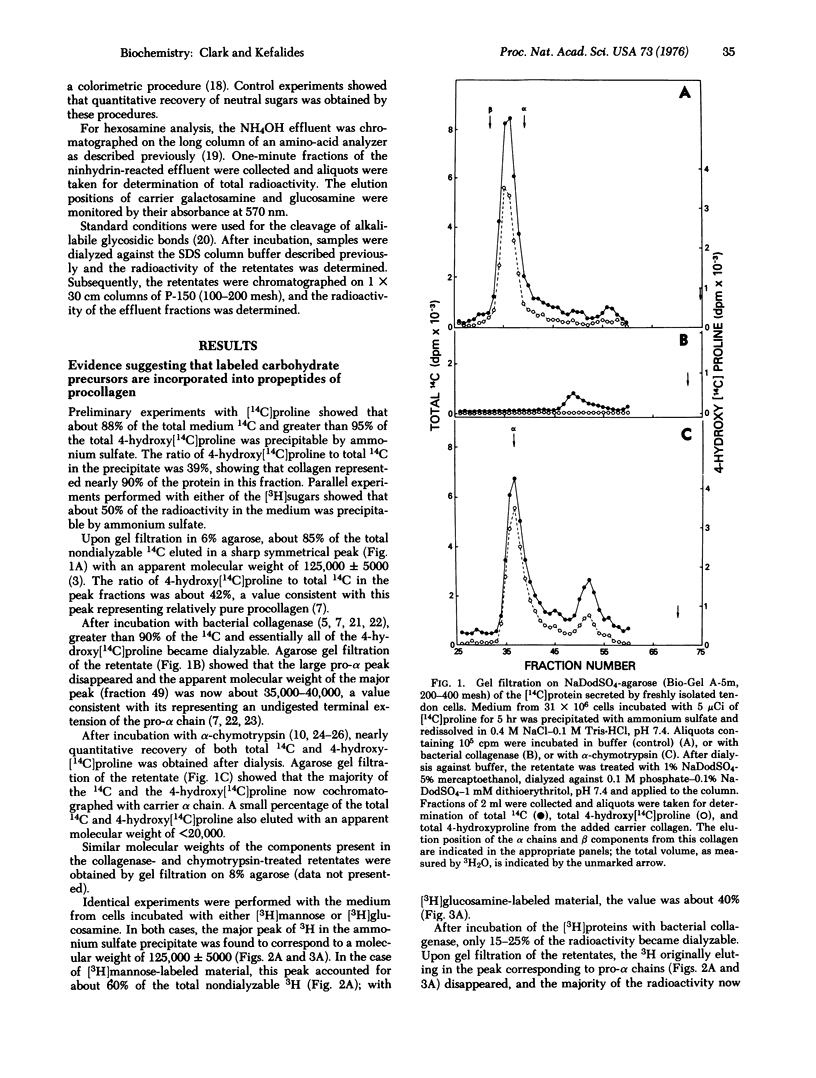
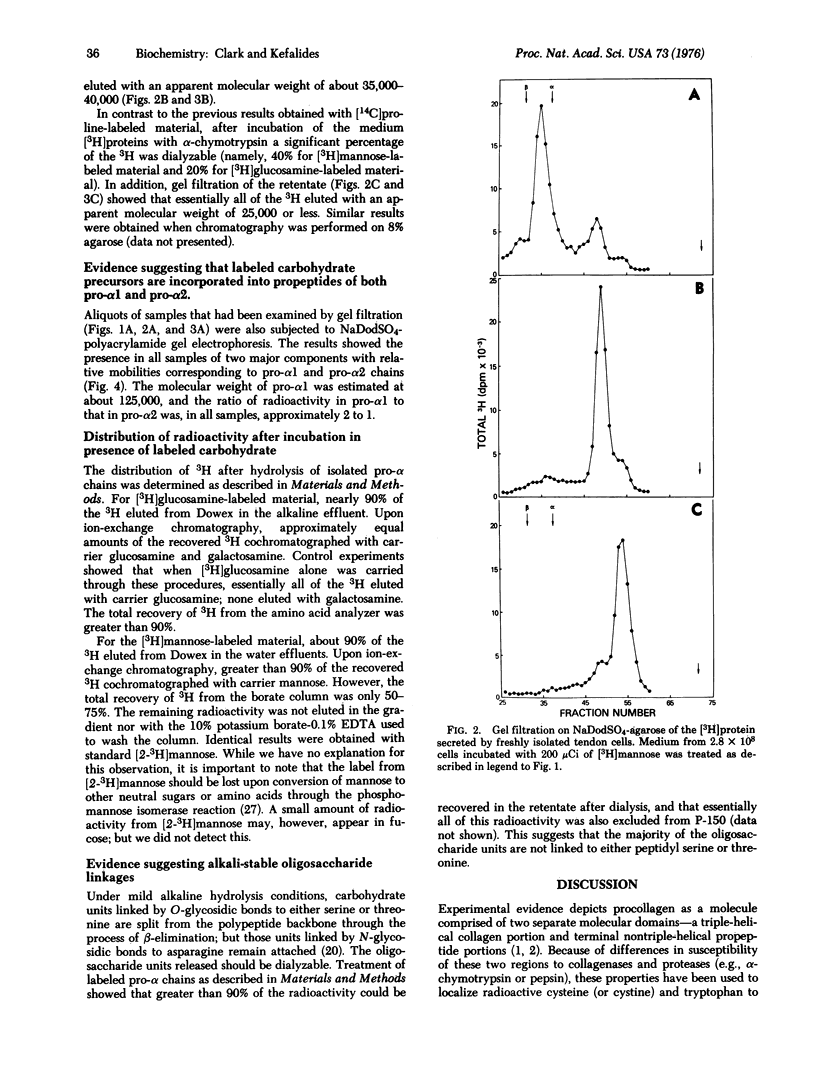
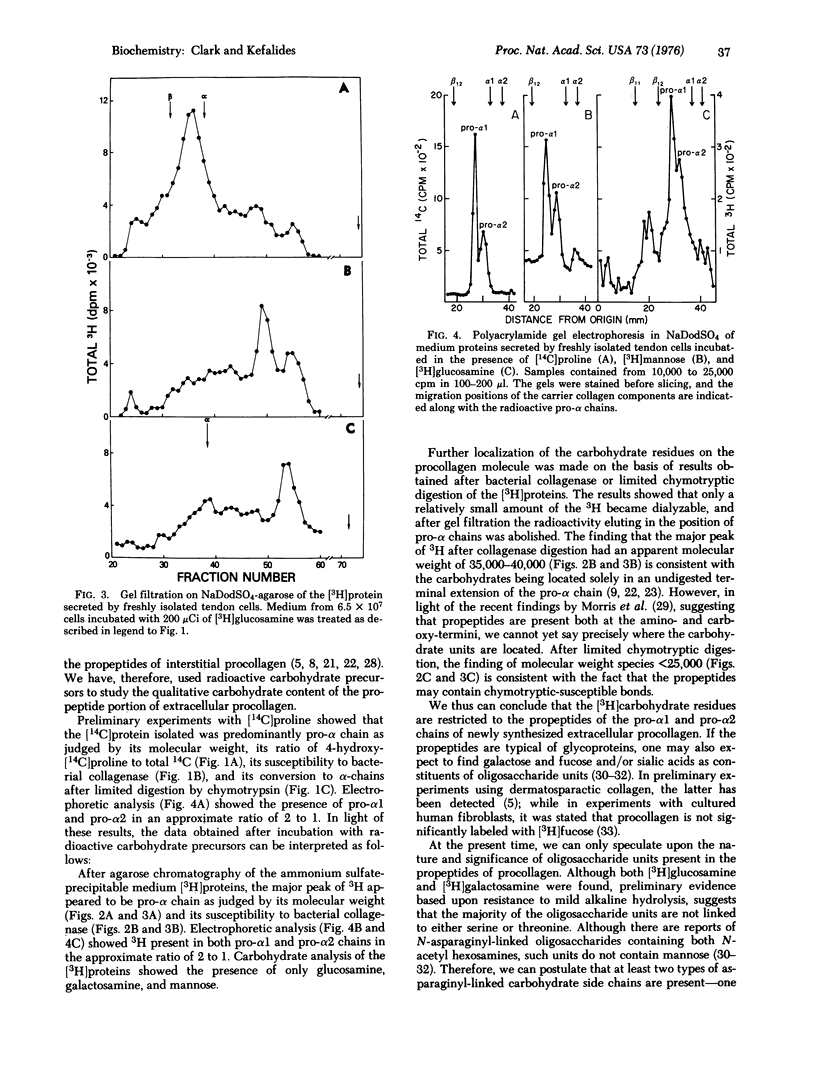
Abstract
Cells obtained from chick embryo tendons incorporate isotopically labeled glucosamine and mannose into the pro-alpha1 and pro-alpha2 chains of procollagen as judged by sodium dodecyl sulfate-gel filtration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The label was further localized to the propeptides of pro-alpha1 and pro-alpha2 by its chromatographic behavior after digestion with bacterial collagenase or alpha-chymotrypsin. Carbohydrate analysis of isolated pro-alpha chains showed the presence of labeled galactosamine in addition to mannose and glucosamine. Resistance to mild alkaline hydrolysis suggested that greater than 90% of the oligosaccharide units are not linked to the propeptide backbone by either serine or threonine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Ehrlich H. P., Wyke A. W. Procollagen: conversion of the precursor to collagen by a neutral protease. Science. 1972 Feb 4;175(4021):544–546. doi: 10.1126/science.175.4021.544. [DOI] [PubMed] [Google Scholar]
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Dehm P., Jimenez S. A., Olsen B. R., Prockop D. J. A transport form of collagen from embryonic tendon: electron microscopic demonstration of an NH 2 -terminal extension and evidence suggesting the presence of cystine in the molecule (chick embryo-tropocollagen-gel filtration). Proc Natl Acad Sci U S A. 1972 Jan;69(1):60–64. doi: 10.1073/pnas.69.