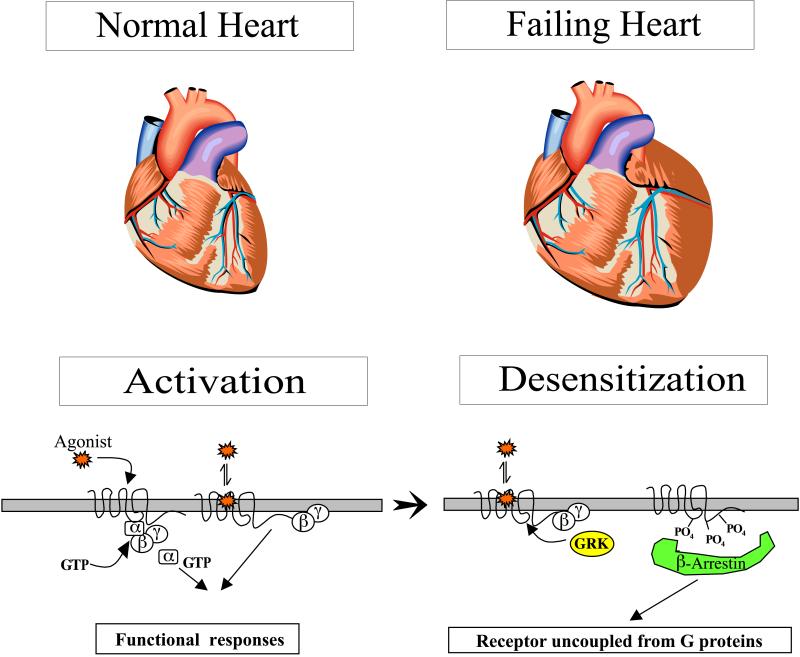
The rate and strength of beating of the heart is under the reciprocal control of the adrenergic (sympathetic) and cholinergic (parasympathetic) systems. Increased strength (inotropy) in cardiac beating in response to blood-borne epinephrine or to neurally delivered norepinephrine is mediated by β-adrenergic receptors. These G protein-coupled receptors have served as a key model for defining the molecular events linking receptor occupancy to effector regulation via G proteins.
As shown schematically in Fig. 1, occupancy of β-adrenergic receptors by agonist ligands, such as the endogenous catecholamines epinephrine and norepinephrine, facilitates interactions with heterotrimeric G proteins. These functional encounters accelerate GTP binding to the α subunit of the G protein, leading to two potential regulators of effector function: GTP-liganded α subunits or dissociated βγ subunit complexes. For β-adrenergic receptors, a principal effector is adenylyl cyclase, and the G protein that couples receptor activation to stimulation of adenylyl cyclase is termed Gs. Depending on the isoform of adenylyl cyclase expressed in the target cell, either the α subunit of Gs or the βγ complex, or both, leads to the enhanced synthesis of cAMP. Cyclic AMP serves as the second messenger for epinephrine by activating cAMP-dependent protein kinase within the cell and effecting a variety of phosphorylation-dependent changes in electrical and chemical functions. Activation of the β-adrenergic receptor system is terminated either by dissociation of the agonist from the receptor or by a number of autoregulatory events, referred to as desensitization or tachyphylaxis (1).
The study of the molecular events underlying desensitization of the β-adrenergic receptor-adenylyl cyclase system has been driven by the realization that a variety of diseases, particularly in the cardiovascular system, manifest diminished responsiveness to catecholamines. Cellular studies have revealed that desensitization of the β-adrenergic receptor system results from functional uncoupling of the receptor from Gs as a result of receptor phosphorylation. The receptor can be phosphorylated by cAMP-dependent protein kinase on a residue in the third intracellular loop of the receptor (2); this can occur as a result of activation of only a trivial fraction of the receptor population, because of amplification in the cAMP signaling cascade, and can account for half of the desensitization that occurs in a target cell (3). However, considerably more experimental attention has been focused on β-adrenergic receptor phosphorylation in the C terminus of the receptor catalyzed by a unique receptor directed kinase, dubbed βARK. The substrate for βARK is the agonist-occupied receptor (1). βARK-catalyzed phosphorylation of the β-receptor leads to receptor association with yet another protein, dubbed β-arrestin, which in turn leads to receptor hyperphosphorylation and functional uncoupling from G proteins. In vitro studies have suggested that arrestin interaction with the β-adrenergic receptor serves as an adapter for receptor endocytosis via clathrin-coated pits; this internalization fosters receptor dephosphorylation and serves as a prelude to recycling to the surface for reactivation. At some point, receptor entry via endocytosis is not followed by resensitization and recycling but, rather, leads to receptor trafficking to lysosomes and ultimate receptor degradation. However, agonist-elicited decreases in receptor density, termed “down-regulation,” do not contribute in a quantitatively significant way to acute desensitization responses when compared with the profound effect of phosphorylation-dependent functional uncoupling of β-receptors from G proteins (3).
