Abstract
Herpes virus (CMV, HSV, VZV) and invasive fungal infections continue to cause significant morbidity and mortality in allogeneic hematopoietic cell transplant (HCT) recipients despite the availability of effective therapies. In this study, we developed an internet-based survey, which was distributed to all HCT centers participating in the CIBMTR program, to gather information on strategies utilized for the prevention of disease due to herpes virus and fungal infections between 1999 and 2003. The survey response rate was 72%, representing 175 programs from 32 countries. Generally, reported center strategies were in accord with the CDC guidelines published in 2000, with 81% of programs using low-dose acyclovir prophylaxis for HSV seropositive patients, 99% of programs reporting use of a CMV prevention strategy during the first 100 days post-transplant for all patients at risk of CMV disease, and 90% of programs using antifungal prophylaxis. Seventy percent of programs reported routine use of a CMV prevention strategy in high-risk patients after day 100. The greatest departure from published guidelines was the use of acyclovir prophylaxis for VZV seropositive recipients in 75% of programs. There were very few reported changes within centers in practices over the study time period. Significant regional variations were found with regard to surveillance procedures and treatment durations. There were no significant differences in treatment practices by center size and very few differences found between those centers that reported treating primarily pediatric patients versus primarily adult patients. In summary, our survey demonstrates overall agreement with published guidelines for the prevention of disease due to herpesviruses and fungal infections with significant regional differences found in duration of antiviral prophylaxis, duration of preemptive therapy, and duration and dosing of antifungal prophylaxis. Center size and age of primary patient population were not associated with many reported differences in strategies.
Introduction
Cytomegalovirus (CMV) infection continues to be a major cause of morbidity and mortality in recipients of allogeneic hematopoietic cell transplants (HCT) despite the development of effective antiviral therapies and the advancement of testing modalities for early diagnosis. (1) Historically, CMV reactivation occurred in 60–80% of seropositive patients with approximately one-third of these patients going on to develop symptomatic CMV disease. (2) The use of CMV prevention strategies with either universal prophylaxis or preemptive therapy for high-risk patients, have significantly decreased the incidence of CMV disease in the first 100 days after transplantation. Concurrent with the reduction of early CMV disease, there has been a significant increase in the incidence of late CMV disease, posing a further challenge in the coordination of care for these patients. (3, 4)
The epidemiology of invasive fungal infections has also changed significantly in the past twenty years. Before the widespread adoption of effective antifungal prophylaxis Candidal infections were the most common invasive fungal infections in the first 100 days after transplant and carried significant morbidity and mortality.(5) Currently, invasive fungal infections occur in less than 5% of patients, with molds comprising the majority of cases. (6)
In 2000, the CDC, the Infectious Disease Society of America, and the American Society of Blood and Bone Marrow Transplantation published evidence-based guidelines for the prevention of opportunistic infections among HCT recipients.(7) Since then, data describing current practices in the surveillance, prophylaxis, and treatment of CMV have been limited to individual centers or countries.(8, 9) In order to more fully understand current prevention practices, we developed a web-based survey to elicit information on center-specific strategies for the prevention of CMV disease, both in the early and late post-transplant periods, fungal disease, and other herpes viruses, such as herpes simplex virus (HSV) and varicella zoster virus (VZV). This survey was distributed to all HCT centers participating in the CIBMTR program allowing for comparison of strategies regionally, as well as by program size, and for adult versus pediatric programs. Additionally, the time period studied (1999–2003) permitted a comparison of practices immediately prior to the availability of evidence-based guidelines to those after the guidelines were published.
Materials and Methods
A web-based survey was designed using Survey Monkey (http://www.surveymonkey.com) to investigate center-specific practices used to prevent HSV, VZV, CMV and fungal diseases in allogeneic transplant recipients during the time period of 1999 to 2003. An email invitation to participate in the survey was distributed to all program directors at CIBMTR-affiliated HCT centers in May 2006. To optimize early survey return rates we offered 4 prizes, the recipients were randomly selected from the centers that returned the survey within the specified time period.
The survey consisted of 8 questions, many of which contained several sublevels [Appendix A]. In general, participants were instructed to select the response that best described their center’s primary prevention strategies – those strategies used in the majority of their patients- and indicate if these changed during the time period 1999–2003. Question 1 inquired about the centers’ strategies for VZV and HSV prevention in seropositive patients. They were specifically asked about the use of low-dose acyclovir (defined here as 5mg/kg every 8 hours), valacyclovir (500 mg twice daily) or famciclovir (500 mg twice daily) and the duration of treatment if used. Question 2 evaluated the use of high-dose acyclovir (defined here as ≥ 800 mg 4–5 times daily) and valacyclovir (6–8g daily) as prophylaxis for CMV reactivation. Questions 3–5 explored the use of ganciclovir or foscarnet based strategies for prevention of CMV reactivation prior to and after Day 100, as demonstrated in Figure 1. Other topics of interest included the use of CMV seronegative or leukoreduced blood products and the use of adoptive cellular immunotherapy. Finally, participants were asked to describe their center’s practices regarding the use of antifungal prophylaxis, the timing of initiation, the duration of use, and the preferred drug and dosage. Space was provided at the end of the survey for qualifying statements. Information regarding number of transplants performed by each center annually was obtained later through direct email inquiries. The protocol was submitted to the IRB at the Fred Hutchinson Cancer Research Institute and received a designation of “Not Human Subjects Research.”
