Abstract
Background
Photodynamic therapy (PDT) has been suggested as an efficient clinical approach for the treatment of dental plaque in the field of dental care. In PDT, once the photosensitizer is irradiated with light of a specific wavelength, it transfers the excitation energy to molecular oxygen, which gives rise to singlet oxygen.
Methodology/Principal Findings
Since plaque disclosing agents usually contain photosensitizers such as rose bengal, erythrosine, and phloxine, they could be used for PTD upon photoactivation. The aim of the present study is to compare the ability of these three photosensitizers to produce singlet oxygen in relation to their bactericidal activity. The generation rates of singlet oxygen determined by applying an electron spin resonance technique were in the order phloxine > erythrosine ≒ rose bengal. On the other hand, rose bengal showed the highest bactericidal activity against Streptococcus mutans, a major causative pathogen of caries, followed by erythrosine and phloxine, both of which showed activity similar to each other. One of the reasons for the discrepancy between the singlet oxygen generating ability and bactericidal activity was the incorporation efficiency of the photosensitizers into the bacterial cells. The incorporation rate of rose bengal was the highest among the three photosensitizers examined in the present study, likely leading to the highest bactericidal activity. Meanwhile, the addition of L-histidine, a singlet oxygen quencher, cancelled the bactericidal activity of any of the three photoactivated photosensitizers, proving that singlet oxygen was responsible for the bactericidal action.
Conclusions
It is strongly suggested that rose bengal is a suitable photosensitizer for the plaque disclosing agents as compared to the other two photosensitizers, phloxine and erythrosine, when used for PDT.
Introduction
Photodynamic therapy (PDT) has been suggested as an efficient clinical approach for the treatment of different types of cancer [1]–[3] and as an alternative to chemical disinfection agents [4]–[7]. In the field of dental care, PDT has been applied to treat endodontic infection including dental plaque [8]–[15], which is structurally and functionally organized multi-species biofilm colonizing tooth surfaces and epithelial cells covering gingival sulcus and periodontal pocket [16].
The PDT consist of mainly there components. These are light, oxygen, and a photosensitizer. Once the photosensitizer is irradiated with light of a specific wavelength, it absorbs photons of the wavelength and transfers the excitation energy to molecular oxygen which is in turn metamorphosed to its diamagnetic form, singlet oxygen [17] with other reactive oxygen species (ROS) formed downstream such as superoxide anion, hydroxyl radicals, and hydrogen peroxide [18]–[20]. Of the ROS, singlet oxygen plays a central role for cytotoxicity in PDT [21], [22], indicating that the larger amount of singlet oxygen the target is exposed to, the more effectively undesired cells such as cancer cells and bacteria are killed.
Plaque disclosing agents, which are used to detect plaques on the tooth surface, usually contain photosensitizers such as rose bengal, erythrosine, and phloxine, all of which are also used for food coloring. Thus, if these plaque disclosing agents are irradiated with light of a suitable wavelength for the photosensitizers, singlet oxygen is validly and locally generated around the plaques disclosed by the agents. The aim of the present study is to compare the ability of these three photosensitizers to produce singlet oxygen upon irradiation with the light at around their maximal absorption wavelengths in relation to their bactericidal activity. To determine the photogenerated singlet oxygen, an electron spin resonance (ESR) technique was applied based on previous studies showing that the relative yield of singlet oxygen generated by various photosensitizers can be evaluated properly by ESR analysis [23]–[25]. To clarify the relation between the singlet oxygen producing ability and the bactericidal activity, incorporation efficiency of these photosensitizers into bacterial cells was also examined.
