Abstract
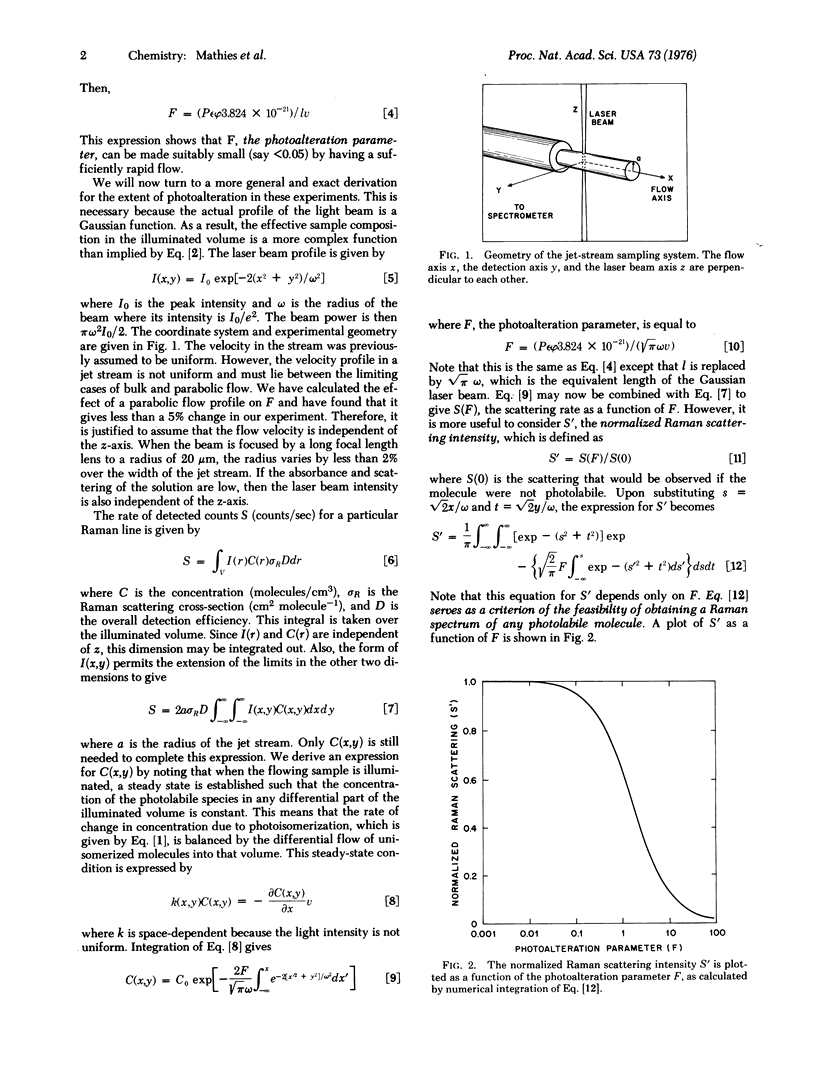
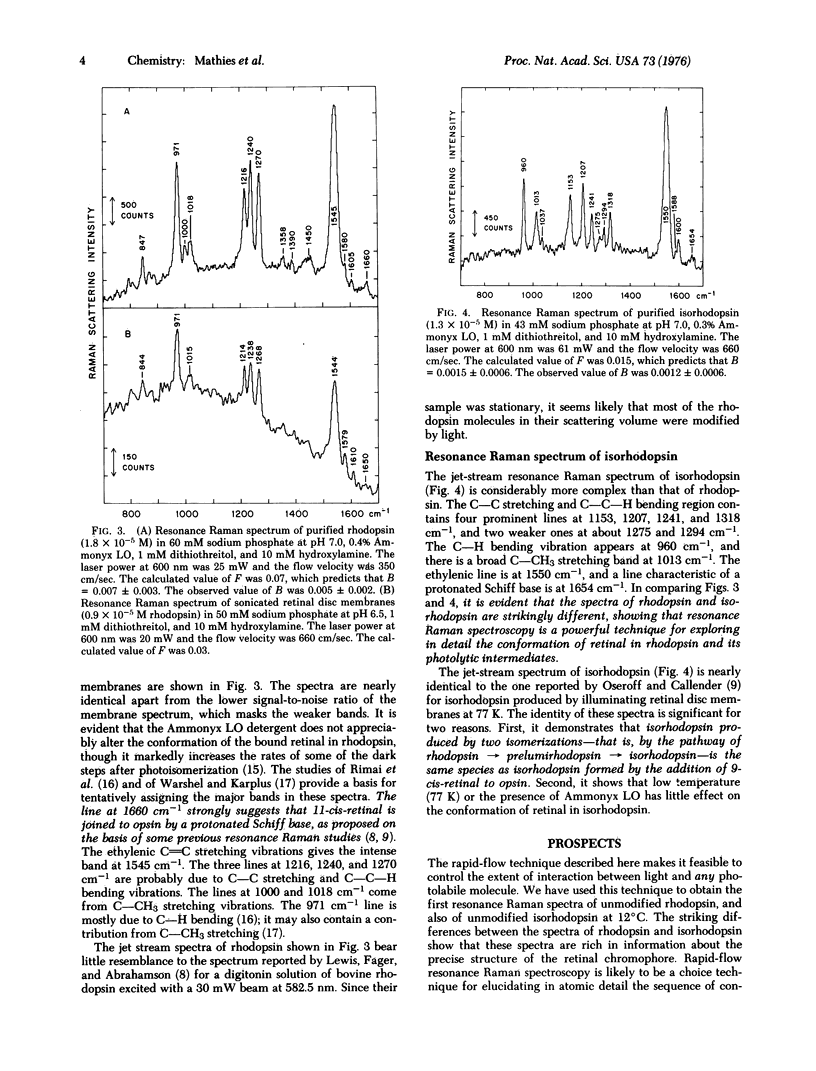
We have devised a method for obtaining the resonance Raman spectrum of a photolabile molecule before it is modified by light. The essence of this technique is that the sample is flowed through the light beam at a sufficiently high velocity so that the fraction of photoisomerized (or photodestroyed) molecules in the illuminated volume is very low. This rapid-flow technique has enabled us to measure the resonance Raman spectrum of unphotolyzed bovine rhodopsin in Ammonyx LO detergent solution and in sonicated retinal disc membranes. The major features of these spectra, which are very similar to one another, are the protonated Schiff base line near 1660 cm-1, the ethylenic line at 1545 cm-1, lines due to skeletal modes at 1216, 1240, and 1270 cm-1, and a line due to C-H bending at 971 cm-1. The resonance Raman spectrum of unphotolyzed isorhodopsin formed by the addition of 9-cis-retinal to opsin was also measured. The spectrum of isorhodopsin is more complex and differs markedly from that of rhodopsin. In isorhodopsin, the ethylenic line is shifted to 1550 cm-1, and there are six lines between 1153 and 1318 cm-1. The rapid-flow technique described here makes it feasible to control the extent of interaction between light and any photolabile molecule. We present a theory for predicting the effective sample composition in the illuminated volume as a function of the flow rate, light intensity, and spectral characteristics of the photolabile species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Zuckerman D. M., Lamola A. A., Jovin T. M. Rhodopsin. Purification and recombination with phospholipids assayed by the metarhodopsin I leads to metarhodopsin II transition. Biochemistry. 1974 Aug 13;13(17):3448–3458. doi: 10.1021/bi00714a005. [DOI] [PubMed] [Google Scholar]