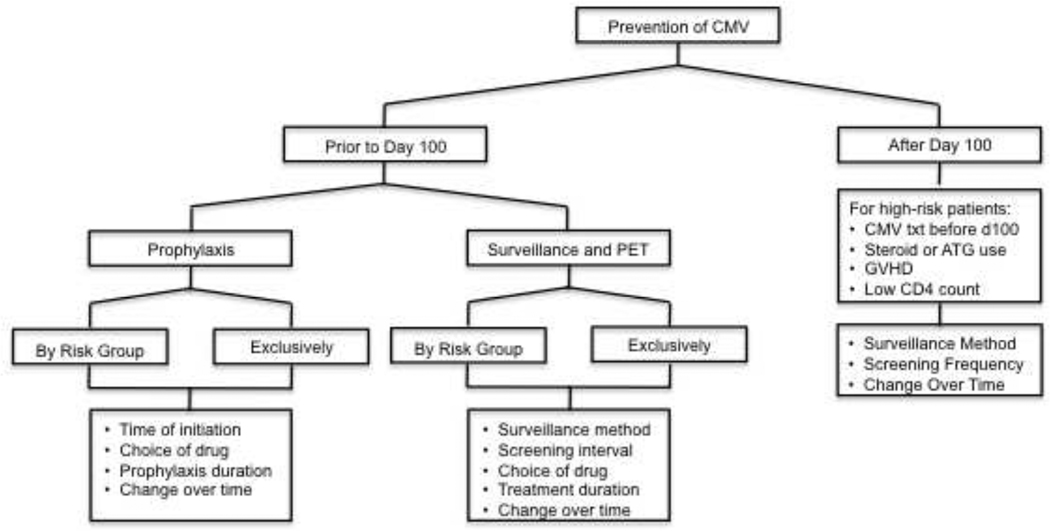
Figure 1. Flow of Survey Topics Regarding CMV Disease Prevention after HCT.
CMV- cytomegalovirus, PET- preemptive therapy, ATG- anti-thymocyte globulin, GVHD- graft versus host disease
For each topic, the survey data were tabulated and analyzed to compare differences in practices based on geographic region, center size, and primary population treated (adult, pediatric, or both). As some of the geographic regions had very few participating centers, several regions were grouped together to facilitate statistical comparisons. Similarly, while we collected more detailed data on program size, the data were ultimately analyzed in two groups: those programs that performed less than or equal to fifty allogeneic stem cell transplants annually and those that performed greater than fifty per year. A Chi-square test or Fisher’s Exact test were used to calculate p-values for the comparisons. Two-sided P values of <0.05 are considered significant.
RESULTS
Participating Centers
The survey web link was sent to the program directors of 244 CIBMTR centers. Responses were received from 175 centers (72%) in 32 countries. The 69 programs that did not respond were from a wide distribution of geographic locations and, we assume, were otherwise similar to the programs that participated. There was wide variation in participant center size with 62% performing 50 or fewer allogeneic transplants annually and 12% performing more than 100 (Table 1). Additionally, 46% of centers treated primarily adult patients while 21% were primarily pediatric centers allowing for comparison of practices between adult and pediatric patients. The survey was completed by a variety of HCT professionals: 136 physicians, of which 54 were the directors of the HCT programs at their institution; 11 nurses or nurse practitioners; and 28 research coordinators and data managers.
Table 1.
Regional Comparison of Patient Age Groups and Center Size of Survey Participants
244 Centers Contacted, 175 Responses (72%), from 32 Countries*
| Regional Location of Survey Participants | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total % (n=175) |
US/CAN % (n=97) |
Europe % (n=40) |
Aust/NZ % (n=13) |
L. America % (n=12) |
Asia % (n=6) |
Middle East % (n=4) |
Africa % (n=3) |
|
| Patient Characteristics | ||||||||
| Primarily Adult | 46.3 | 47.4 | 50.0 | 61.5 | 33.3 | 16.7 | 25.0 | 33.3 |
| Primarily Pediatric | 21.1 | 23.7 | 17.5 | 38.5 | 8.3 | 16.7 | 0.0 | 0.0 |
| Both Adult and Pediatric | 32.6 | 28.9 | 32.5 | 0.0 | 58.3 | 66.7 | 75.0 | 66.7 |
| No of allogeneic transplants/year ** | ||||||||
| <25 | 33.7 | 29.9 | 22.5 | 61.5 | 75.0 | 33.3 | 0.0 | 66.7 |
| 26–50 | 28.0 | 30.9 | 32.5 | 23.1 | 16.7 | 0.0 | 25.0 | 0.0 |
| 51–100 | 20.0 | 20.6 | 32.5 | 7.7 | 0.0 | 16.7 | 0.0 | 0.0 |
| 101–200 | 9.7 | 8.2 | 10.0 | 0.0 | 0.0 | 33.3 | 75.0 | 0.0 |
| >200 | 2.3 | 4.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Missing data | 6.3 | 6.2 | 2.5 | 7.7 | 8.3 | 16.7 | 0.0 | 33.3 |
Countries represented by region (number of responding programs): US/Can--United States (90), Canada (7); Europe-- Germany (13)United Kingdom (7), Spain (5), Belgium (3), Sweden (2), Finland (2), Italy (2), Switzerland (1), Norway (1), Denmark (1), Poland (1), Portugal (1), Czech Republic (1); Australia/NZ-- Australia (10), New Zealand (3); Latin America-- Brazil (3), Argentina (3), Uruguay (3), Venezuela (2), Mexico (1); Middle East-- Israel (2), Iran (1), Pakistan (1); Asia-- India (2), China, Hong Kong (1), Japan (1), Taiwan (1), Korea (1); Africa-- South Africa (3)
Data regarding center size were obtained via direct correspondence after survey was completed
HSV and VZV Prophylaxis
Eighty-one percent of programs reported using low-dose acyclovir, valacyclovir, or famciclovir for prophylaxis in either VZV seropositive (VZV+) or VZV seronegative/HSV seropositive patients (VZV-/HSV+) (Table S1). This proportion increases to 94.2% when those programs using high-dose acyclovir or ganciclovir for CMV prophylaxis were included. Nevertheless, there were still 10 programs that did not use any prophylaxis for VZV+ or VZV-/HSV+ patients. Of the thirty-four programs (23.9%) that did not report using low-dose acyclovir prophylaxis for VZV+ patients, eight were using high-dose acyclovir and seven were using ganciclovir prophylaxis for CMV seropositive patients. American/Canadian and Latin American programs reported using low dose acyclovir in 88.7% and 91.7% of respondents, respectively. This was significantly different from other regions, where low-dose acyclovir use was reported between 53.8–72.5% of respondents (p=0.006) (Table S1). There were no significant differences in use of low-dose acyclovir based on pediatric vs. adult patient populations or program size (Table S2). There were also no changes in treatment strategies reported in either VZV+ or VZV-/HSV+ patients over the time period studied.