Results
Determination of photogenerated singlet oxygen by ESR analysis of nitroxide radical
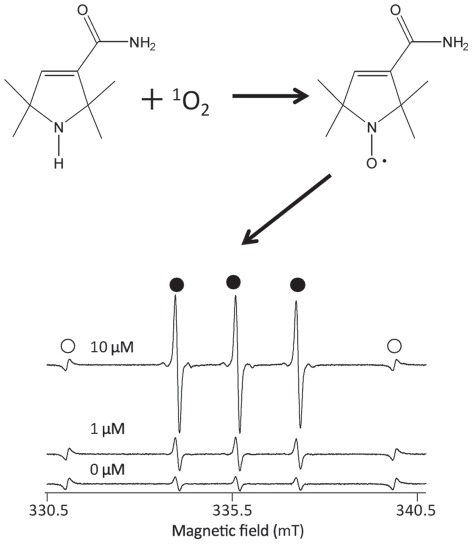
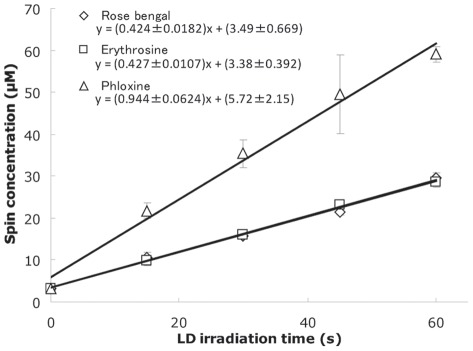
Representative ESR spectra of the laser-irradiated mixtures containing different concentration of rose bengal and 2,2,5,5-tetramethyl-3-pyrroline-3-carboxamide (TPC) are shown in Fig. 1. When a mixture containing 450 mM TPC and 1 or 10 µM rose bengal was irradiated by the laser light with a wavelength of 532 nm for 60 s, a typical signal of the nitroxide radical generated during the oxidation of TPC by singlet oxygen was observed dependently on the concentration of rose bengal as described in our previous study [23]. When the singlet oxygen producing abilities of the three photosensitizers, rose bengal, erythrosine, and phloxine at a fixed concentration of 10 μM were examined, the amounts of nitroxide radical increased linearly with the laser irradiation time at least up to 60 s (Fig. 2). The generation rates of nitroxide radical which corresponded to the slope values of the regression lines for the two variables, time and spin concentration, were 0.42±0.02 (mean ± standard error) µMs−1, 0.43±0.01 µMs−1, and 0.94±0.06 µMs−1 for rose bengal, erythrosine, and phloxine, respectively, indicating that the yield of the radical was in the order phloxine > erythrosine ≒ rose bengal.
Figure 1. A schema showing oxidation of a sterically hindered amine TPC (2,2,5,5-tetramethyl-3-pyrroline-3-carboxamide) by singlet oxygen to give rise to the nitroxide radical, and representative ESR spectra of the laser-irradiated mixtures containing rose bengal and a sterically hindered amine TPC.
The mixture containing 450 mM TPC and 0, 1 or 10 µM rose bengal was irradiated by the laser light with a wavelength of 532 nm for 60 s. Open circle and closed circle indicate Mn2+ marker and the nitroxide radical, respectively.
Figure 2. The yields of the nitroxide radical from the laser-irradiated photosensitizers.
The mixture containing 450 mM TPC and 10 µM photosensitizer was irradiated by the laser light with a wavelength of 532 nm for 15, 30, 45 and 60 s. Each value represents the mean of triplicate determinations with standard deviation. The slope and intercept of each regression line are expressed as the mean with standard error.