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehm P., Olsen B. R., Prockop D. J. Antibodies to chick-tendon procollagen. Affinity purification with the isolated disulfide-linded NH2-terminal extensions and reactivity with a component in embryonic serum. Eur J Biochem. 1974 Jul 1;46(1):107–116. doi: 10.1111/j.1432-1033.1974.tb03602.x. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Biosynthesis of cartilage procollagen. Eur J Biochem. 1973 May;35(1):159–166. doi: 10.1111/j.1432-1033.1973.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Denduchis B., Kefalides N. A., Bezkorovainy A. The chemistry of sheep anterior lens capsule. Arch Biochem Biophys. 1970 Jun;138(2):582–589. doi: 10.1016/0003-9861(70)90384-x. [DOI] [PubMed] [Google Scholar]
- Furthmayer H., Timpl R., Stark M., Lapière C. M., Kühn K. Chemical properties of the peptide extension in the palpha 1 chain of dermatosparactic skin procollagen. FEBS Lett. 1972 Dec 1;28(2):247–250. doi: 10.1016/0014-5793(72)80723-3. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Epstein E. H., Jr, Sherr C. J. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3655–3659. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Prockop D. J. Further evidence for a transport form of collagen. Its extrusion and extracellular conversion to tropocollagen in embryonic tendon. FEBS Lett. 1971 Oct 1;17(2):245–248. doi: 10.1016/0014-5793(71)80156-4. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation and characterization of cyanogen bromide peptides from basement membrane collagen. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1151–1158. doi: 10.1016/0006-291x(72)90955-2. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Johnson G. S., White B., Scocca J. An accelerated system for analysis of neutral sugars in complex carbohydrates. Anal Biochem. 1971 Oct;43(2):640–643. doi: 10.1016/0003-2697(71)90301-0. [DOI] [PubMed] [Google Scholar]