Among those centers that used low-dose acyclovir prophylaxis, there was remarkable regional variation in treatment duration (p=0.008) (Table 2 and S1). For VZV+ patients, American/Canadian centers tend to treat for longer duration with 40.2% of programs treating for at least 6 months and 17.5% of programs treating for at least one year. Alternately, excluding programs that were using high dose acyclovir for CMV prophylaxis, 21.8% of American centers did not use any prophylaxis for VZV prevention. A higher percentage of European centers used low-dose acyclovir in VZV+ patients, but for shorter durations with the majority treating for less than 4 months. There were no significant differences in the duration of low-dose acyclovir prophylaxis for VZV+ patients in pediatric vs. adult patients or by program size (Table S2).
Table 2.
Duration of Low-Dose Acyclovir Prophylaxis* for Varicella and HSV by Region
| Do Not Treat** | 1 month | 3–4 months | 6 months*** | ≥ 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VZV+ | VZV−/HSV+ | VZV+ | VZV−/HSV+ | VZV+ | VZV−/HSV+ | VZV+ | VZV−/HSV+ | VZV+ | VZV−/HSV+ | ||
| US/CAN | % (n=97) | 26.8 | 7.2 | 6.2 | 25.8 | 11.3 | 20.6 | 24.7 | 23.7 | 17.5 | 13.4 |
| Europe | % (n=40) | 10.0 | 0.0 | 20.0 | 30.0 | 32.5 | 32.5 | 3.1 | 2.1 | 0.0 | 0.0 |
| Aus/NZ | % (n=13) | 23.1 | 7.7 | 15.4 | 30.8 | 15.4 | 23.1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Latin America | % (n=12) | 25.0 | 0.0 | 25.0 | 33.3 | 25.0 | 33.3 | 1.0 | 1.0 | 1.0 | 1.0 |
| Asia | % (n=6) | 16.7 | 16.7 | 33.3 | 50.0 | 16.7 | 16.7 | 1.0 | 0.0 | 0.0 | 0.0 |
| Middle East | % (n=4) | 25.0 | 25.0 | 0.0 | 0.0 | 50.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| South Africa | % (n=3) | 33.3 | 33.3 | 0.0 | 0.0 | 33.3 | 33.3 | 0.0 | 0.0 | 0.0 | 0.0 |
Includes acyclovir less than or equal to 5mg/kg every 8 hours, valacyclovir 500mg twice daily, or famciclovir 500mg twice daily
Excludes those programs that use high-dose Acyclovir prophylaxis for CMV seropositive patients
Treatment duration equal to 6 months or until discontinuation of immunosuppression
For VZV-/HSV+ patients, low-dose acyclovir prophylaxis was used at more centers with a trend toward shorter treatment durations in most regions. American/Canadian, and Latin American programs treated for longer durations than other regions, with 35.6% and 20.0% of programs respectively treating for as long as 12 months (Table S1). Only one program outside of these regions treated for as long (p=0.017). Most programs treated only until engraftment or for 3–4 months after transplantation. Again, there were no significant differences in practices among programs that primarily treated pediatric vs. adult patients or by program size (Table S2).
CMV Prevention in seropositive patients
High-dose Acyclovir or Valacyclovir Prophylaxis
Fifty-five programs (31.4%) reported using high-dose acyclovir or valacyclovir for prevention of CMV disease in at-risk patients during the study time period (Table S1). European programs used this strategy in 47.5% of programs, significantly more than American/Canadian programs (23.7%, p=0.008). However, when comparing responses among all regions, the differences are not statistically significant (p=0.063). This strategy was more commonly utilized in centers that treated primarily pediatric patients (54.1%) versus those that treated primarily adult patients (22.2%) (p=0.002) (Table S2). There were no significant differences noted based on the size of the transplant center.
There was notable variation in duration of treatment among those centers using high-dose acyclovir or valacyclovir with 32.7% of programs treating for one month or until engraftment, 40% treating for 3–4 months, and 23.6% treating for at least 6 months or until immune reconstitution. Regional differences were noted with a majority (57.9%) of European programs treating for 3–4 months, American/ Canadian programs treating only until engraftment 40.9% of the time, and extending treatment for at least 12 months 27.3% of the time, and other regions treating for 3–4 months a majority of the time (p=0.005) (Table S1). There were no significant differences in treatment duration by age of patient population or program size (Table S2). Fifty (92%) of the programs reported using the same practices consistently over the study time period.
Ganciclovir or Foscarnet Prophylaxis
By 2003, 167 of 175 respondents reported using a ganciclovir based CMV prevention strategy, either prophylaxis or preemptive therapy, and 1 program used a foscarnet-based strategy. Forty -eight (27%) centers reported using ganciclovir (n=47) or foscarnet (n=1) based prophylactic therapy in at least some allogeneic transplant recipients between 1999 and 2003 (Table S1). While there was no significant difference in the use of this strategy by region, pediatric vs. adult centers or center size, there was a suggestion of higher use of this strategy in centers in Australia/New Zealand. Seven of thirteen programs (53.9%) in Australia/New Zealand used ganciclovir prophylaxis for some portion of the study period compared with 21.6% of US/Canadian and 25% of European programs. However, by 2003, only 38.5% of centers in Australia and New Zealand were still using this strategy. Seventy percent of centers that used ganciclovir prophylaxis reported consistent use of prophylaxis for each year of the study period, with the remainder switching to a preemptive strategy between 2000 and 2002.
Ganciclovir Prophylaxis Initiation
Two thirds of centers using ganciclovir based prophylaxis for prevention of CMV initiated treatment at the time of engraftment (n= 26) while one third started prophylaxis pre-transplant (n=13), many commenting that therapy was interrupted on Day 0 and reinitiated after engraftment. One center started pre-transplant if the transplant recipient was CMV seropositive but waited until engraftment if only the donor was CMV seropositive. These findings were consistent across regions, patient populations, and center sizes. All practices were stable over the time period the center was using a prophylactic strategy.
