Abstract
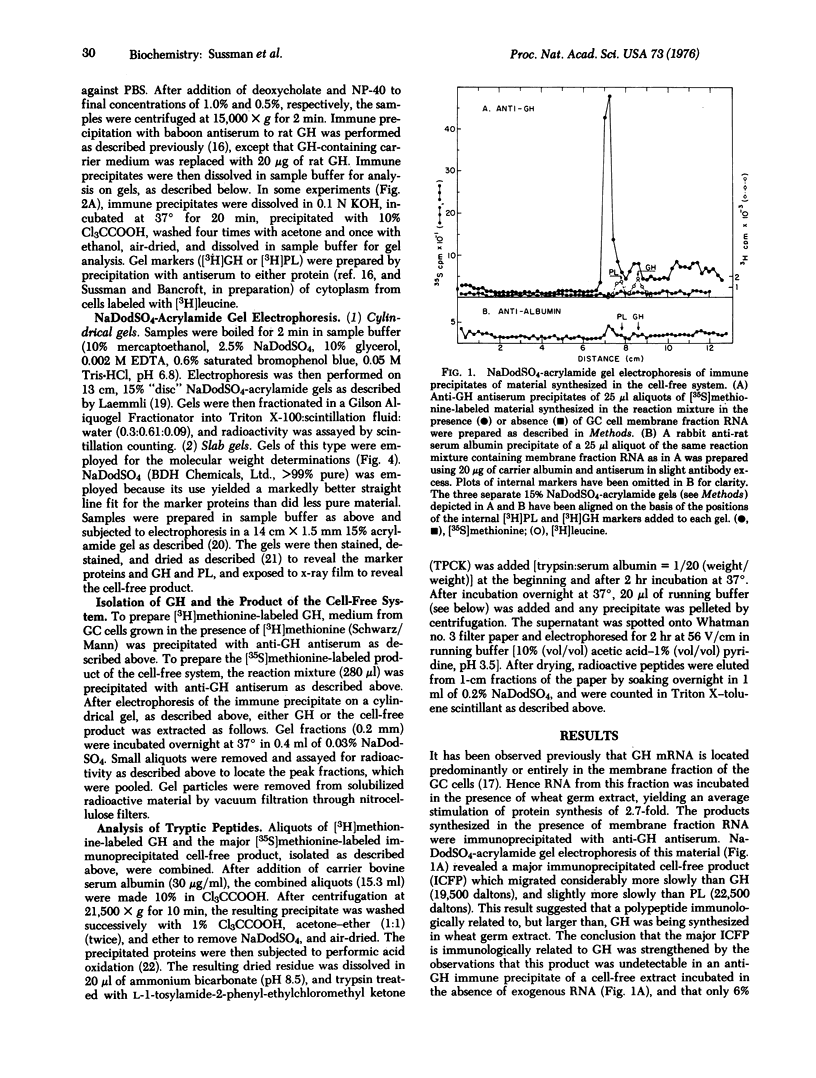
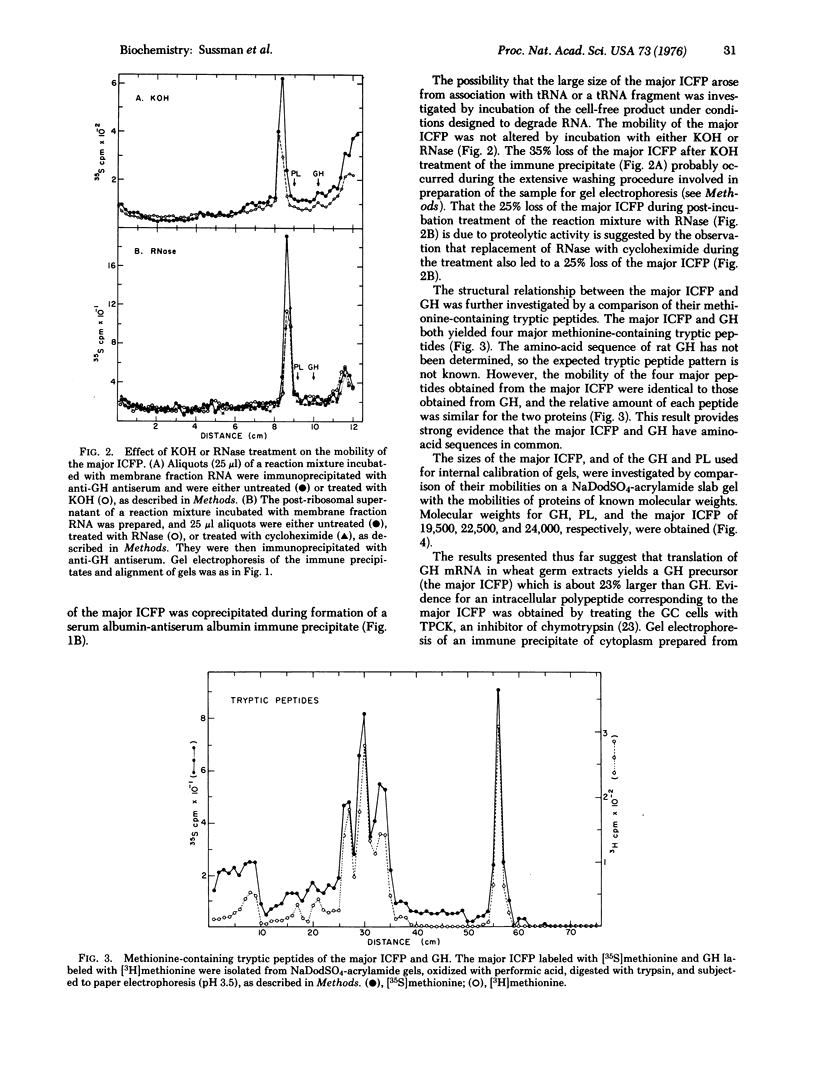
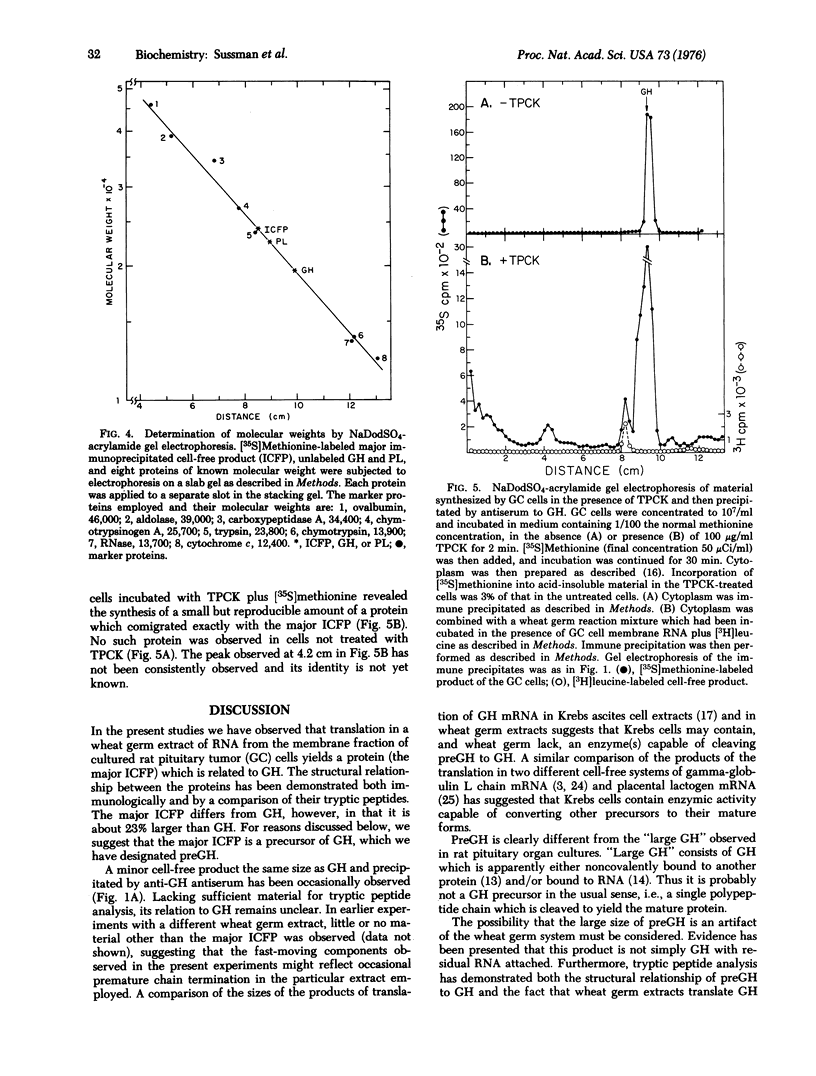
Membrane fraction RNA isolated from rat pituitary tumor (GC) cells has been translated in a wheat germ extract. A product was synthesized which was immunologically related to growth hormone, but which migrated more slowly than growth hormone upon sodium dodecyl sulfate-acrylamide gel electrophoresis. The mobility of the cell-free product on gels of this type was unchanged by treatment with either KOH or RNase. The mobilities during paper electrophoresis of the methionine-containing tryptic peptides obtained from the cell-free product were identical to those obtained from growth hormone synthesized and secreted by the GC cells. Molecular weights for growth hormone and the cell-free product of 19,500 and 24,000, respectively, were determined by gel electrophoresis of these proteins together with marker proteins of known molecular weights. No protein with the properties of the cell-free product was detected after a 2 min incubation of the GC cells with [35S]methionine. However, treatment of the GC cells, with a protease inhibitor, L-1-tosylamide-2-phenyl-ethylchloromethyl ketone (TPCK), led to the appearance of a new polypeptide, immunologically related to growth hormone, and with a mobility on gels identical to that of the cell-free product. These results strongly imply that the cell-free product represents a growth hormone precursor (pregrowth hormone) which is rapidly converted to growth hormone in pituitary cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft F. C. Measurement of growth hormone synthesis by rat pituitary cells in culture. Endocrinology. 1973 Apr;92(4):1014–1021. doi: 10.1210/endo-92-4-1014. [DOI] [PubMed] [Google Scholar]
