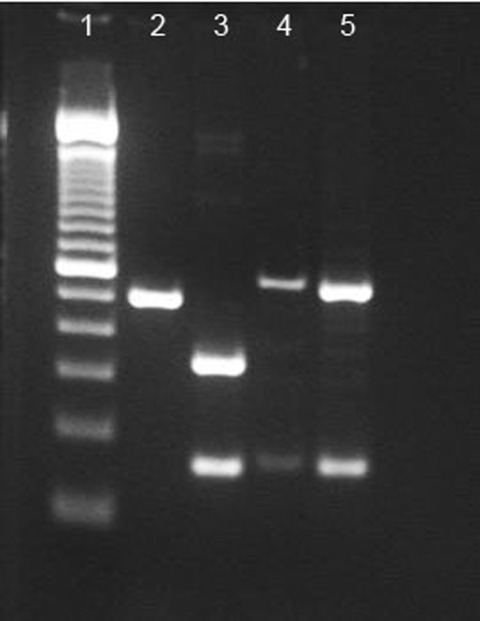
To characterize the tprI gene, PCR amplicons were digested with the restriction enzyme BsrDI (New England Biolabs, Pickering, Ontario, Canada). The tprI gene from the clinical specimen was digested into 2 fragments of 334 bp and 159 bp (Figure), whereas tprI amplicons from the syphilis control strain were not digested and remained as a single band of 493 bp. DNA sequencing of the tprI gene from the clinical specimen confirmed the presence of a BsrDI site (NN/CATTGC at position 1759–1766), which is typical for T. pallidum subsp. endemicum; no restriction site was observed in the syphilis control strain or in GenBank sequences for T. pallidum subsp. pertenue (Table 2).
Figure.
BsrDI digest of tprI and BsrDI/BsEI double digest of tprC from the oral ulcer swab specimen and the syphilis control strain Nichols. Lanes from left to right: 1, 100-bp ladder; 2, Nichols tprI product not digested with BsrDI (493-bp band); 3, clinical specimen tprI product digested with BsrDI (334-bp and 159-bp bands); 4, Nichols tprC digested with BsrDI/BsiEI (547-bp and 160-bp bands); 5, clinical specimen tprC digested with BsrDI/BsiEI (547-bp and 160-bp bands).
Table 2. Comparison of nucleotide sequences of tprI and tprC genes detected in clinical specimens from cases of endemic syphilis, Canada, 2011*.
| Strain† | tprI gene sequence at nt positions 1740–1766, 5′ → 3′ | GenBank accession no. |
|---|---|---|
| Clinical strain | CTC CGA TGT TCC CTA CAT GGG CAT TGC | JN674562 |
| Bosnia A | CTC CGA TGT TCC CTA CAT GGG CAT TGC | DQ886678 |
| Nichols‡ | TGC TGA CGC TCC TTA CAT GGG TAT TGC | NC_000919 |
| Samoa D | TGC TGA CGC TCC TTA CAT GGG TAT TGC | DQ886680 |
| tprC gene sequence at nt positions 1704–1736, 5′ → 3′ | ||
| Clinical strain | GGT GCT CTC CGA TGT TCC CTA CAT GGG CAT TGC | JN674563 |
| Bosnia A | GGT GCT CTC CGA TGT TCC CTA CAT GGG CAT TGC | DQ886673 |
| Nichols‡ | CGT GCT TGC TGA CGC TCC TTA CAT GGG CAT TGC | NC_000919 |
| Samoa D | GGT GCT CTC CGA TGT TCC CTA CAT GGG TAT TAC | DQ886671 |
*Boldface indicates differences in nucleotide sequences. BsrDI restriction sites are underlined. †Sequences were obtained from GenBank: Bosnia A (T. pallidum subsp. endemicum), Samoa D (T. pallidum subsp. pertenue) and syphilis control strain Nichols (T. pallidum subsp. pallidum). ‡Sequence also confirmed in this study.
To characterize the tprC genes, we simultaneously digested PCR amplicons by using restriction enzymes BsrDI and BsiEI (New England Biolabs). Digestion of DNA from T. pallidum subsp. endemicum strains with BsrDI yielded 2 bands, 547 bp and 160 bp. The syphilis control strain also showed bands at 547 bp and 160 bp after digestion with BsrDI, because of a site found in only one T. pallidum subsp. pallidum strain (Figure). The restriction fragment length polymorphism patterns for the tprC genes from the clinical specimen and the syphilis control strain were identical (Figure). However, DNA sequencing of the tprC gene from the clinical specimen revealed a >99% match to tprC GenBank sequences from other T. pallidum subsp. endemicum strains, a ≈97% match to T. pallidum subsp. pallidum Nichols strain (the syphilis control strain), and a ≈98% match to GenBank sequences for T. pallidum subsp. pertenue (Table 2). Furthermore, DNA sequence confirmed the presence of a BsrDI restriction site in the tprC gene, which would be absent in subspecies pertenue. Characterization of the arp, tprI, and tprC genes identified the etiology of these clinical cases as T. pallidum subspecies endemicum.