Ganciclovir Prophylaxis Duration
Thirty-seven (77.2%) of the 48 programs using ganciclovir prophylaxis, continued treatment for 3 months, while 18.8% of programs treated for between 3–6 months and 6.3% treated for >6 months (Table S1). There were no significant differences noted by region, pediatric versus adult patients, or center size. “Other” responses were noted in only 2 programs included treating until CMV specific immune response was detected and treating until CD4 count was >200. Again, all practices were stable over each year the center was using a prophylactic strategy.
Ganciclovir or Foscarnet Preemptive Therapy
Table 3 compares the use of prophylactic versus preemptive therapy in all participating centers by patient risk group in 2003. During the previous study years 1999–2002, ten programs switched from a prophylactic to a preemptive treatment strategy. Sixty-eight percent of programs endorsed using a strategy of preemptive therapy alone, compared with only 5.1% who endorsed using ganciclovir-based prophylaxis alone. Several programs (22.3%) reported using both strategies in the same time period, possibly indicating that the center policies allowed physicians to choose which strategy was used.
Table 3.
CMV Preventin: Ganciclovir Prophylaxis versus Preemptive Therapy by Risk Group in 2003
| Ganciclovir Prophylaxis | Preemptive Therapy | Physician Preference | Missing information | |
|---|---|---|---|---|
| n= 175 programs | Alone (%) | Alone (%) | (%) | (%) |
| HLA identical siblings | 4.6 | 77.7 | 11.4 | 6.3 |
| Unrelated Donors or Partially HLA-mismatched Related | 5.7 | 65.1 | 16.0 | 13.1 |
| Recipients of Non-myeloablative Conditioning | 4.0 | 69.7 | 8.6 | 17.7 |
For HLA identical siblings, preemptive therapy alone was used in 77.7% of centers, while prophylaxis alone was used in 4.6% and physician preference in 11.4%. In transplants involving unrelated donors or partially HLA-mismatched related donors, preemptive therapy alone was used in 65.1% of programs, prophylaxis alone in 5.7%, and physician preference in 16.0% of programs. The survey asked respondents whether a particular strategy was used for a given risk group, without asking whether that center actually performed transplantation in that risk group during the time period studied. Thus if the strategy was not affirmed in the risk group it could either be because they did not use that strategy in that group or because they did not perform transplantations in that particular risk group. Therefore, we only had an approximate denominator and the proportions do not all add up to 100%. A similar pattern was seen for recipients of non-myeloablative conditioning where 69.7% of programs used preemptive therapy alone, 4.0% used prophylaxis alone, and 8.6% used physician preference. The survey was not designed to obtain reliable data on this issue for T-cell depleted grafts and haploidentical donors.
There were no significant regional differences in the use of preemptive therapy in transplants involving HLA identical siblings, unrelated or HLA-mismatched related donors, or recipients of non-myeloablative conditioning. There were also no significant differences in the proportion of programs using preemptive therapy among pediatric and adult centers or by center size (Table S1, S2).
Among those programs using a strategy of preemptive therapy, 62.0% of programs performed surveillance on all patients while 36.1% of programs screened only patients who were seropositive at the time of their transplant and those seronegative patients who received a graft from a seropositive donor. Only 1.9% of programs performed surveillance exclusively on patients who were seropositive at the time of their transplantation and at highest risk of reactivation. Again, there were significant regional differences in the patient populations selected for routine surveillance (Table S1). European and Latin American programs tended to subject all transplant recipients to surveillance, regardless of CMV status (81.6% and 90.0%, respectively), while American/Canadian and Australian/New Zealand programs seronegative recipients from seronegative donors (D-/R-) were less likely to be screened (51.6% and 55.6%, respectively) (p<0.001). There were no differences in patients selected for surveillance based on patient population or center size (Table S2).
Method of CMV Surveillance
A variety of tests were used to detect subclinical CMV among those programs using a preemptive strategy. Forty-six percent of programs used CMV antigen (pp65) testing, while 28.5% used a plasma CMV DNA PCR test and 22.2% used a whole blood or white blood cell based CMV DNA pcr test (Supplemental data). Two percent of programs used CMV shell vial testing. These trends were stable across regions, patient population and center size. Eighty-seven percent of programs reported using the same detection method over the entire study period. Of the 13 programs that changed the testing method, all but one program switched from a pp65 antigen test to a PCR test.
Frequency of Surveillance
The great majority of programs (91.1%) performed CMV surveillance testing at least weekly during the first 100 days post-transplant in the years they were utilizing a strategy of preemptive therapy. Eight programs (5.1%) performed surveillance testing twice weekly and seven programs employed a de-escalating strategy of surveillance testing weekly initially and then less frequently over time. The strategies were stable over the time period that the center was using preemptive therapy and there were no significant differences based on region, patient population or center size.
Duration of Treatment
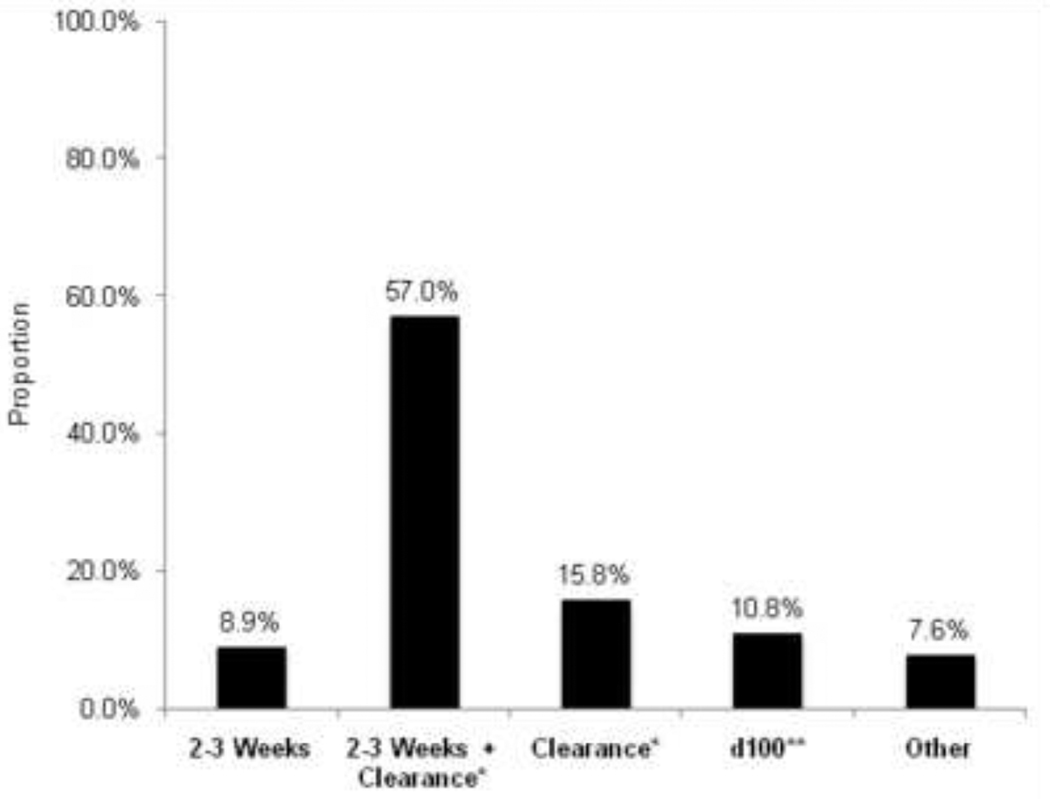
There was considerable variation in the duration of treatment once CMV was detected by surveillance (Figure 2), with the majority of programs treated for at least 2–3 weeks and clearance of CMV DNA or antigen from blood. There were no significant differences by region, patient population, or center size.