- Bancroft F. C., Wu G. J., Zubay G. Cell-free synthesis of rat growth hormone. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3646–3649. doi: 10.1073/pnas.70.12.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns A., Janssen P., Bloemendal H. The molecular weight of the 14S calf lens messenger RNA. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1157–1164. doi: 10.1016/s0006-291x(74)80100-2. [DOI] [PubMed] [Google Scholar]
- Boime I., Boguslawski S., Caine J. The translation of a human placental lactogen mRNA fraction in heterologous cell-free systems: the synthesis of a possible precursor. Biochem Biophys Res Commun. 1975 Jan 6;62(1):103–109. doi: 10.1016/s0006-291x(75)80411-6. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Harrison T. M., Mathews M. B., Milstein C. Translation of messenger RNA for immunoglobulin light chains in a cell-free system from Krebs II ascites cells. FEBS Lett. 1972 Jun 15;23(2):244–248. doi: 10.1016/0014-5793(72)80352-1. [DOI] [PubMed] [Google Scholar]
- Church R. L., Pfeiffer S. E., Tanzer M. L. Collagen biosynthesis: synthesis and secretion of a high molecular weight collagen precursor (procollagen). Proc Natl Acad Sci U S A. 1971 Nov;68(11):2638–2642. doi: 10.1073/pnas.68.11.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. J., Milstein C. The translation in vitro of mRNA for immunoglobulin heavy chains. Eur J Biochem. 1973 Jul 2;36(1):1–7. doi: 10.1111/j.1432-1033.1973.tb02877.x. [DOI] [PubMed] [Google Scholar]
