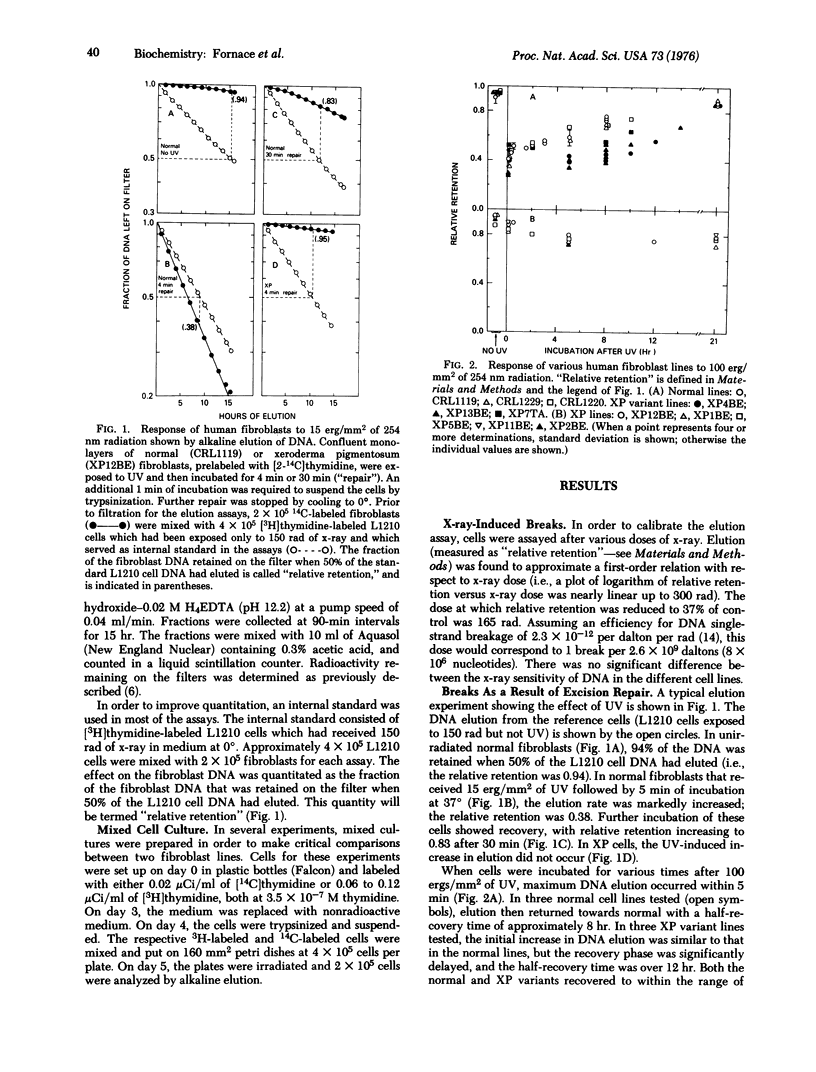
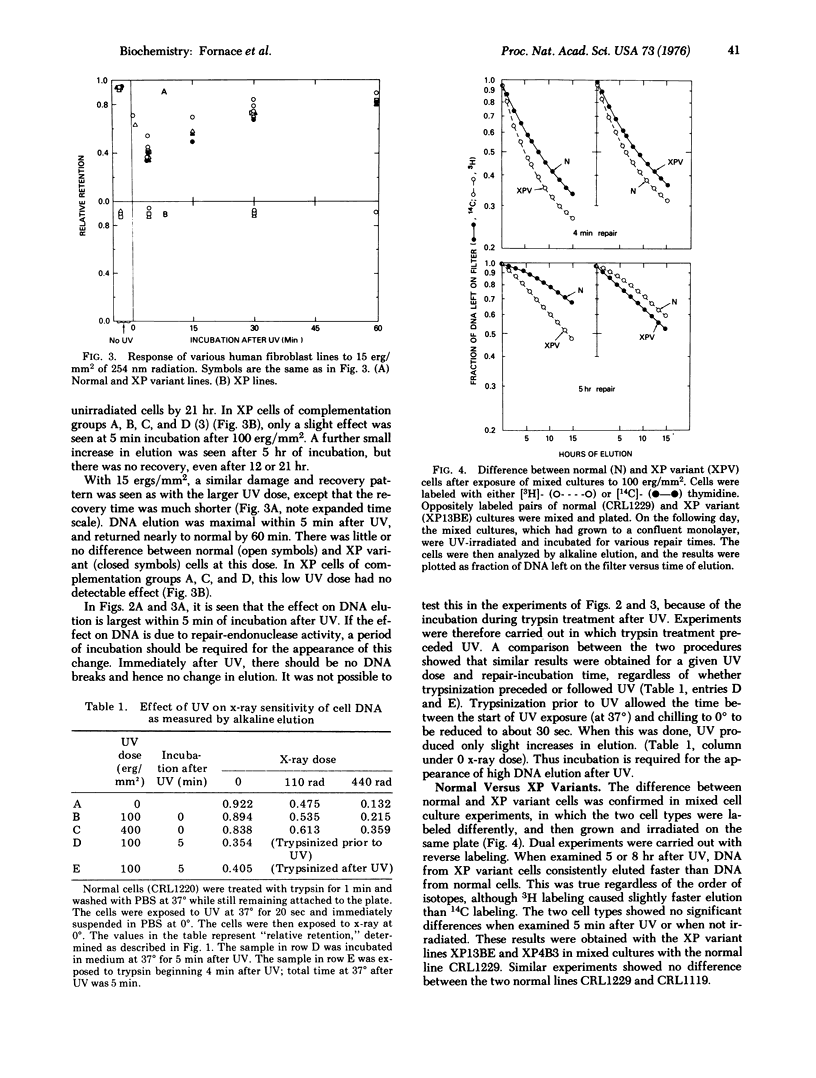
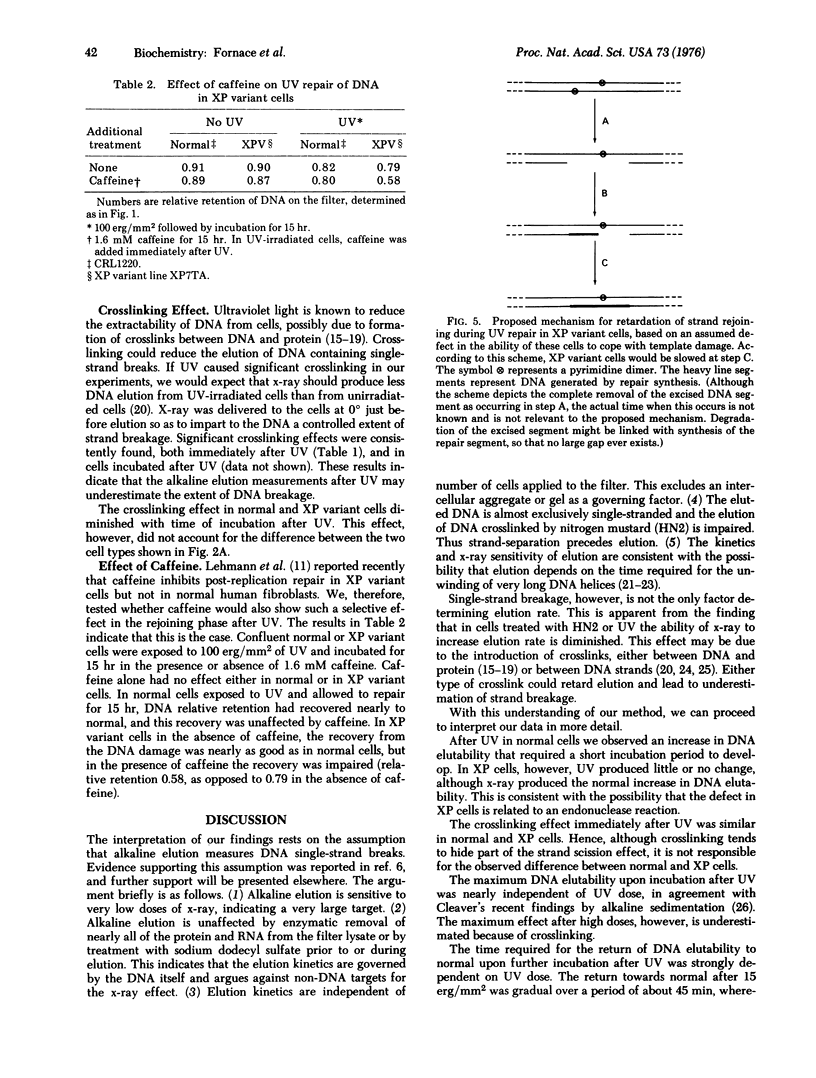
Abstract
The method of DNA alkaline elution was applied to a study of the formation and resealing of DNA single-strand breaks after irradiation of human fibroblasts with ultraviolet light (UV). The general features of the results were consistent with current concepts of DNA excision repair, in that breaks appeared rapidly after UV, and resealed slowly in normal fibroblasts, whereas breaks did not appear in those cells of patients with xeroderma pigmentosum (XP) that are known to have defects in DNA repair synthesis. The appearance of breaks required a short post-UV incubation, consistent with the expected action of an endonuclease. Cells of the variant form of XP characterized by normal DNA repair synthesis exhibited normal production of breaks after UV, but were slower than normal cells in resealing these breaks. This difference was enhanced by caffeine. A model is proposed to relate this finding with a previously described defect in post-replication repair in these XP variant cells. DNA crosslinking appears to cause an underestimate in the measurement of DNA breakage after UV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnström G., Erixon K. Radiation induced strand breakage in DNA from mammalian cells. Strand separation in alkaline solution. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Mar;23(3):285–289. doi: 10.1080/09553007314550311. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Steps in DNA chain elongation and joining after ultra-violet irradiation of human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Nov;22(5):417–424. doi: 10.1080/09553007214551301. [DOI] [PubMed] [Google Scholar]
