Abstract
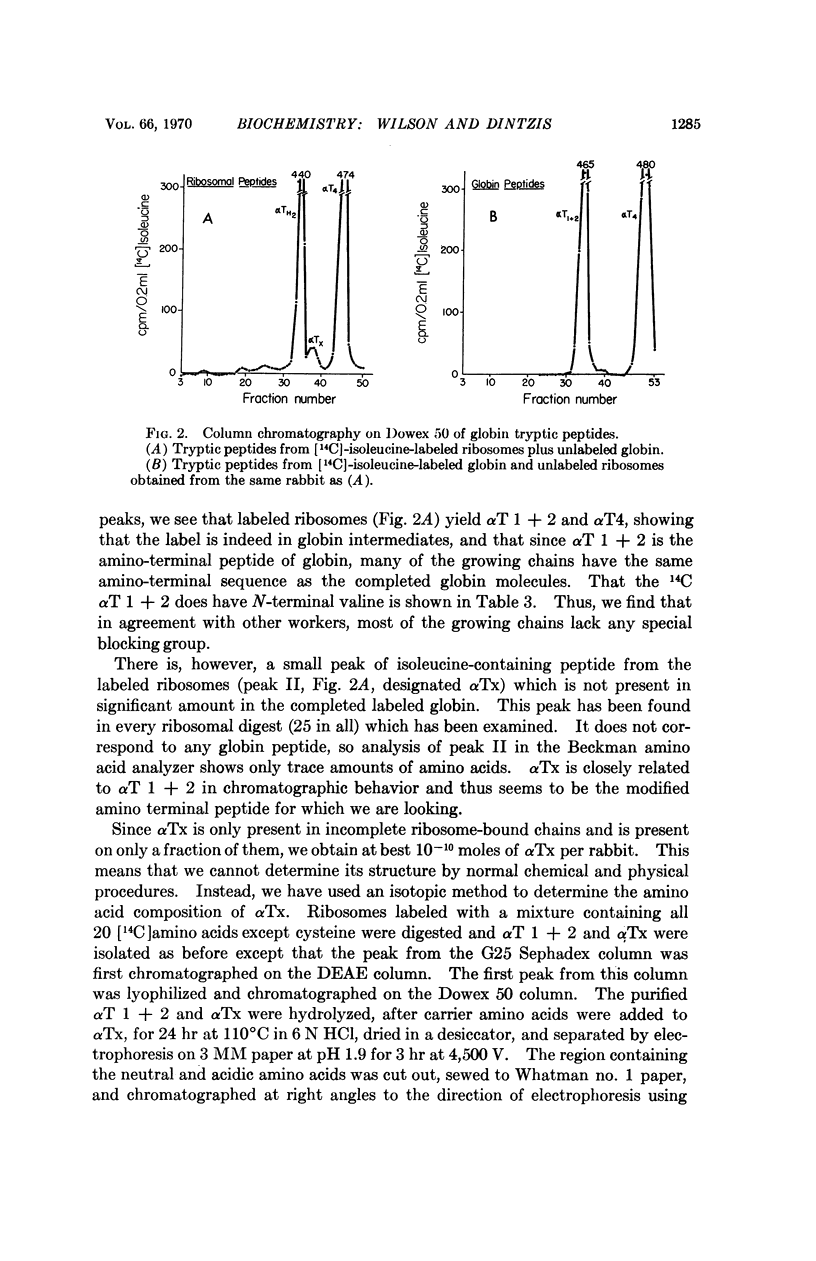
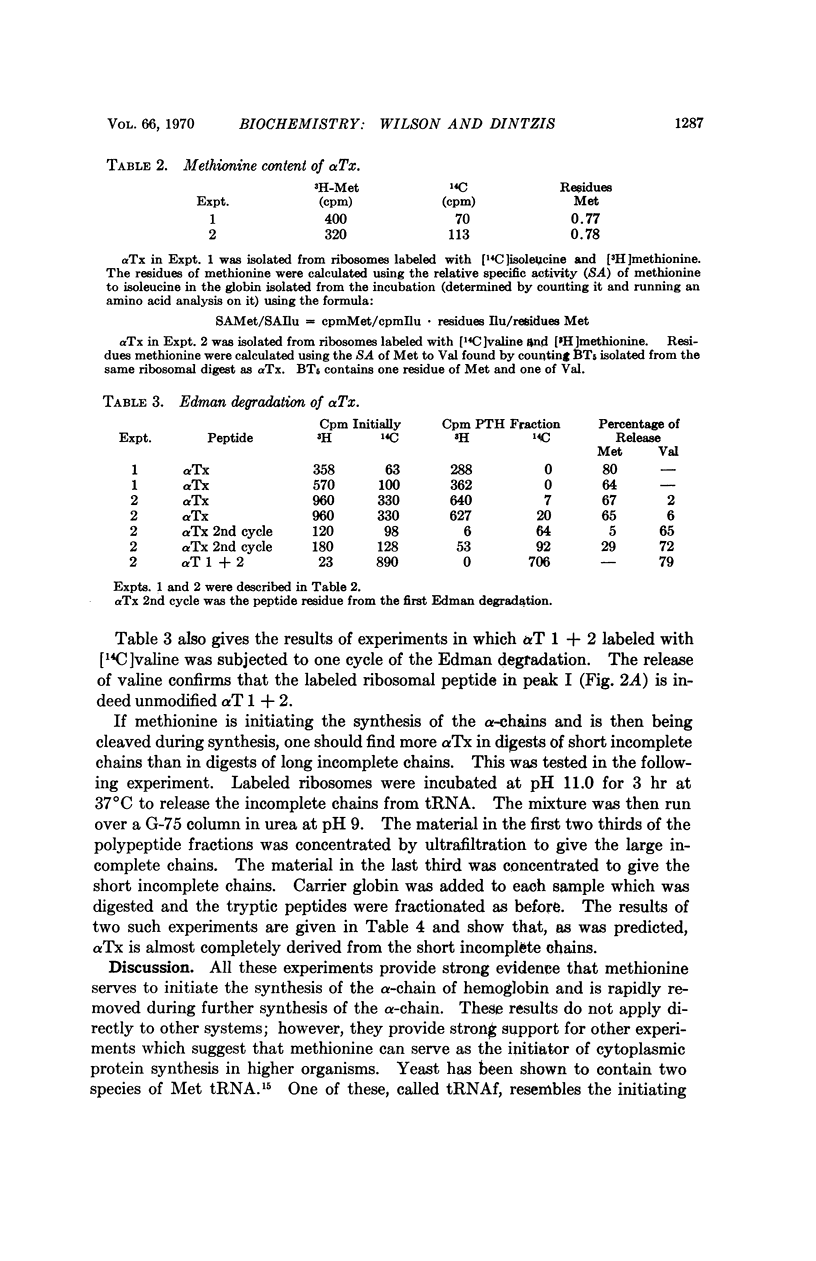
About 15% of the nascent α-chains isolated from in vivo labeled rabbit reticulocytes have methionine as their amino terminal amino acid. The methionine is predominantly on the shortest nascent chains, suggesting that methionine initiates the synthesis of rabbit hemoglobin and is rapidly removed during further synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. M. On the release of the formyl group from nascent protein. J Mol Biol. 1968 May 14;33(3):571–589. doi: 10.1016/0022-2836(68)90307-0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- DOOLITTLE R. F. CHARACTERIZATION OF LAMPREY FIBRINOPEPTIDES. Biochem J. 1965 Mar;94:742–750. doi: 10.1042/bj0940742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galper J. B., Darnell J. E. The presence of N-formyl-methionyl-tRNA in HeLa cell mitochondria. Biochem Biophys Res Commun. 1969 Jan 27;34(2):205–214. doi: 10.1016/0006-291x(69)90633-0. [DOI] [PubMed] [Google Scholar]
- Gonano F., Baglioni C. Initiation of hemoglobin synthesis with rabbit and E. coli tRNA in a reticulocyte cell-free system. Eur J Biochem. 1969 Nov;11(1):7–11. doi: 10.1111/j.1432-1033.1969.tb00732.x. [DOI] [PubMed] [Google Scholar]
- MARCKER K., SANGER F. N-FORMYL-METHIONYL-S-RNA. J Mol Biol. 1964 Jun;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Culp W. J., Hardesty B. Deacylated transfer ribonucleic acid as a factor for peptide chain initiation in rabbit reticulocyte systems. J Biol Chem. 1968 Dec 