Bactericidal activity of laser-irradiated photosensitizers
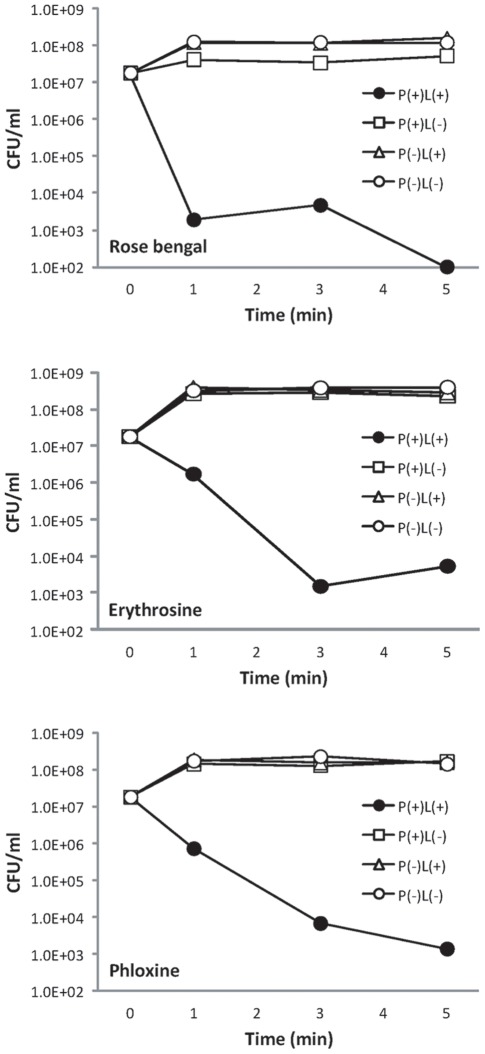
All of the photosensitizers excited by laser irradiation killed effectively the bacterial strain used in the present study in an irradiation-time dependent manner, and the numbers of bacteria were reduced by 3 or more logarithmic order within 3 min (Fig. 3). Of the photosensitizers, rose bengal exerted the highest activity against the bacterial strain with 3 logarithmic reduction within a short time as 1 min, and phloxine and erythrosine showed almost the same activity with approximately 1 logarithmic reduction within 1 min. Under the other three conditions expressed as P(+)L(−): photosensitizer alone, P(−)L(+): laser irradiation alone, and P(−)L(−): no treatment, almost no bactericidal effects were observed.
Figure 3. Number of viable S. mutans in the suspension after each treatment.
P(+)L(+), P(+)L(−), P(−)L(+), and P(−)L(−) stand for laser irradiation of each photosensitizer, photosensitizer alone, laser irradiation alone, and no treatment, respectively. Under the conditions of P(+)L(+) and P(+)L(−), the suspension contained 10 μM rose bengal, phloxine, or erythrosine. Each value represents the mean of nonuplicate determinations.
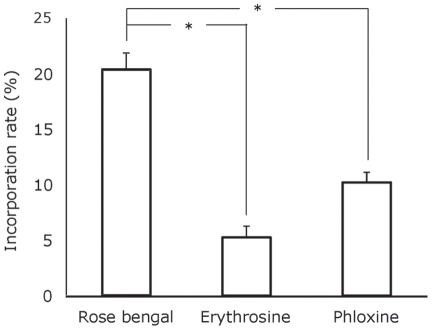
To further clarify the relation between the singlet oxygen producing ability and the bactericidal activity, incorporation efficiency of these photosensitizers into bacterial cells was also examined (Fig. 4). Of the three photosensitizers examined in the present study, the highest incorporation rate was obtained in rose bengal, followed by phloxine and erythrosine. That is, 20% of the rose bengal added to the bacterial suspension was incorporated into the bacterial cells, and 10% and 5% for the phloxine and the erythrosine, respectively. Statistically significant differences between the two groups were found in all combinations.
Figure 4. Incorporation rates of photosensitizers into the bacterial cells.
The bacterial suspension containing 10 μM rose bengal, phloxine, or erythrosine was incubated at room temperature for 3 min. Each value represents the mean of triplicate determinations with standard deviation. Statistically significant differences between the two groups are shown as * (p<0.05).
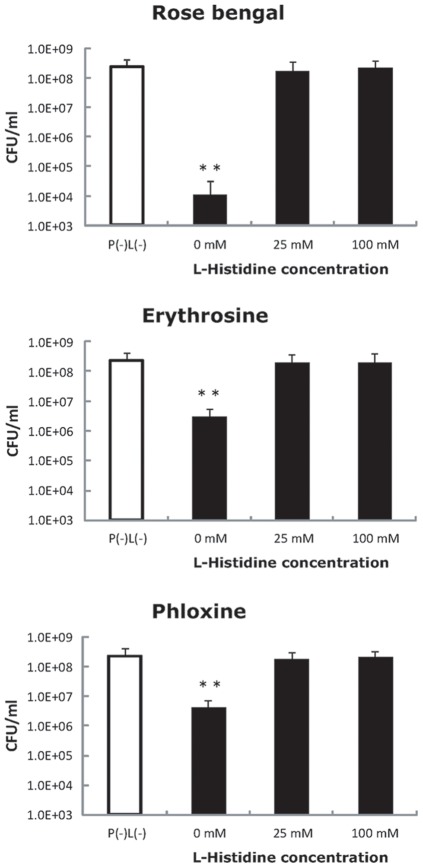
To confirm if the bactericidal effect obtained under the condition of P(+)L(+) was attributable to singlet oxygen generated by the laser-irradiated photosensitizers, effect of L-histidine, a singlet oxygen quencher, was examined (Fig. 5). Addition of not only 100 mM but 25 mM L-histidine completely abolished the bactericidal effect of laser-irradiated photosensitizers.