- Burk P. G., Lutzner M. A., Clarke D. D., Robbins J. H. Ultraviolet-stimulated thymidine incorporation in xeroderma pigmentosum lymphocytes. J Lab Clin Med. 1971 May;77(5):759–767. [PubMed] [Google Scholar]
- Cleaver J. E. Sedimentation of DNA from human fibroblasts irradiated with ultraviolet light: possible detection of excision breaks in normal and repair-deficient xeroderma pigmentosum cells. Radiat Res. 1974 Feb;57(2):207–227. [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol. 1972 Mar;58(3):124–128. doi: 10.1111/1523-1747.ep12538913. [DOI] [PubMed] [Google Scholar]
- Day R. S. Xeroderma pigmentosum variants have decreased repair of ultraviolet-damaged DNA. Nature. 1975 Feb 27;253(5494):748–749. doi: 10.1038/253748a0. [DOI] [PubMed] [Google Scholar]
- Glisin V. R., Doty P. The cross-linking of DNA by ultraviolet radiation. Biochim Biophys Acta. 1967 Jul 18;142(2):314–322. doi: 10.1016/0005-2787(67)90614-4. [DOI] [PubMed] [Google Scholar]
- Habazin V., Han A. Ultra-violet-light-induced DNA-to-protein cross-linking in HeLa cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(6):569–575. doi: 10.1080/09553007014550711. [DOI] [PubMed] [Google Scholar]
- Jolley G. M., Ormerod M. G. An improved method for measuring cross-links in the DNA of mammalian cells: the effect of nitrogen mustard. Biochim Biophys Acta. 1973 May 10;308(2):242–251. doi: 10.1016/0005-2787(73)90154-8. [DOI] [PubMed] [Google Scholar]
- Jung E. G. Das pigmentierte Xerodermoid. Ein Defekt der Rekombinations-Erholung von UV Schäden. Arch Dermatol Forsch. 1971;241(1):33–43. [PubMed] [Google Scholar]
- Kohn K. W., Friedman C. A., Ewig R. A., Iqbal Z. M. DNA chain growth during replication of asynchronous L1210 cells. Alkaline elution of large DNA segments from cells lysed on filters. Biochemistry. 1974 Sep 24;13(20):4134–4139. doi: 10.1021/bi00717a011. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Spears C. L., Doty P. Inter-strand crosslinking of DNA by nitrogen mustard. J Mol Biol. 1966 Aug;19(2):266–288. doi: 10.1016/s0022-2836(66)80004-9. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod M. G., Stevens U. The rejoining of x-ray-induced strand breaks in the DNA of a murine lymphoma cell (L5178Y). Biochim Biophys Acta. 1971 Feb 25;232(1):72–82. doi: 10.1016/0005-2787(71)90492-8. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Rydberg B. The rate of strand separation in alkali of DNA of irradiated mammalian cells. Radiat Res. 1975 Feb;61(2):274–287. [PubMed] [Google Scholar]
- SMITH K. C. Dose dependent decrease in extractability of DNA from bacteria following irradiation with ultraviolet light or with visible light plus dye. Biochem Biophys Res Commun. 1962 Jul 3;8:157–163. doi: 10.1016/0006-291x(62)90255-3. [DOI] [PubMed] [Google Scholar]
- Simukova N. A., Budowsky E. I. Conversion of non-covalent interactions in nucleoproteins into covalent bonds: UV-induced formation of polynucleotide-protein crosslinks in bacteriophage Sd virions. FEBS Lett. 1974 Jan 15;38(3):299–303. doi: 10.1016/0014-5793(74)80077-3. [DOI] [PubMed] [Google Scholar]
- Smith K. C. A mixed photoproduct of thymine and cysteine: 5-S-cysteine, 6-hydrothymine. Biochem Biophys Res Commun. 1970;39(6):1011–1016. doi: 10.1016/0006-291x(70)90658-3. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Kinetics of the photochemical addition of [35S] cysteine to polynucleotides and nucleic acids. Biochemistry. 1968 Mar;7(3):1033–1037. doi: 10.1021/bi00843a023. [DOI] [PubMed] [Google Scholar]
- Uhlenhopp E. L., Zimm B. H. Viscoelastic characterization of single-stranded DNA from Escherichia coli. Biophys J. 1975 Mar;15(3):223–232. doi: 10.1016/S0006-3495(75)85813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