- Friedman E., Friedman J., Gershon S. Dopamine synthesis: stimulation by a hypothalamic factor. Science. 1973 Nov 23;182(4114):831–832. doi: 10.1126/science.182.4114.831. [DOI] [PubMed] [Google Scholar]
- Gozes I., Schmitt H., Littauer U. Z. Translation in vitro of rat brain messenger RNA coding for tubulin and actin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):701–705. doi: 10.1073/pnas.72.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindas-Mügge I., Lane C. D., Kreil G. Insect protein synthesis in frog cells: the translation of honey bee promelittin messenger RNA in Xenopus oocytes. J Mol Biol. 1974 Aug 15;87(3):451–462. doi: 10.1016/0022-2836(74)90096-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Yaffe D. Determination of actin messenger RNA in cultures of differentiating embryonic chick skeletal muscle. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4467–4471. doi: 10.1073/pnas.71.11.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pong S. S., Nuss D. L., Koch G. Inhibition of initiation of protein synthesis in mammalian tissue culture cells by L-1-tosylamido-2-phenylethyl chloromethyl ketone. J Biol Chem. 1975 Jan 10;250(1):240–245. [PubMed] [Google Scholar]
- Prives C. L., Aviv H., Paterson B. M., Roberts B. E., Rozenblatt S., Revel M., Winocour E. Cell-free translation of messenger RNA of simian virus 40: synthesis of the major capsid protein. Proc Natl Acad Sci U S A. 1974 Feb;71(2):302–306. doi: 10.1073/pnas.71.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos B. A., Okano K., Deftos L. J. Evidence for a pro-calcitonin. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1134–1140. doi: 10.1016/0006-291x(74)90430-6. [DOI] [PubMed] [Google Scholar]