Figure 5. Influence of L-histidine, a singlet oxygen quencher, on the bactericidal effects of laser-irradiated photosensitizers.
L-Histidine (25 and 100 mM) was added to the bacterial suspension containing 10 μM rose bengal, phloxine, or erythrosine, followed by laser-irradiation for 60 s. Each vealue represents the mean of nonuplicate determinations with standard deviation. Statistically significant differences from the corresponding P (−)L(−) group are shown as ** (p<0.01).
Discussion
Nitroxide radical generation through oxidation of TPC is illustrated in Fig. 1. When singlet oxygen is photogenerated, the proton is subtracted from the amine moiety of TPC concomitant with oxygen accretion reaction yielding the corresponding nitroxide radical as detected by the ESR analysis (Fig. 1). Since the amount of nitroxide radical increased with laser-irradiation time (Fig. 2), it was confirmed that singlet oxygen was generated time-dependently on the laser-irradiation of the photosensitizers, rose bengal, phloxine, and erythrosine. Regarding the singlet oxygen generation efficiency, it was proved that of the three photosensitizes phloxine has the highest ability to generate singlet oxygen because the slope value of the regression line as shown in Fig. 2 was the highest followed by those of rose bengal and erythrosine. Therefore, if the bactericidal effect of laser-irradiated photosensitizers had been solely dependent on the amount of photogenerated singlet oxygen, phloxine would have shown the highest bactericidal activity. However, since rose bengal but not phloxine showed the highest bactericidal activity (Fig. 3), factors other than singlet oxygen also seemed to be involved in the bactericidal activity. The most likely factor is incorporation efficiency of the photosensitizers into the bacterial cells. Indeed, rose bengal was most efficiently incorporated into the bacterial cells as compared to the other two photosensitizers (Fig. 4), likely leading to the highest bactericidal activity. Meanwhile, the bactericidal activity of laser-irradiated phloxine was almost the same as that of erythrosine, even though both the singlet oxygen producing ability and the incorporation efficiency of phloxine were superior to those of erythrosine. One of the possibilities is that erythrosine itself would have cytotoxic effect on the bacterial cells in some degree. However, no bactericidal effect of erythrosine was observed under the condition of P(+)L(−), suggesting that, even if erythrosine is cytotoxic, cytotoxic property of erythrosine is negligible. The other possibility is localization of the incorporated photosensitizers in the bacterial cells. That is, if a photosensitizer is mainly localized only around the cell wall, photogenerated singlet oxygen fails to damage enzymes, nucleic acids, and subcellular organelles in the cytoplasm. The aspect of the localization at cellular level is the next issue to be studied in the future. Finally, to prove that the bactericidal effect of the excited photosensitizers would be attributable to photogenerated singlet oxygen, effect of L-histidine, a singlet oxygen quencher, was examined. Since the addition of 25 mg/ml of L-histidine cancelled the bactericidal activity of any of the three excited photosensitizers (Fig. 5), it is proved that singlet oxygen is responsible for the bactericidal action elicited by the laser-irradiated photosensitizers. From these, it is strongly suggested that rose bengal is a suitable photosensitizer for the plaque disclosing agents as compared to the other two photosensitizers, phloxine and erythrosine, when used for PDT.
Materials and Methods
Reagents
Reagents were purchased from the following sources: TPC, and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL) from Sigma Aldrich (St. Louis, MO, USA); rose bengal, erythrosine, and phloxine from Wako Pure Chemical Industries (Osaka, Japan). All other reagents used were of analytical grade.