The boy was administered 600,000 IU of penicillin intramuscularly; 4 weeks later, the ulcer and hoarseness had resolved, and the boy drooled only occasionally. All household members were subsequently treated with penicillin intramuscularly (Table 1).
Conclusions
We suspect the 1-year-old child acquired her infection through close contact with her 3-year-old brother and other siblings, who themselves acquired endemic syphilis while in the Republic of Senegal. The use of molecular techniques greatly assisted in confirming the clinical diagnosis.
Control programs of the World Health Organization and the United Nations Children’s Fund using long-acting penicillin helped reduce the incidence of endemic syphilis and yaws by ≈95% during the 1950s and 1960s. Unfortunately, changes in the administration and delivery of these programs led to an increase in the prevalence of nonvenereal treponematoses during the 1970s (2,5,6). A risk remains for importation of disease into areas where it is not endemic and, subsequently, for local transmission of the etiologic agent.
Diagnosis of imported cases and cases resulting from local transmission is confounded by a lack of experienced clinicians in the diagnosis of nonvenereal treponematoses, and the inability of serologic methods to differentiate these disease entities from venereal syphilis and from each other. Definitive diagnosis is also hampered by widespread unavailability of molecular diagnostics. This deficit necessitates an integrated effort to offer a reproducible reference service to all care providers in a timely and reliable manner to ensure the best clinical outcome, as well as appropriate follow-up for infection control and public health purposes.
Biography
Dr Fanella is a pediatric infectious diseases physician at the University of Manitoba. His research interests include methicillin-resistant Staphylococcus aureus infections, antibiotic stewardship, blastomycosis, and tropical medicine.
Footnotes
Suggested citation for this article: Fanella S, Kadkhoda K, Shuel M, Tsang R. Local Transmission of imported endemic syphilis, Canada, 2011. Emerg Infect Dis [serial on the Internet]. 2012 Jun [date cited]. http://dx.doi.org/10.3201/eid1806.111421
References
- 1.Farnsworth N, Rosen T. Endemic treponematosis: review and update. Clin Dermatol. 2006;24:181–90. 10.1016/j.clindermatol.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 2.Antal GM, Lukehart SA, Meheus AZ. The endemic treponematoses. Microbes Infect. 2002;4:83–94. 10.1016/S1286-4579(01)01513-1 [DOI] [PubMed] [Google Scholar]
- 3.Endemic treponematoses in the 1980s [editorial]. Lancet. 1983;2:551–2. [PubMed] [Google Scholar]
- 4.Kapembwa MS. Endemic treponematoses. In: Cook GC, Zumla A, editors. Manson’s tropical diseases. New York: Elsevier; 2009. p. 1139–48. [Google Scholar]
- 5.Yakinci C, Ozcan A, Aslan T, Demirhan B. Bejel in Malatya, Turkey. J Trop Pediatr. 1995;41:117–20. [DOI] [PubMed] [Google Scholar]
- 6.Perine PL, Hopkins DR, Niemel PLA, St John RK, Causse G, Antal GM, eds. Handbook of endemic treponematoses: yaws, endemic syphilis, and pinta. Geneva: World Health Organization; 1984. [cited 2011 Aug 21]. http://whqlibdoc.who.int/publications/1984/9241541768.pdf
- 7.Martin IE, Tsang RSW, Sutherland K, Tilley P, Read R, Anderson B, et al. Molecular characterization of syphilis in patients in Canada: azithromycin resistance and detection of Treponema pallidum DNA in whole-blood samples versus ulcerative swabs. J Clin Microbiol. 2009;47:1668–73. 10.1128/JCM.02392-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper KN, Liu H, Ocampo PS, Steiner BM, Martin A, Levert K, et al. The sequence of the acidic repeat protein (arp) gene differentiates venereal from nonvenereal Treponema pallidum subspecies, and the gene has evolved under strong positive selection in the subspecies that causes syphilis. FEMS Immunol Med Microbiol. 2008;53:322–32. 10.1111/j.1574-695X.2008.00427.x [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Rodes B, George R, Steiner B. Molecular characterization and analysis of a gene encoding the acidic repeat protein (Arp) of Treponema pallidum. J Med Microbiol. 2007;56:715–21. 10.1099/jmm.0.46943-0 [DOI] [PubMed] [Google Scholar]
- 10.Centurion-Lara A, Molini BJ, Godornes C, Sun E, Hevner K, Van Voorhis WC, et al. Molecular differentiation of Treponema pallidum subspecies. J Clin Microbiol. 2006;44:3377–80. 10.1128/JCM.00784-06 [DOI] [PMC free article] [PubMed] [Google Scholar]