Diminished responsiveness of the β-adrenergic receptor system to catecholamines has been unequivocally demonstrated in chronic congestive heart failure, a life-threatening disease affecting more than 4 million Americans. Heart failure can be defined as a decline in heart function below that required for maintaining adequate cardiac output to meet the metabolic demands of body tissues. Classic literature suggests that a fully functioning β-adrenergic system is not essential to achieve normal cardiac function in healthy hearts performing under “basal” conditions (4). In contrast, the β-adrenergic system is essential for response to stress and for supporting circulatory function in heart failure. The decreased responsiveness in the β-adrenergic system in heart failure, despite elevated circulating levels of catecholamines, has been correlated with changes in a number of the molecular players in the β-adrenergic system or their interactions (cf. Fig. 1). The molecular changes noted in failing human hearts include diminished receptor density (5), especially for the β1-adrenergic receptor subtype and its mRNA template (6); reduced stimulation of adenylyl cyclase by β-adrenergic agonists without changes in enzyme content or activation by agonists at other receptors (5); an increase in the mRNA, protein, and catalytic activity levels of βARK kinase (6); and an increase in Gi, the G protein that mediates the functional antagonism of cAMP production (7). Many of these phenomena are recapitulated in animal models of congestive heart failure, such as that achieved by electrical pacing in chronically instrumented rabbits (8) or dogs (9).
Strategies for therapeutic intervention could target either the underlying cause of heart failure or the consequences of that failure. Diminished β-adrenergic responsiveness, although certainly a hallmark of the disease, is not necessarily the precipitating dysfunction. Reduction in the sensitivity of the arterial baroreflex response to blood pressure, and resultant decline in tonic baroreflex suppression of central nervous system-dependent sympathetic activity, lead to sustained elevation in plasma norepinephrine. This elevated norepinephrine is a likely candidate for evoking desensitization of the β-adrenergic system characteristic of the disease state. Although the introduction of β-adrenergic receptor blockers in the treatment of heart failure was initially based on nonintuitive empirical evidence from clinical trials (10), protection against sustained activation of the β-adrenergic receptor system (and concomitant desensitization of that system) may very well contribute to the therapeutic efficacy of these agents.
Transgenic mice have provided an opportunity to test hypotheses regarding the molecular changes that precipitate congestive heart failure, and those that might represent useful therapeutic interventions, by, for example, gene therapy (11). Milano et al. (12) reported the development of transgenic mouse lines overexpressing the β2-adrenergic receptor by 55- to 195-fold in the heart, driven by the cardiac selective α-myosin heavy chain promoter. This expression increased in a nearly linear manner over the first 2 months of life, in parallel with α-myosin heavy chain expression (12). Receptor overexpression was paralleled by increased basal- as well as agonist (isoproterenol)-induced adenylyl cyclase activity. These animals manifested increased left atrial isometric tension and increased left ventricular function when assessed within ≈2 months of age. In fact, baseline cardiac function was elevated to that characteristic of control animals treated with isoproterenol, suggesting that the increased basal cAMP production was sufficient to induce a maximal inotropic response. Elevated baseline responses appeared to be attributable to receptor isomerization into an active state (i.e., capable of activating G proteins) in these animals dramatically overexpressing the β2-adrenergic receptor in the heart, even in the absence of agonist. The interpretation of these studies was that augmenting adrenergic function by in vivo gene transfer might represent a viable, therapeutic alternative for the treatment of patients suffering from congestive heart failure. In fact, adenoviral-mediated in vitro gene transfer of either the human β2-adrenergic receptor or an inhibitor of βARK into isolated myocytes from rabbits chronically paced to produce hemodynamic failure resulted in a restoration of agonist-stimulated cAMP production to these target cells (8), providing credence to the hypothesis that increasing the effectiveness of the β-adrenergic receptor signaling pathway may improve the functional performance of the heart.
This conclusion, however, seems difficult to reconcile with the clinical experience that β-adrenergic receptor blockade inhibits the clinical progression of disease and improves outcomes in this patient population (13). In contrast, β-adrenergic receptor agonists are useful only for the short-term support of the circulation in patients with advanced heart failure, presumably because of the development of tolerance (i.e., desensitization) to these agents. Why, then, would cardiac-specific overexpression of β-adrenergic receptors appear to offer such desirable consequences, such as increased inotropy of the heart, even considering that these results were obtained in an otherwise healthy animal?