- Rossman T., Norris C., Troll W. Inhibition of macromolecular synthesis in Escherichia coli by protease inhibitors. Specific reversal by glutathione of the effects of chloromethyl ketones. J Biol Chem. 1974 Jun 10;249(11):3412–3417. [PubMed] [Google Scholar]
- Russell J. H., Geller D. M. Rat serum albumin biosynthesis: evidence for a precursor. Biochem Biophys Res Commun. 1973 Nov 1;55(1):239–245. doi: 10.1016/s0006-291x(73)80085-3. [DOI] [PubMed] [Google Scholar]
- Schmeckpeper B. J., Adams J. M., Harris A. W. Detection of a possible precursor of immunoglobulin light chain in MOPC 41 A plasmacytoma cells. FEBS Lett. 1975 Apr 15;53(1):95–98. doi: 10.1016/0014-5793(75)80691-0. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Stachura M. E., Frohman L. A. "Large" growth hormone: ribonucleic acid-associated precursor of other growth hormone forms in rat pituitary. Endocrinology. 1974 Mar;94(3):701–712. doi: 10.1210/endo-94-3-701. [DOI] [PubMed] [Google Scholar]
- Stachura M. E., Frohman L. A. Large growth hormone: evidence for the association of growth hormone with another protein moiety in the rat pituitary. Endocrinology. 1973 Jun;92(6):1708–1713. doi: 10.1210/endo-92-6-1708. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Peptide hormones. Annu Rev Biochem. 1974;43(0):509–538. doi: 10.1146/annurev.bi.43.070174.002453. [DOI] [PubMed] [Google Scholar]
- Zanini A., Giannattasio G., Meldolesi J. Studies on in vitro synthesis and secretion of growth hormone and prolactin. II. Evidence against the existence of precursor molecules. Endocrinology. 1974 Jan;91(1):104–111. doi: 10.1210/endo-94-1-104. [DOI] [PubMed] [Google Scholar]