An experimental laser device for excitation of photosensitizers
To determine a wavelength of laser light for excitation of rose bengal, erythrosine, and phloxine, maximal absorption wavelengths of the three photosensitizers were spectrophotometrically determined. That is, 550 nm, 530 nm, and 540 nm for rose bengal, erythrosine, and phloxine, respectively, were obtained, indicating that the wavelengths of 530 to 550 nm are suitable to properly excite all of the three photosensitizers. An experimental laser device equipped with the second harmonic of Nd-YAG laser (wavelength: 532 nm) and a laser power meter was made (PAX Co. Ltd., Sendai, Japan) as described in our previous study [23]. An output power of the laser was set at 20 mW in the present study. When a semi-micro cuvette containing 200 µl of sample is set in the experimental device, the area of the sample irradiated by the laser is almost 5×5 mm resulting in the energy dose of 80 mW/cm2. The light path of the cuvette was 10 mm.
Determination of photogenerated singlet oxygen by ESR analysis of nitroxide radical
A sterically hindered amine, TPC, was used without further purification. The TPC was dissolved in ultrapure water to make a 500 mM solution. Each photosensitizer (rose bengal, erythrosine, and phloxine) was dissolved in ultrapure water to make 100 µM solutions. Then, 180 µl of TPC solution and 20 µl of each photosensitizer solution were mixed in a disposable plastic semi-micro cuvette to make final concentrations of 450 mM for TPC, and 10 µM for the photosensitizer. In an experiment conducted to check the ESR spectra, final concentrations of 1 and 10 µM rose bengal were used. Immediately after mixing, the cuvette was set in the experimental laser device. The sample in the cuvette was irradiated by the laser light for 60 sec. After the laser irradiation, the sample was transferred to a quartz cell and ESR spectrum was recorded on an X-band ESR spectrometer (JES-FA-100, JEOL, Tokyo, Japan). The measurement conditions for ESR were as follows: field sweep, 330.50–340.50 mT; field modulation frequency, 100 kHz; field modulation width, 0.05 mT; amplitude, 80; sweep time, 2 min; time constant, 0.03 sec; microwave frequency, 9.420 GHz; microwave power, 4 mW. To calculate the spin concentration of the nitroxide radical, which was generated through oxidation of TPC by singlet oxygen, 20 µM TEMPOL was used as a standard sample and the ESR spectrum of manganese (Mn2+) which was equipped in the ESR cavity was used as an internal standard. The spin concentration was determined using Digital Data Processing (JEOL, Tokyo, Japan). All tests were performed in triplicate.
Bactericidal test
Stock culture strains of Streptococcus mutans JCM 5705 was purchased from Japan Collection of Microorganisms, RIKEN BioResource Center (Wako, Japan). The bacterial strain was cultured on Brain Heart Infusion (BHI) agar (Becton Dickinson Labware, Franklin lakes, NJ, USA) anaerobically using Anaero Pack (Mitsubishi Gas Chemical Company, Tokyo, Japan) at 37°C. The bacterial suspension was prepared in sterile physiological saline from cultures grown on BHI agar at 37°C for 2 days. The suspension was adjusted to approximately 2×107 colony forming units (CFU)/ml. The bactericidal potency of the laser-irradiated photosensitizer was examined. An aliquot (180 µl) of the bacterial suspension and 20 µl of each photosensitizer solution prepared as described above were mixed in a disposable plastic semi-micro cuvette to make final concentration of 10 µM for the photosensitizer. Immediately after mixing, the cuvette was set in the experimental laser device. The sample in the cuvette was irradiated by the laser light for 1, 3, and 5 min. After the irradiation, a ten-fold serial dilution of the mixture was prepared using sterile physiological saline and 10 µl of the dilution was seeded onto BHI agar to determine the number of viable bacterial cells in the suspension. The agar medium was cultured for 48 h under the conditions as described above. The number of CFU/ml was then determined. The bactericidal effect of laser irradiation of each photosensitizer (expressed as P(+)L(+)) was compared to the effect of; (1) photosensitizer alone, P(+)L(−), (2) laser irradiation alone, P(−)L(+), and (3) no treatment, P(−)L(−). For the condition of L (−), the samples were kept without the laser irradiation in the clean bench to avoid contamination. For the condition of P(−), sterile physiological saline was added into the reaction system instead of the photosensitizer solution. All tests were repeatedly conducted nine times.