Figure 2. Duration of Preemptive Therapy for CMV when Detected by Surveillance Testing (n=158).
* Clearance of CMV (pp65) antigenemia or CMV DNAemia
** day 100 post-transplant
Prevention of Late CMV Disease
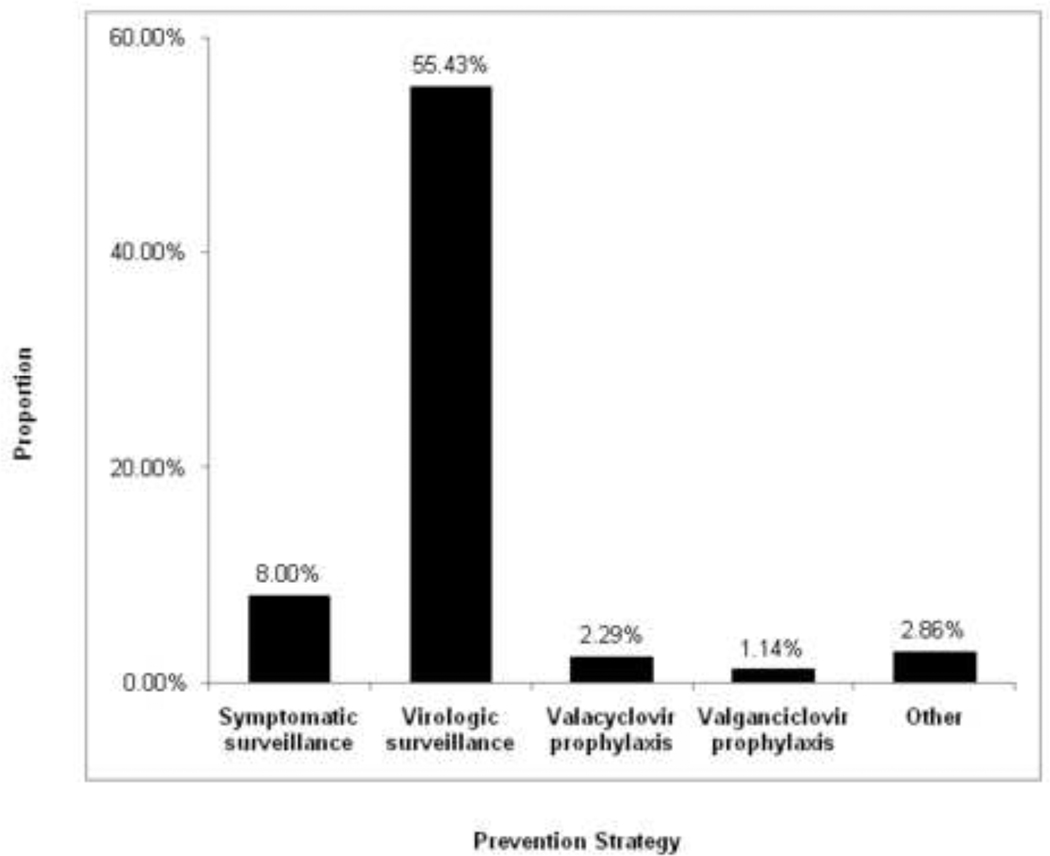
Approximately 60% of respondents (108 centers) reported using either a prophylactic or preemptive strategy with high-risk transplant recipients after day 100 (Figure 3). Ninety-seven centers (55.4%) used a strategy of virologic surveillance with preemptive therapy. The majority of these programs (55.0%) performed surveillance testing twice monthly. Thirty-four centers (27.9%) tested at least weekly, while 22 (18.0%) centers performed testing on a more variable schedule. Among the centers utilizing a prophylactic approach, 4 programs treated with high-dose valacyclovir, 2 used valganciclovir, and 1 used oral ganciclovir.
Figure 3. CMV Prevention Strategy after Day 100 Post-Transplant (n=175).
Sixty-seven programs (38.3%) did not use either surveillance with preemptive therapy or prophylaxis to prevent CMV disease after day 100 during the study period. There were no significant differences noted based on region, patient population or center size (Table S1 and S2).
