Abstract
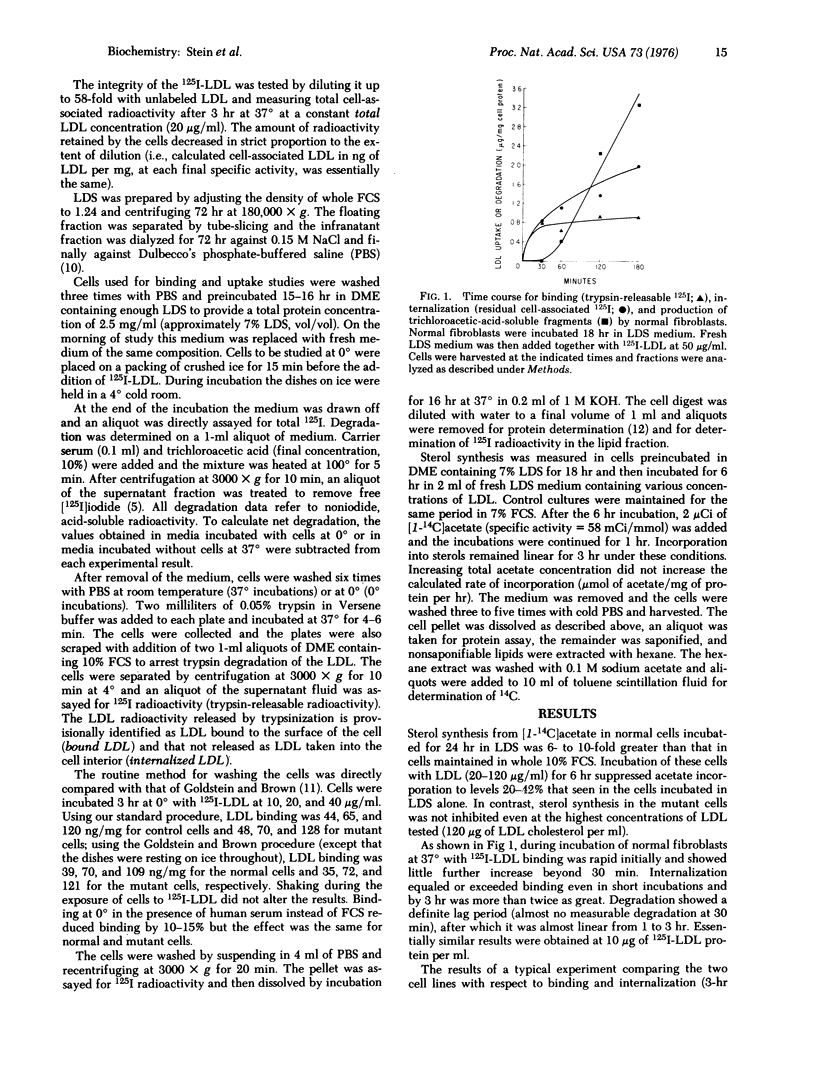
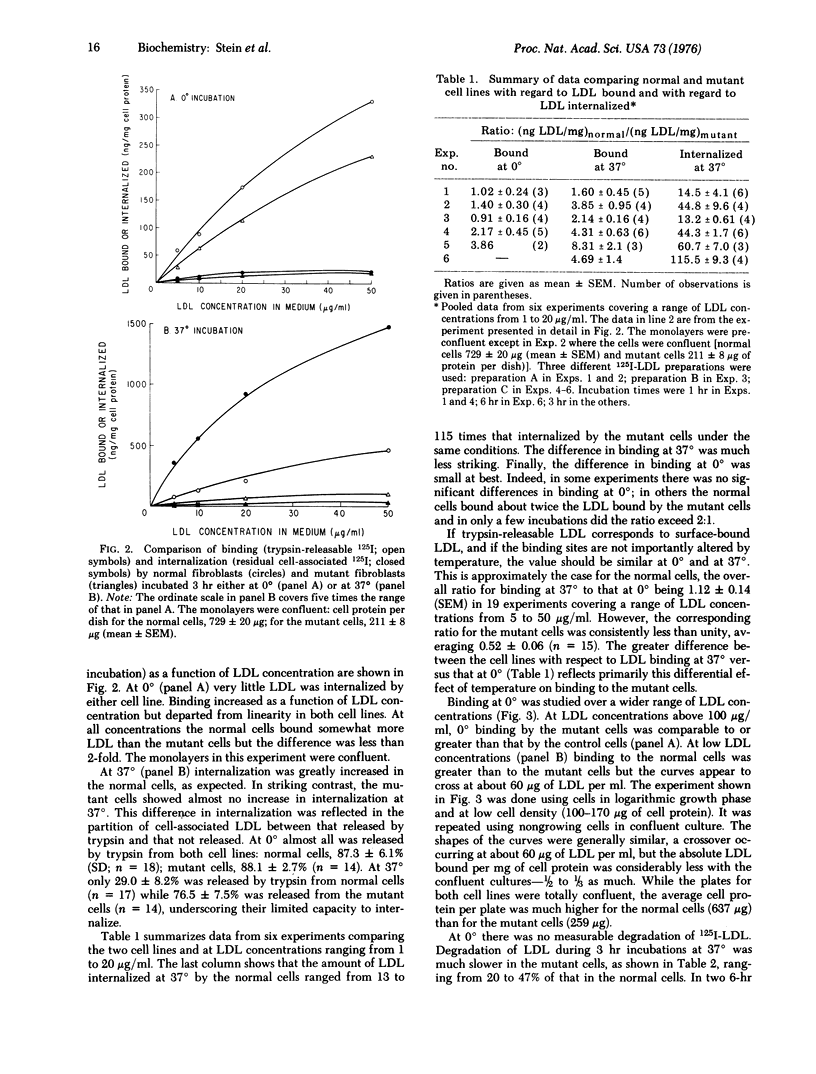
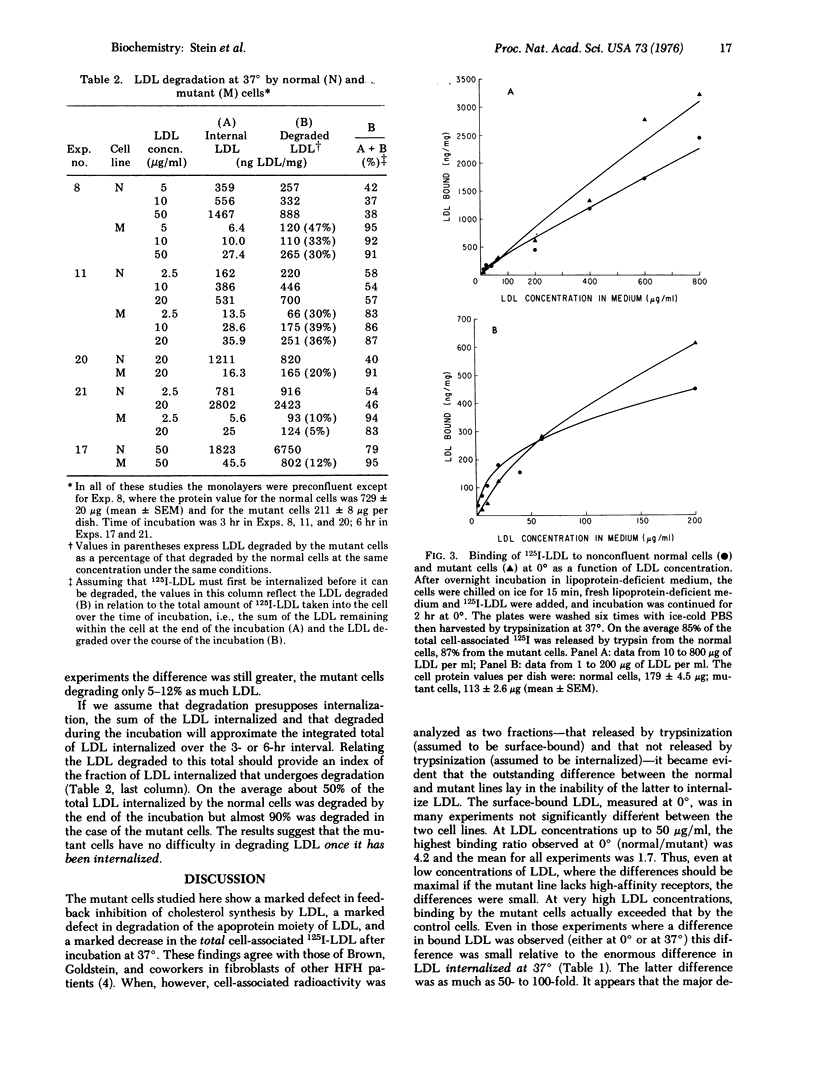
Skin fibroblasts from a patient with homozygous familial hypercholesterolemia (HFH) were compared with normal skin fibroblasts with regard to binding, internalization, and degradation of iodinated human low density lipoprotein (LDL). Like other cell lines from HFH patients, the mutant cells showed no suppression of sterol synthesis by LDL. Surface binding, measured at 0 degrees to eliminate the appreciable internalization that was shown to occur at 37 degrees, was on the average slightly less for HFH cells than normal cells at low LDL concentrations but comparable or even greater at high LDL concentrations (greater than 60 mug of LDL protein per ml). A major defect observed was in the rate of internalization of LDL at 37 degrees, which was only 1-10% of that in normal cells. LDL degradation was also markedly reduced but not to the same extent. Thus, a larger fraction of the LDL taken up appeared to be degraded by the mutant cells. The most striking