- Lehnhardt W. F., Winzler R. J. Determination of neutral sugars in glycoproteins by gas-liquid chromatography. J Chromatogr. 1968 May 7;34(4):471–479. doi: 10.1016/0021-9673(68)80091-3. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Morris N. P., Fessler L. I., Weinstock A., Fessler J. H. Procollagen assembly and secretion in embryonic chick bone. J Biol Chem. 1975 Jul 25;250(14):5719–5726. [PubMed] [Google Scholar]
- Rose I. A. Preparation of phosphoenolpyruvate and pyruvate specifically labeled with deuterium and tritium. Methods Enzymol. 1975;41:110–115. doi: 10.1016/s0076-6879(75)41028-x. [DOI] [PubMed] [Google Scholar]
- Schofield J. D., Prockop D. J. Procollagen-a precursor form of collagen. Clin Orthop Relat Res. 1973 Nov-Dec;(97):175–195. doi: 10.1097/00003086-197311000-00026. [DOI] [PubMed] [Google Scholar]
- Schofield J. D., Uitto J., Prockop D. J. Formation of interchain disulfide bonds and helical structure during biosynthesis of procollagen by embryonic tendon cells. Biochemistry. 1974 Apr 23;13(9):1801–1806. doi: 10.1021/bi00706a004. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Anderson J. C., Jackson D. S. The incorporation of L-[1-3-H]= fucose into non-collagenous glycoproteins secreted by human fibroblasts in culture. Biochem Soc Trans. 1975;3(1):138–140. doi: 10.1042/bst0030138. [DOI] [PubMed] [Google Scholar]