Use of CMV Negative and/or Leukoreduced Blood Products
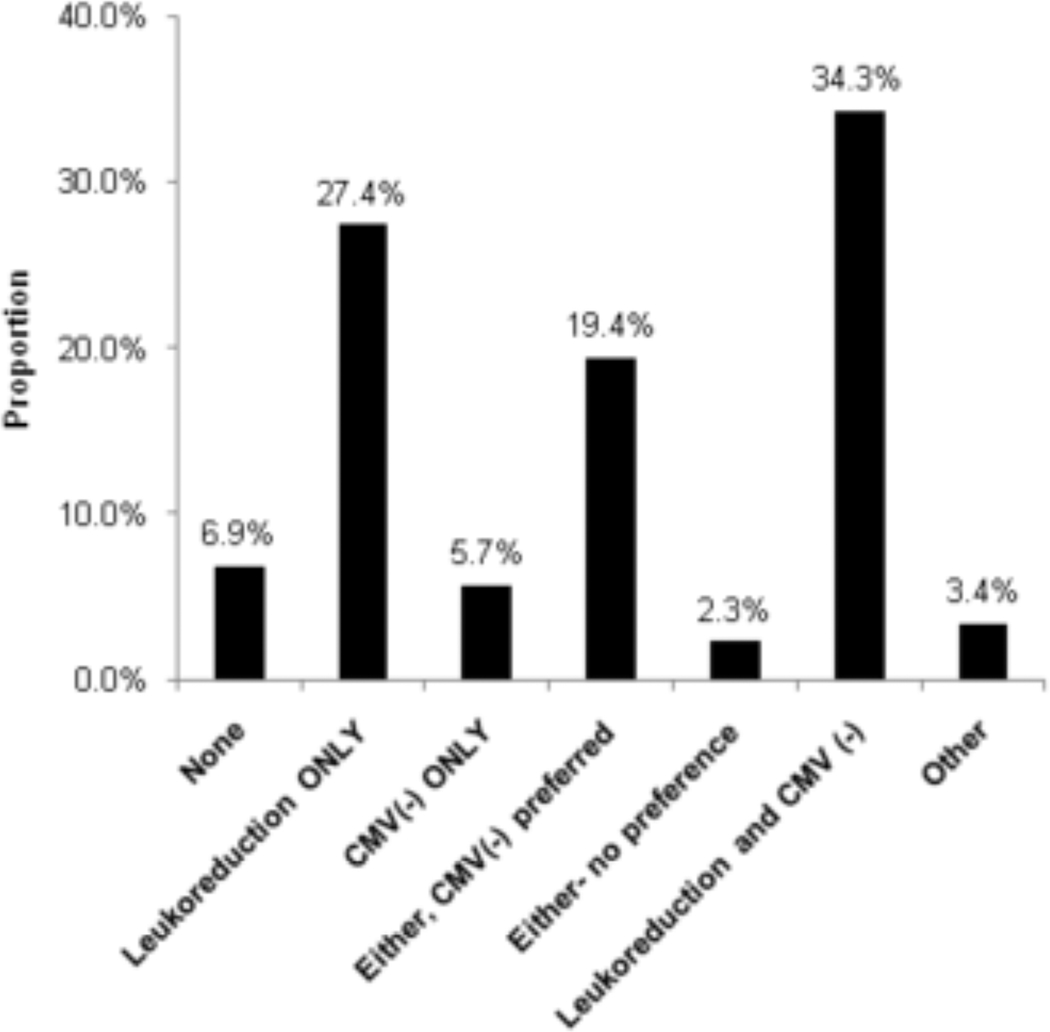
Most programs were using some strategy to reduce CMV transmission via blood products during the study period (Figure 4). Sixty programs (34.3%) used both CMV seronegative blood products and leukoreduction. Forty-eight programs (27.4%) used leukoreduced blood products only. Thirty-four programs (19.4%) used CMV seronegative blood products preferentially, but used leukoreduced blood products when CMV seronegative products were not available. A much smaller number of centers (5.7%) used CMV seronegative blood only, and 2.3% used one or the other with no preference. Centers that treat primarily adults were more likely to use both CMV negative blood products and leukoreduction as compared to centers that treated primarily pediatric patients and centers that treated both (46.2% vs. 28.6% and 28.6%, respectively, p=0.016) (Table S1). Twelve centers (6.9%) did not have any program strategy for using CMV seronegative blood products or leukoreduction in allogeneic transplant recipients.
Figure 4. Strategies for Prevention of CMV Transmission via Blood Products (n=175).
CMV(-) indicates blood products from CMV seronegative donors
Adoptive Cellular Immunotherapy
Only 7 centers (4.0%) reported using adoptive cellular immunotherapy in allogeneic transplant recipients during the years 1999–2003. One program utilized adoptive cellular immunotherapy as prophylaxis, two programs as preemptive therapy, and four programs utilized it only after initial failure of pharmacologic preemptive therapy.
Antifungal Prophylaxis
Ninety percent of program respondents reported using systemic antifungal prophylaxis in allogeneic transplant recipients (Table S1). While there were no differences in reported antifungal use by center size or patient population, there were significant regional variations. Of the 19 programs that did not use antifungal prophylaxis, 9 were in European countries, 2 were in South Africa, and 3 were in Canada- representing 22.5 %, 33.3%, and 42.8% of the region’s or country’s programs respectively. In comparison, only 3% of programs in the United States, and no programs in Australia, New Zealand, or Latin America reported not using antifungal prophylaxis during the study period (Table S1).