This enigma is partly resolved by the recent findings of Engelhardt et al. (14). These investigators used the α-myosin heavy chain promoter to achieve cardiac-specific expression of the β1-adrenergic receptor subtype in transgenic animals, at levels (5- to 15-fold) greater than normal levels but considerably less than those attained for the β2-adrenergic receptor subtype by Milano et al. (12). Overexpression of receptors led to the expected increases in heart rate and contractility when compared with control animals at 12 weeks. However, morphological analysis indicated that marked myocyte hypertrophy accompanied by fibrosis developed in the transgenic hearts within a few weeks after birth. Perhaps as a consequence of these morphological changes, the increase in myocardial performance noted early in life declined beyond the time point of 16 weeks to a level well below 50% of that seen in wild-type animals by 35 weeks of age. Loss of contractility measured in 35-week-old transgenic mice was paralleled by a reduction of left ventricular function measured by MRI and by the development of evidence of heart failure in mice at later dates. This study affirms the widely held perception that the damaged myocardium has limited response. The precise mechanism through which increased β1-adrenergic receptor expression leads to myocardial hypertrophy, fibrosis, and dysfunction over time is unclear but likely involves calcium-dependent pathways recently recognized to play an essential role in the development of cardiac hypertrophy and failure (15). These findings, which also may have been observed with overexpression of β2-adrenergic receptors had the studies of Milano et al. (12) been extended beyond 8–10 weeks of age, suggest that the long-term β-adrenergic stimulation is in fact toxic to the myocardium. Consequently, the attenuated cardiac responses to catecholamines achieved by reduction in receptor density and enhanced desensitization are actually cardioprotective!
What general principles, then, do these studies offer for the rational selection of therapeutic targets in chronic diseases that may be initiated by a series of molecular events distinct from those that cause the symptoms of the disease and may have arisen to compensate for the precipitating events? In this example, the β-adrenergic receptor system appears to have been optimally designed for “fight or flight”: i.e., acute reflexes. The beneficial effects of activation of this system achieved in the short term are not realized over the long term, where compensatory responses prevail. An analogous example is the renin–angiotensin system, where long-term inhibition of this system has more beneficial therapeutic effects than activation, which has only short-term benefit (16). Rational selection of therapeutic targets requires deciding whether it is wiser to modify signaling pathways mediating short-term responses or to influence the activity of chronic compensatory responses to achieve the desired clinical outcome.
Footnotes
The companion to this commentary begins on page 7059 in issue 12 of volume 96.
References
- 1.Haussdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 2.Clark R B, Friedman J, Dixon R A, Strader C D. Mol, Pharmacol. 1989;36:343–348. [PubMed] [Google Scholar]
- 3.Lohse M J, Benovic J L, Caron M G, Lefkowitz R J. J Biol Chem. 1987;265:3202–3209. [PubMed] [Google Scholar]
- 4.Gaffney T E, Braunwald E. Am J Med. 1963;34:320–324. doi: 10.1016/0002-9343(63)90118-9. [DOI] [PubMed] [Google Scholar]
- 5.Bristow M R, Ginsburg R, Monobe W, Cubiciotti R S, Sageman W S, Lurie K, Billingham M E, Harrison D E, Stinson E B. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 6.Ungerer M, Bohm M, Elce J S, Erdmann E, Lohse M J. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 7.Feldman A M, Cates A E, Veazey W B, Hershberger R E, Bristow M R, Baughman K L, Baumgartner W A, Van Dop C. J Clin Invest. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akhter S A, Skaer C A, Kypson A P, McDonald P H, Peppel K C, Glower D D, Lefkowitz R J, Koch W. Proc Natl Acad Sci USA. 1997;94:12100–12105. doi: 10.1073/pnas.94.22.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiuchi K, Shannon R P, Komamura K, Cohen D J, Bianchi C, Homcy C J, Vatner S F, Vatner D E. J Clin Invest. 1993;91:907–914. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly R A, Smith T W. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1995. pp. 809–838. [Google Scholar]
- 11.Frans W M, Mueller O J, Hartong R, Frey N, Katus H A. J Mol Med. 1997;75:115–129. doi: 10.1007/s001090050096. [DOI] [PubMed] [Google Scholar]
- 12.Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 13.Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel J P. Circulation. 1998;98:1184–1191. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt S, Hein L, Wiesmann F, Lohse M J. Proc Natl Acad Sci USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molkentin J D, Lu J R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown N J, Vaughan D E. Circulation. 1998;97:1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]