defect observed, then, was not in surface binding of LDL but in rate of LDL internalization. While this might be secondary to a defect in specific binding sites of LDL, the magnitude of the observed differences in binding at low temperature seems too small to account for the huge differences in internalization (13- to 115-fold).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigan J., Bhathena S. J., Schreiner M. E. Control of sterol synthesis and of hydroxymethylglutaryl CoA reductase in skin fibroblasts grown from patients with homozygous type II hyperlipoproteinemia. J Lipid Res. 1975 Mar;16(2):151–154. [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Brannan P. G., Bohmfalk H. A., Brunschede G. Y., Dana S. E., Helgeson J., Goldstein J. L. Use of mutant fibroblasts in the analysis of the regulation of cholesterol metabolism in human cells. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):425–436. doi: 10.1002/jcp.1040850409. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brunschede G. Y., Brown M. S. Genetic heterogeneity in familial hypercholesterolemia: evidence for two different mutations affecting functions of low-density lipoprotein receptor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1092–1096. doi: 10.1073/pnas.72.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moutafis C. D., Myant N. B. The metabolism of cholesterol in two hypercholesterolaemic patients treated with cholestyramine. Clin Sci. 1969 Oct;37(2):443–454. [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Molecular biology of cellular membranes with applications to immunology. Adv Immunol. 1974;19(0):1–66. doi: 10.1016/s0065-2776(08)60251-5. [DOI] [PubMed] [Google Scholar]
- Sniderman A. D., Carew T. E., Steinberg D. Turnover and tissue distribution of 125-I-labeled low density lipoprotein in swine and dogs. J Lipid Res. 1975 Jul;16(4):293–299. [PubMed] [Google Scholar]
- Williams C. D., Avigan J. In vitro effects of serum proteins and lipids on lipid synthesis in human skin fibroblasts and leukocytes grown in culture. Biochim Biophys Acta. 1972 Mar 23;260(3):413–423. doi: 10.1016/0005-2760(72)90056-2. [DOI] [PubMed] [Google Scholar]