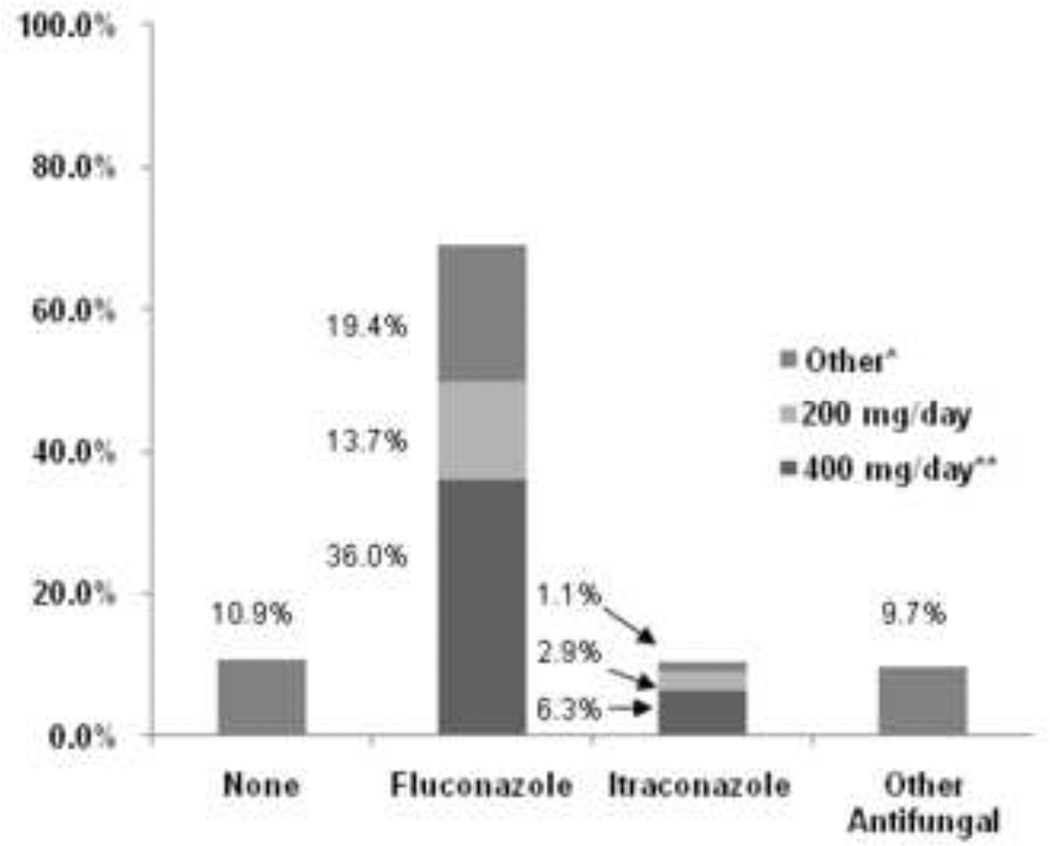
The majority of programs (69.1%) chose fluconazole as their primary agent for prophylaxis, with 36% using a dose of 400 mg/day or the weight-based equivalent of 4–6 mg/kg/day, 13.7% using 200 mg/day, and 19.4% reporting either lower doses or a dosing range (Figure 5). Approximately 10% of programs used itraconazole as their primary agent for prophylaxis and another 10% reported the use of other antifungal agents. Only 7 programs (4.4%) reported a change in the primary drug used for antifungal prophylaxis between 1999 and 2003, all switching from fluconazole to itraconazole.
Figure 5. Antifungal Prophylaxis Utilization, Primary Drug and Dose (n=175).
* Other antifungal prophylaxis regimens included: amphotericin B doses ranging from 0.1 –0.6 mg/kg/day, liposomal amphotericin B 3 mg/kg twice weekly, voriconazole 200–400 mg/day, caspofungin 50mg/m2/day, or a sequence of two different agents.
** or the weight based equivalent of 4–6 mg/kg/day
Antifungal prophylaxis was initiated at the time of the pre-transplant conditioning regimen in 63% of centers, whereas 27% of centers started antifungal prophylaxis at the time of transplant-Day 0 (+/− 1 day) (Table S1). Antifungal prophylaxis was continued until engraftment in 23% of programs. Forty-eight percent of centers reported use of antifungal prophylaxis until Day 75–100 post-transplant and 29% reported some other duration. There were no significant differences in duration of treatment by region, center size, or patient population (Table S1,S2). Of the centers that used antifungal prophylaxis, 98% reported consistent use of their chosen duration of treatment between 1999 and 2003. Two programs started using antifungal prophylaxis in 2001 and 2003 respectively, and one program stopped using antifungal prophylaxis after 2002.
DISCUSSION
The development of sensitive diagnostic testing and effective antiviral and antifungal therapies has done much to decrease the morbidity and mortality due to CMV, invasive fungal infections, and other herpes viruses after HCT. With the publication of evidence-based guidelines by the CDC in 2000, physicians and transplant centers were provided with detailed recommendations for the prevention and treatment of opportunistic infections in HCT. However, to our knowledge, there have been no studies investigating the adoption of these recommendations globally. With this large, multinational survey we had a unique opportunity to perform such a study using a simple, web-based survey tool. With a response rate of 72%, representing 175 programs in 32 different countries, we were able to obtain a detailed view of the strategies utilized both before and after publication of these guidelines.
In accord with the CDC recommendations, approximately one-quarter of programs did not use long term low-dose suppressive acyclovir in VZV seropositive patients (DIII).(7) However, three-quarters of programs departed from the recommendation by treating for varying durations, with approximately one-fifth of the programs treating for the first year after transplantation. We speculate that this practice was influenced by a randomized, placebo-controlled trial of long term acyclovir for prevention of VZV disease which had been completed and presented in abstract form at international meetings, but was not published at the time the guidelines were written. In the most recent guidelines this has been upgraded to a BI level recommendation.(10) We observed statistically significant regional variation in the treatment strategy utilized for VZV+/HSV− patients. For example, in Europe, 90% of centers treated for some duration of time, varying from 1–4 months, but no centers treated for as long as one year. In contrast, one-third of North American centers did not offer any prophylaxis and one-third treated for the entire first year (Table S1). Again, this division likely reflects evidence-based transitions of practice prior to their inclusion in the published guidelines.
A majority of centers utilized low-dose acyclovir prophylaxis to HSV+ patients after HCT, which is consistent with CDC recommendations (AI). However, given the proven efficacy, excellent safety profile, and the low cost of acyclovir prophylaxis, it was surprising that there were 10 programs that did not appear to be using any strategy for HSV disease prevention. There was significant regional variation in the duration of acyclovir prophylaxis. The CDC guidelines recommend continuation of acyclovir until engraftment or resolution of mucositis, whichever is longer (BIII), while recommending against treatment for longer than 1 month (DIII).(7) In our study we found that a similar number of programs were in accord with this recommendation as departed from it by treating HSV+/VZV- patients for much longer durations. Approximately one-quarter of programs treated for at least 6 months or discontinuation of immunosuppression, and about one-eighth treated for one year. This practice is encouraged by evidence that long-term suppressive therapy with low-dose acyclovir (800 mg twice daily or 500mg twice daily of valacyclovir) reduces the incidence of HSV disease and the development of acyclovir resistant HSV.(11)
All but two of the participating programs placed all patients at risk for post-transplant CMV disease on a CMV disease prevention program for the first 100 days post-transplant, with high-dose acyclovir, prophylactic ganciclovir, or surveillance with preemptive therapy (AI). Surveillance with preemptive ganciclovir or foscarnet therapy was used by 90.8% of centers in at least some patients, compared to 31.4% of programs using high-dose acyclovir and 27.6% using ganciclovir prophylaxis. There were no significant differences in the use of these strategies across all regions. However, there was considerable regional variation in the duration of prophylaxis and the duration of treatment after detection with surveillance. Approximately one-quarter of programs indicated that their center used both a strategy of ganciclovir prophylaxis and surveillance with preemptive therapy during the same time period. This could be interpreted as a center policy allowing physicians to choose between two equally recommended strategies. However, it is also possible that the survey questions were misinterpreted - a limitation common to all survey studies.