Effect of singlet oxygen quencher on bactericidal activity
To further examine if bactericidal effects of laser-irradiated phostosensitozers were attributable to the generated singlet oxygen, L-histidine, a singlet oxygen quencher [26], [27], was added to the reaction mixture of bacterial suspension and photosensitizer. More in detail, 100 μl of the bacterial suspension (S. mutans JCM 5705), 20 μl of photosensitizer, and 80 μl of L-histidine was mixed in a disposable plastic semi-micro cuvette to make final concentrations of 2.5×108 CFU/ml for the bacteria, 10 μM for the photosensitizer, and 25 or 100 mM for L-histidine. Immediately after mixing, the cuvette was set in the experimental laser device. The sample in the cuvette was irradiated by the laser light for 60 sec. After the irradiation, the number of CFU/ml in the suspension was determined as described above. All tests were repeatedly conducted nine times. Statistical analysis for the significant differences from the mean values under the condition of P(−)L(−) was done by Dunnett's multiple comparison test following logarithmic conversion. P values of <0.05 were considered significant.
Incorporation efficiency of photosensitizer into bacterial cells
An aliquot (1800 μl) of S. mutans suspension (approximately 108 CFU/ml) and 200 μl of 100 μM photosensitizer were mixed and incubated for 3 min at room temperature under a light shielding condition. Then the bacteria were harvested by centrifugation at 4000 x g for 5 min. Following decantation of the supernatant, the pellet was washed twice by physiological saline. For the extraction of the photosensitizer, 200 μl of 99.5% ethanol was added to the resultant bacterial pellet, followed by vigorous agitation. Following centrifugation, absorbance of the supernatant was determined at the maximal absorbance wavelength of each photosensitizer. Incorporation rates of the photosensitizers were calculated by the following equation. That is (incorporated amount of photosensitizer/added photosensitizer) ×100. All tests were performed in triplicate. Statistical analysis for the incorporation rates was done by Tukey-Kramer's multiple comparison test. P values of <0.05 were considered significant.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Ministry of Education, Science, Sports and Culture, Japan, Grant-in-Aid for Young Scientists (B), 21791932, 2010. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 2.Juarranz A, Jaen P, Sanz-Rodriguez F, Cuevas J, Gonzalez S. Photodynamic therapy of cancer. Basic principles and applications. Clin Transl Oncol. 2008;10:148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 3.Simone CB, Friedberg JS, Glatstein E, Stevenson J, Sterman DH, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis. 2012;4:63–75. doi: 10.3978/j.issn.2072-1439.2011.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43:755–67. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira Gonzales F, Maisch T. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol. 2012;116:1–10. doi: 10.1016/j.funbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Vera DM, Haynes MH, Ball AR, Dai DT, Astrakas C et al. Photochem Photobiol (in press); 2012. Strategies to potentiate antimicrobial photoinactivation by overcoming resistant phenotypes(dagger). doi: 10.1111/j.1751-1097.2012.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilela S F, Junqueira JC, Barbosa JO, Majewski M, Munin E, et al. Arch Oral Biol (in press; 2012. Photodynamic inactivation of Staphylococcus aureus and Escherichia coli biofilms by malachite green and phenothiazine dyes: An in vitro study. doi: 10.1016/j.archoralbio.2011.12.002) [DOI] [PubMed] [Google Scholar]
- 8.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, et al. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 10.Garcia VG, Fernandes LA, Macarini VC, de Almeida JM, Martins TM et al. Treatment of experimental periodontal disease with antimicrobial photodynamic therapy in nicotine-modified rats. J Clin Periodontol. 2011;38:1106–1114. doi: 10.1111/j.1600-051X.2011.01785.x. [DOI] [PubMed] [Google Scholar]
