Abstract
Radioisotopically labeled T4-proteins extracted from purified capsids and phage and from infected cells were separated by gel electrophoresis in the presence of sodium dodecyl sulfate and a reducing reagent. They were identified by autoradiography and by counting of the fractionated gels.
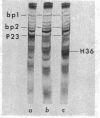
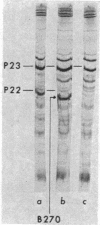
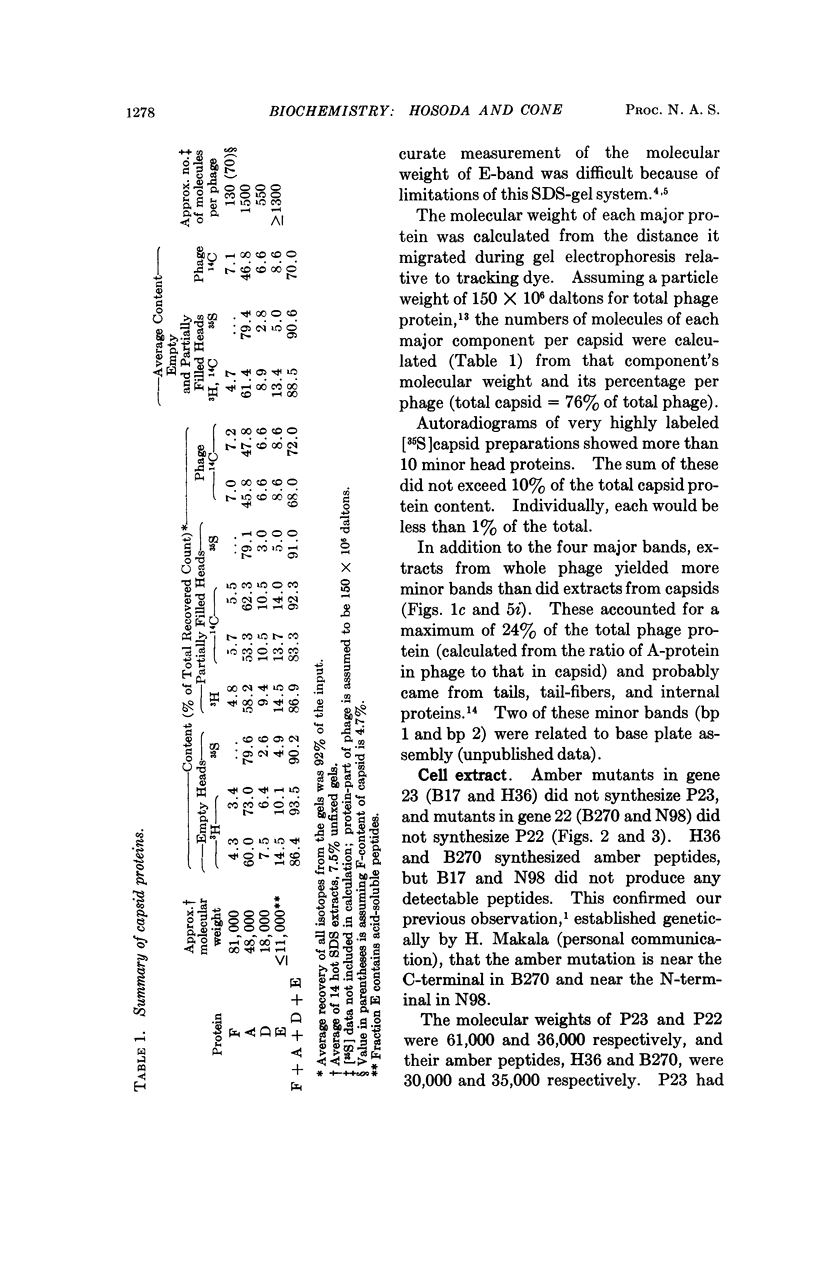
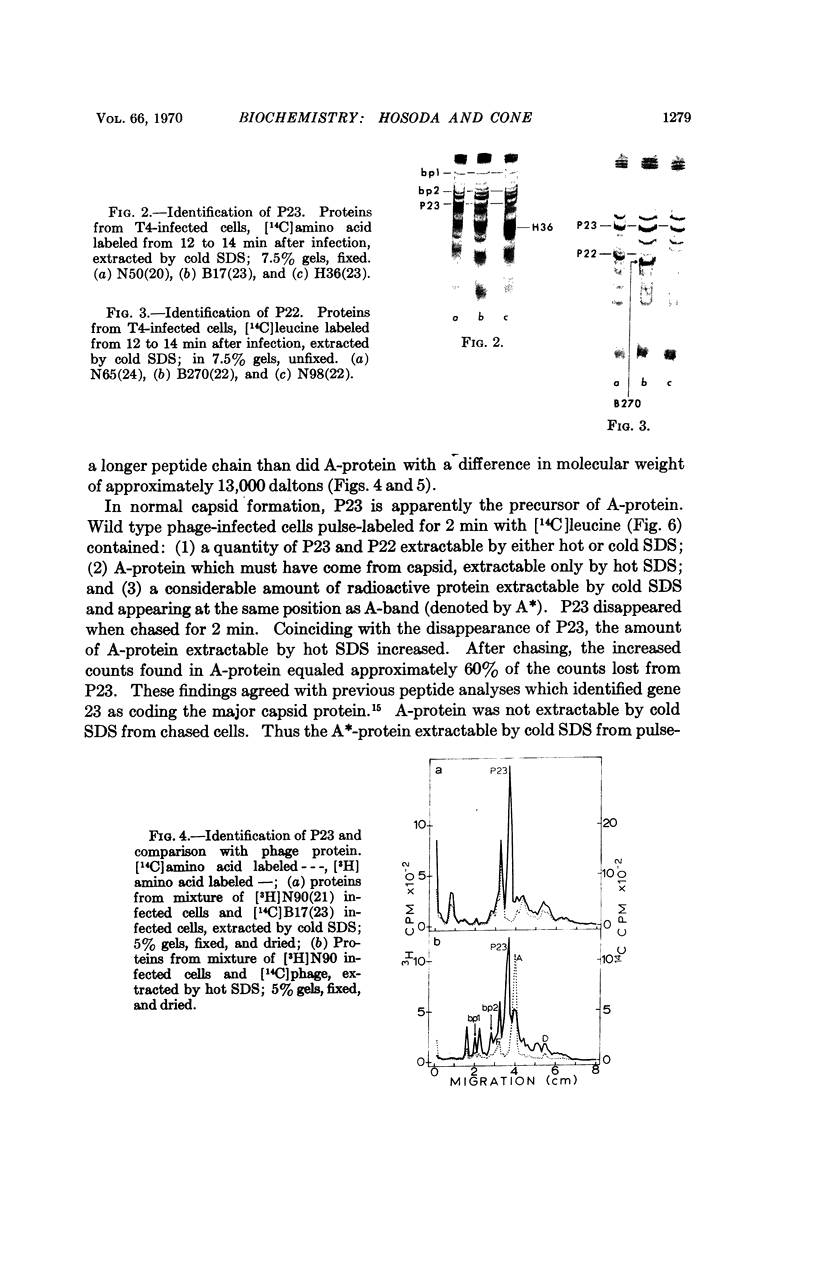
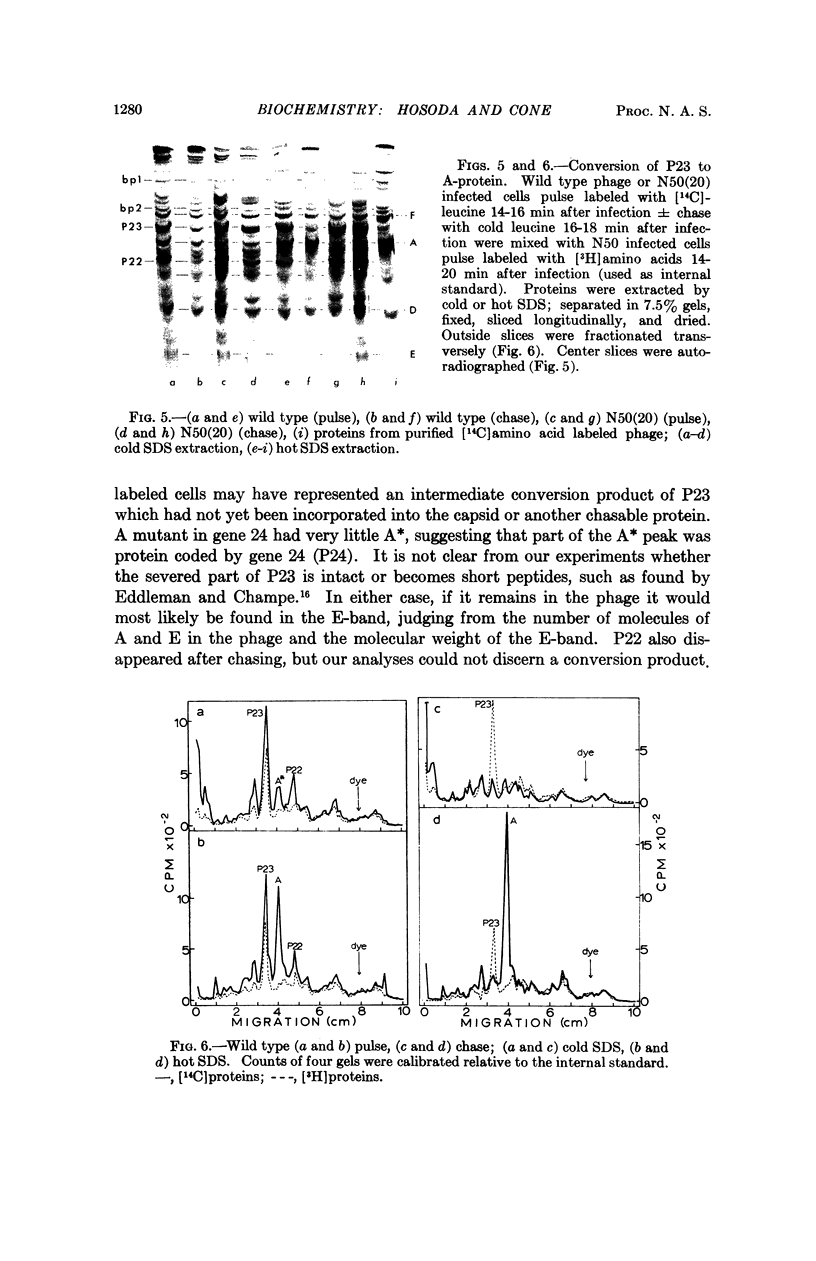
Four major protein bands (F, A, D, and E) were detected in capsid and phage. These accounted for more than 90% of the total capsid protein and 70% of the phage protein (60% of the total capsid protein was in A-band). Coelectrophoresis of [14C]proteins from capsids and [3H]proteins from phage-infected cells indicated that the protein coded by gene 23 (P23) was a peptide chain approximately 25% longer than A-protein. Pulse-chase experiments combined with differential extraction indicated that conversion