- Carey P. R., Schneider H. Resonance Raman spectra of chymotrypsin acyl enzymes. Biochem Biophys Res Commun. 1974 Apr 8;57(3):831–837. doi: 10.1016/0006-291x(74)90621-4. [DOI] [PubMed] [Google Scholar]
- Crouch R., Purvin V., Nakanishi K., Ebrey T. Isorhodopsin II: artificial photosensitive pigment formed from 9,13-dicis retinal. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1538–1542. doi: 10.1073/pnas.72.4.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. B., Shriver D. F., Klotz I. M. Resonance Raman studies of the electronic state of oxygen in hemerythrin. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2582–2584. doi: 10.1073/pnas.70.9.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf A., Whittenberger B. P., Goff S. P., Waggoner A. S. The spectral properties of some visual pigment analogs. Exp Eye Res. 1973 Dec 24;17(6):591–606. doi: 10.1016/0014-4835(73)90088-2. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland J. T., Watters K. L., Petersen R. L. Resonance Raman investigation of an enzyme-inhibitor complex. Biochemistry. 1975 Feb 11;14(3):624–630. doi: 10.1021/bi00674a025. [DOI] [PubMed] [Google Scholar]
- Mendelsohn R. Resonance Raman spectroscopy of the photoreceptor-like pigment of Halobacterium halobium. Nature. 1973 May 4;243(5401):22–24. doi: 10.1038/243022a0. [DOI] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Strekas T. C., Spiro T. G. Resonance-raman evidence for anomalous heme structures in cytochrome c' from Rhodopseudomonas palustris. Biochim Biophys Acta. 1974 Jun 7;351(2):237–245. doi: 10.1016/0005-2795(74)90186-x. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Stryer L. Induced optical activity of the metarhodopsins. Biochemistry. 1971 Aug 17;10(17):3250–3254. doi: 10.1021/bi00793a014. [DOI] [PubMed] [Google Scholar]
- Warshel A., Karplus M. Calculation of pi-pi excited state conformations and vibronic structure of retinal and related molecules. J Am Chem Soc. 1974 Sep 4;96(18):5677–5689. doi: 10.1021/ja00825a001. [DOI] [PubMed] [Google Scholar]


