Abstract
Objective
To evaluate the prognostic significance of histologic grade on survival of ovarian serous cancer in Denmark during nearly 30 years.
Methods
Using the nationwide Danish Pathology Data Bank, we evaluated 4,317 women with ovarian serous carcinoma in 1978–2006. All pathology reports were scrutinized and tumors classified as either low-grade serous carcinomas (LGSC) or high-grade serous carcinomas (HGSC). Tumors in which the original pathology reports were described as well-differentiated were classified as LGSC, and those that were described as moderately or poorly differentiated were classified as HGSC. We obtained histologic slides from the pathology departments for women with a diagnosis of well-differentiated serous carcinoma during 1997–2006, which were then reviewed by expert gynecologic pathologists. Data were analyzed using Kaplan-Meier methods and Cox proportional hazards regression analysis with follow-up through June 2009.
Results
Women with HGSC had a significantly increased risk of dying (HR=1.9; 95% CI: 1.6–2.3) compared with women with LGSC while adjusting for age and stage. Expert review of 171 women originally classified as well-differentiated in 1997–2006 were interpreted as LGSC in 30% of cases, whereas 12% were interpreted as HGSC and 50% as serous borderline ovarian tumors (SBT). Compared with women with confirmed LGSC, women with SBT at review had a significantly lower risk of dying (HR=0.5; 95% CI: 0.22–0.99), and women with HGSC at review had a non-significantly increased risk of dying (HR=1.6; 95% CI: 0.7–3.4).
Conclusions
A binary grading system is a significant predictor of survival for ovarian serous carcinoma.
Keywords: Ovarian serous carcinoma, binary grading system, overall survival, population-based, registry-based, expert gynecologic pathology slide review
Introduction
Ovarian cancer is the sixth most common malignancy in the Western world and the leading cause of death from gynecologic cancers [1]. 5-year survival rates range from 90% in women with disease confined to the ovaries to less than 10% in women with distant metastases [2]. Two thirds of ovarian cancers are diagnosed after the disease has spread beyond the ovaries because most patients are asymptomatic in low stages [3,4]. Ovarian serous carcinoma is the most common histologic type of ovarian cancer, representing about 50% of cases [5,6], and accounts for the vast majority of deaths [7].
Staging according to the International Federation of Gynecology and Obstetrics (FIGO) is the most important prognostic factor in determining survival of women with cancer of the ovaries [8,9]. Although several studies have shown that histologic grade is of prognostic importance [3,8,10–25], these studies utilized different grading systems, included various stages of disease, generally included only a small number of cases and did not specify the histologic type. Nevertheless, histologic grade is considered important in treatment decisions [7].
Traditionally, ovarian cancer has been graded as well-, moderately, and poorly differentiated but recently, a 2-tier system which divides serous carcinoma into low-grade serous carcinomas (LGSC) and high-grade serous carcinomas (HGSC) has been proposed [26]. The system is based primarily on the assessment of nuclear atypia using the mitotic index as a secondary feature [27]. The 3-tier system is highly subjective and implies that serous carcinoma progresses from well- to moderately and then poorly differentiated carcinoma. In contrast, the 2-tier system has been shown to be highly reproducible and is supported by molecular biologic studies indicating that LGSC and HGSC develop along different pathways [28–30], not as a progression from well- to moderately and poorly differentiated carcinoma. This has important clinical implications as evidenced by the lack of sensitivity of LGSC to the standard cytotoxic chemotherapy that is effective for HGSC. Molecular genetic studies have shown that LGSC are characterized by mutations of the KRAS, BRAF or ERBB2 genes and are relatively genetically stable. In contrast, HGSC are characterized by TP53 mutations and a high level of genetic instability. The majority of ovarian serous carcinomas are HGSC (90%), whereas LGSC comprise about 10% [31].
Only a few studies have examined histologic grade as a prognostic factor for women diagnosed with ovarian serous carcinoma using the 2-tier system [4,7,26,32,33]. Three studies [4,26,32] found that women with LGSC had a significantly better survival compared with women with HGSC, whereas two studies [7,33] did not; however, the latter two studies were based on only few cases of LGSC, and one of the studies [7] approached statistical significance.