of P23 into A-protein and alteration of the protein coded by gene 22 (P22) appeared to be vital steps in formation of normal capsids. Mutations in several other genes known to prevent normal capsid formation inhibited conversion of P23 to A-protein and alteration of P22.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor M. B., Roslansky P. F. Gel electrophoresis of the head proteins of T-even phage. Virology. 1970 Feb;40(2):251–259. doi: 10.1016/0042-6822(70)90400-9. [DOI] [PubMed] [Google Scholar]
- Eddleman H. L., Champe S. P. Components in T4-infected cells associated with phage assembly. Virology. 1966 Nov;30(3):471–481. doi: 10.1016/0042-6822(66)90123-1. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coliphage T2. I. Preparation, assay, and some chemical properties. J Gen Physiol. 1957 May 20;40(5):809–825. doi: 10.1085/jgp.40.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Levinthal C. Protein synthesis by Escherichia coli infected with bacteriophage T4D. Virology. 1968 Apr;34(4):709–727. doi: 10.1016/0042-6822(68)90092-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. V. The components of the T4 capsid and of other, capsid-related structures. Virology. 1968 Mar;34(3):549–561. doi: 10.1016/0042-6822(68)90074-3. [DOI] [PubMed] [Google Scholar]
- LEVINE L., BARLOW J. L., VAN VUNAKIS H. An internal protein in T2 and T4 bacteriophages. Virology. 1958 Dec;6(3):702–717. doi: 10.1016/0042-6822(58)90116-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Beguin F., Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970 Jan 14;47(1):69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- Mass M., Fine R., Murakami W. T. Protein composition of polyoma virus. J Mol Biol. 1968 Aug 28;36(1):167–177. doi: 10.1016/0022-2836(68)90227-1. [DOI] [PubMed] [Google Scholar]
- SARABHAI A. S., STRETTON A. O., BRENNER S., BOLLE A. CO-LINEARITY OF THE GENE WITH THE POLYPEPTIDE CHAIN. Nature. 1964 Jan 4;201:13–17. doi: 10.1038/201013a0. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]