25;243(24):6343–6352. [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmimoff H., Arnstein H. R. The initiation of haemoglobin synthesis in rabbit reticulocytes. Biochem J. 1969 Oct;115(1):113–124. doi: 10.1042/bj1150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Meyer R., Eisenstadt J. M., Brawerman G. Involvement of N-formylmethionine in initiation of protein synthesis in cell-free extracts of Euglena gracilis. J Mol Biol. 1967 May 14;25(3):571–574. doi: 10.1016/0022-2836(67)90210-0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver. J Mol Biol. 1968 Dec 14;38(2):241–243. doi: 10.1016/0022-2836(68)90409-9. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Nishimura S., Ukita T. Purification of histidine-specific transfer RNA from yeast. Biochim Biophys Acta. 1967;145(3):605–612. doi: 10.1016/0005-2787(67)90119-0. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Sekiya T., Ukita T. Selective utilization of nonformylatable species of methionyl-tRNA's from Escherichia coli and yeast in a reticulocyte cell-free system. Biochim Biophys Acta. 1970 Feb 18;199(2):559–561. doi: 10.1016/0005-2787(70)90108-5. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Ukita T., Nishimura S. Characterization of two species of methionine transfer ribonucleic acid from bakers' yeast. J Biol Chem. 1968 Nov 10;243(21):5761–5768. [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. Initiation of the alpha chain of rabbit hemoglobin. Cold Spring Harb Symp Quant Biol. 1969;34:313–317. doi: 10.1101/sqb.1969.034.01.038. [DOI] [PubMed] [Google Scholar]