- Sharon N. Glycoproteins. Sci Am. 1974 May;230(5):78–86. doi: 10.1038/scientificamerican0574-78. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Taubman M. B., Goldberg B. Isolation of a disulfide-stabilized, three-chain polypeptide fragment unique to the precursor of human collagen. J Biol Chem. 1973 Oct 25;248(20):7033–7038. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins: their biochemistry, biology and role in human disease. N Engl J Med. 1969 Nov 6;281(19):1043–concl. doi: 10.1056/NEJM196911062811905. [DOI] [PubMed] [Google Scholar]
- Stark M., Lenaers A., Lapiere C., Kühn K. Electronoptical studies of procollagen from the skin of dermatosparaxic calves. FEBS Lett. 1971 Nov 1;18(2):225–227. doi: 10.1016/0014-5793(71)80450-7. [DOI] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. Improved method for hydroxyproline analysis in tissue hydrolyzates. Anal Biochem. 1971 Feb;39(2):487–491. doi: 10.1016/0003-2697(71)90438-6. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Intracellular hydroxylation of non-helical protocollagen to form triple-helical procollagen and subsequent secretion of the molecule. Eur J Biochem. 1974 Apr 1;43(2):221–230. doi: 10.1111/j.1432-1033.1974.tb03403.x. [DOI] [PubMed] [Google Scholar]
- Von der Mark K., Bornstein P. Characterization of the pro 1 chain of procollagen. Isolation of a sequence unique to the precursor chain. J Biol Chem. 1973 Apr 10;248(7):2285–2289. [PubMed] [Google Scholar]
- WEIMER H. E., MOSHIN J. R. Serum glyco protein concentrations in experimental tuberculosis of guinea pigs. Am Rev Tuberc. 1953 Oct;68(4):594–602. doi: 10.1164/art.1953.68.4.594. [DOI] [PubMed] [Google Scholar]