There were no significant differences in treatment practices by center size as determined by the number of transplants performed annually, comparing those centers that perform more than fifty allogeneic transplants per year to those that perform less than fifty per year. Moreover, with a few exceptions, no differences in practices existed between those centers that treated primarily pediatric patients, those that treated primarily adult patients, and those that treated both. The most interesting exception was the increased use of high-dose acyclovir prophylaxis in patients at risk for CMV disease in one-half of centers that treated primarily pediatric patients compared with only one-quarter of those that treated primarily adults and those that treated both. It is possible that this prophylactic strategy is used less commonly in centers that treat adults because of the weight-based dosing of the parenteral acyclovir formulation, which could be deemed prohibitively expensive for adult patients at some centers.
While most programs reported center strategies that were consistent with the recommendations for prevention of CMV reactivation in the first 100 days after HCT, many fewer programs continue to monitor for CMV after day 100, with 30% of programs reporting that they do not routinely use either prophylaxis or surveillance with preemptive therapy for high-risk patients after day 100. However the most recent guidelines have increased the rating to a BII level based on a non-randomized study where patients at high risk for CMV disease continued to undergo surveillance for 26 weeks after HCT. (12, 13) In this study, twenty-seven percent of patients required preemptive treatment after day 100, however, there were no cases of CMV disease. It is likely that an updated query would find a greater number of programs continuing their CMV prevention programs beyond day 100. The variation in frequency of surveillance testing observed in this study is indicative of the paucity of clear evidence to guide providers in these decisions. (10)
All but 12 of the 175 participating programs used some method of decreasing the risk of CMV transmission via blood products, an AI level recommendation, by using either blood products from CMV seronegative donors, leukoreduced blood products, or both.
This survey-based study is, to our knowledge, the first to compare center-specific practices for the prevention of herpes virus infections after HCT in a large number of programs from 32 different countries. Because of the high response rate, and the diversity of the participating programs, we were also able to make comparisons by patient age population and by center size. However, the large number of comparisons performed increases the probability of finding statistically significant differences when there is no true difference. This statistical problem places a limit on the inferences that should be made about these, primarily descriptive, data. Another limitation of this study is that the survey asked the respondents about the prevention strategies used by their centers a few years in the past, thus their answers are potentially subject to recall bias. Also, while the survey reflects institutional practices actual adherence to these stated practices was unknown. As with many survey studies, it is also possible that, despite careful attention to design of the questions, misinterpretation by the respondents could have affected the accuracy of our findings. Finally, prevention strategies in specific transplant risk groups could not be performed.
In conclusion, in this large, multinational survey, center-specific practices regarding prevention and treatment of herpes virus infections were examined. Most, but not all, responding centers reported provision of HSV, VZV and CMV prevention and treatment regimens prior to the publication of the CDC guidelines in 2000. While several programs reported switching from a CMV antigen based surveillance method to PCR-based detection, overall, very few centers reported significant changes in their prevention and treatment strategies between 1999 and 2003. Additionally, with the exception of increased use of high-dose acyclovir for CMV prophylaxis at centers that treated primarily pediatric patients, there were no differences in center strategies based on patient age or the number of transplants performed annually. There is an ongoing need for high-quality clinical studies to clarify many of these questions as reflected in the significant regional differences observed in the following areas: (1) duration of antiviral prophylaxis for HSV and VZV seropositive patients, (2) duration of antiviral prophylaxis for patients at risk of CMV disease, (3) selection of patients to undergo CMV surveillance testing, (4) duration of preemptive therapy, (5) and strategies for prevention of late CMV disease.
Supplementary Material
Acknowledgments
Financial disclosure: The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government. This article received additional support from NIHCA 18029, HL093294, NIH CA 15704.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103:2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 2.Bowden RA, Ljungman P, Paya CV. Transplant infections. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 3.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 4.Zaia JA, Gallez-Hawkins GM, Tegtmeier BR, et al. Late cytomegalovirus disease in marrow transplantation is predicted by virus load in plasma. J Infect Dis. 1997;176:782–785. doi: 10.1086/517301. [DOI] [PubMed] [Google Scholar]
- 5.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation--a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 6.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45:1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2000;6:659–713. doi: 10.1016/S1083-8791(00)70002-4. 715; 717-627; quiz 729-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser GA, Walker II. Cytomegalovirus prophylaxis and treatment after hematopoietic stem cell transplantation in Canada: a description of current practices and comparison with Centers for Disease Control/Infectious Diseases Society of America/American Society for Blood and Marrow Transplantation guideline recommendations. Biol Blood Marrow Transplant. 2004;10:287–297. doi: 10.1016/j.bbmt.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Preface. Bone Marrow Transplant. 2009;44:453–455. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]
- 11.Erard V, Wald A, Corey L, Leisenring WM, Boeckh M. Use of long-term suppressive acyclovir after hematopoietic stem-cell transplantation: impact on herpes simplex virus (HSV) disease and drug-resistant HSV disease. J Infect Dis. 2007;196:266–270. doi: 10.1086/518938. [DOI] [PubMed] [Google Scholar]
- 12.Peggs KS, Preiser W, Kottaridis PD, et al. Extended routine polymerase chain reaction surveillance and pre-emptive antiviral therapy for cytomegalovirus after allogeneic transplantation. Br J Haematol. 2000;111:782–790. [PubMed] [Google Scholar]
- 13.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.