- 11.Herrera D. Photodynamic therapy for chronic periodontitis. Evid Based Dent. 2011;12:78–79. doi: 10.1038/sj.ebd.6400810. [DOI] [PubMed] [Google Scholar]
- 12.Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontol 2000. 2011;55:143–166. doi: 10.1111/j.1600-0757.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 13.Papastamou V, Nietzsch T, Staudte H, Orellana G, Sigusch BW. Photoinactivation of F. nucleatum and P. gingivalis using the ruthenium-based RD3 sensitizer and a conventional halogen lamp. Arch Oral Biol. 2011;56:264–268. doi: 10.1016/j.archoralbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Rolim JP, de-Melo MA, Guedes SF, Albuquerque-Filho FB, de Souza JR, et al. The antimicrobial activity of photodynamic therapy against Streptococcus mutans using different photosensitizers. J Photochem Photobiol B. 2012;106:40–46. doi: 10.1016/j.jphotobiol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Nagata JY, Hioka N, Kimura E, Batistela VR, Terada RSS, et al. Photodiagnosis Photodyn Ther (in press; 2012. Antibacterial photodynamic therapy for dental caries: Evaluation of the photosensitizers used and light source properties. doi: 10.1016/j.pdpdt.2011.11.006) [DOI] [PubMed] [Google Scholar]
- 16.Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 17.Clo E, Snyder JW, Ogilby PR, Gothelf KV. Control and selectivity of photosensitized singlet oxygen production: challenges in complex biological systems. Chembiochem. 2007;8:475–481. doi: 10.1002/cbic.200600454. [DOI] [PubMed] [Google Scholar]
- 18.Weishaupt KR, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976;36:2326–2329. [PubMed] [Google Scholar]
- 19.Thomas JP, Hall RD, Girotti AW. Singlet oxygen intermediacy in the photodynamic action of membrane-bound hematoporphyrin derivative. Cancer Lett. 1987;35:295–302. doi: 10.1016/0304-3835(87)90131-5. [DOI] [PubMed] [Google Scholar]
- 20.Niziolek-Kierecka M, Pilat A, Korytowski W, Girotti AW. Apoptosis-accommodating ac of nitric oxide in photodynamically stressed tumor cells. Photochem Photobiol. 2010;86:681–686. doi: 10.1111/j.1751-1097.2010.00712.x. [DOI] [PubMed] [Google Scholar]
- 21.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem Photobiol. 1998;68:370–6. [PubMed] [Google Scholar]
- 22.Yamamoto J, Yamamoto S, Hirano T, Li S, Koide M, et al. Monitoring of singlet oxygen is useful for predicting the photodynamic effects in the treatment for experimental glioma. Clin Cancer Res. 2006;12:7132–7139. doi: 10.1158/1078-0432.CCR-06-0786. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Ishiyama K, Ikai H, Kanno T, Sasaki K, et al. Reevaluation of analytical methods for photogenerated singlet oxygen. J Clin Biochem Nutr. 2011;49:87–95. doi: 10.3164/jcbn.10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwamoto Y, Yoshioka H, Yanagihara Y, Mifuchi I. Electron spin resonance detection of singlet oxygen produced in irradiated acriflavine solutions. Chem Pharm Bull. 1985;33:5529–5533. [Google Scholar]
- 25.Zang L, Zhang Z, Misra H. EPR studies of trapped singlet oxygen (1O2) generated during photoirradiation of Hypocrellin A. Photochem. Photobiol. 1990;52:677–683. doi: 10.1111/j.1751-1097.1990.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 26.Martins J, Almeida L, Laranjinha J. Simultaneous production of superoxide radical and singlet oxygen by sulphonated chloroaluminum phthalocyanine incorporated in human low-density lipoproteins: implications for photodynamic therapy. Photochem Photobiol. 2004;80:267–273. doi: 10.1562/2004-03-26-RA-124. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Li L, Zhang Y, Spitz DR, Buettner GR, et al. Inactivation of primary antioxidant enzymes in mouse keratinocytes by photodynamically generated singlet oxygen. Antioxid Redox Signal. 2006;8:1307–1314. doi: 10.1089/ars.2006.8.1307. [DOI] [PubMed] [Google Scholar]