The aim of this population-based study was to assess the prognostic significance of histologic grade using the 2-tier system on overall survival of women diagnosed with ovarian serous carcinoma in Denmark over a period of nearly 30 years. In addition, an expert review was performed on a subset of cases to add information about the binary grading system.
Material and Methods
Identification of women with ovarian serous carcinoma
From the Danish Pathology Data Bank, we identified all women diagnosed with primary ovarian serous cancer from January 1, 1978 through December 31, 2006. The population-based and nationwide Danish Pathology Data Bank was established in 1997 and includes all cytologic and histologic diagnoses performed at pathology departments in Denmark [34]. All pathology departments are mandated to report to the national Pathology Data Bank daily through an online-system. Most pathology departments have to a certain degree also transferred information to the Pathology Data Bank from 1997 and back to 1978. However, data from this period are not complete [35]. The Pathology Data Bank uses the Systematized Nomenclature of Medicine (SNOMED) that is based on codes for topography and morphology. In our study, women were identified by SNOMED topography codes starting with T87 and T86910, T86920, T86921 and T86922 and the SNOMED morphology codes M84413, M84603, M84613 and M90143. Apart from the codes for topography and morphology, the pathology descriptions in text are also included in the Pathology Data Bank. An increasing degree of completeness is also seen for these descriptions over time [35].
Histologic grade and stage
We retrieved the pathology reports for all women diagnosed with ovarian serous carcinoma from the Pathology Data Bank. An expert gynecologic pathologist (J.J) went through all the pathology reports and determined the grade of the tumor as either LGSC if the original diagnosis was well-differentiated carcinoma or HGSC if the original diagnosis was moderately or poorly differentiated carcinoma. If the original pathology report qualified for both LGSC and HGSC, the tumor was classified as HGSC. In several cases, it was not possible to determine whether the tumor was LGSC or HGSC because it was not stated in the pathology report or there was no text pathology description available. If based on the interpretation of the original report the tumor qualified as both LGSC and unknown grade, the tumor was classified as unknown grade since the tumor may have been HGSC. A report, in which the tumor was interpreted as HGSC and unknown grade, was classified as HGSC.
To obtain information about stage at diagnosis, we linked our cohort of women with ovarian serous carcinomas with the Danish National Patient Registry using the personal identification number (PIN) as key identifier. The National Patient Registry is a nationwide registry of virtually all somatic discharges in Danish hospitals since 1977 [36]. The National Patient Registry uses the FIGO classification system to classify stage of disease (stage I–IV). For women where the FIGO stage was not available in the National Patient Registry, we linked our cohort with the Danish Cancer Registry. The nationwide Cancer Registry has collected information on all incident cancer cases in Denmark since 1943 and includes information from hospital departments, general practitioners and specialists [37]. To classify stage of disease, the Cancer Registry uses the FIGO classification system as well as the categories localized disease, regional spread and distant metastases. For women where the FIGO stage was not available in the Cancer Registry, we used localized disease, regional spread and distant metastases to classify stage. To be able to use all information and thereby to gain maximum statistical power in our analyses, we used a combination of the two groupings and categorized stage of disease for all women with ovarian serous carcinoma as localized disease (including FIGO stage I), regional spread (including FIGO stage II and III) or distant metastases (including FIGO stage IV).
Follow-up procedure
All residents in Denmark since April 1, 1968 have been assigned the unique PIN. These numbers are recorded in the nationwide computerized Danish Civil Registration System which contains information about name, sex, addresses, migration and death as well as dates of death if such events occurred [38]. The PIN is included in all health registries in Denmark and can be used as a key identifier to insure accurate linkage of information between registries. All women diagnosed with ovarian serous cancer in our study were followed through linkage with the Civil Registration System and vital status (alive, emigrated or dead) was determined for each woman through June 23, 2009.
Collection of histologic slides
We collected the microscopic slides from the various pathology departments throughout Denmark for all women with a diagnosis of well-differentiated ovarian serous carcinoma diagnosed in 1997–2006. The slides were reviewed by two expert gynecologic pathologists (R.J.K and R.V) who were blinded to the women’s vital status at the end of the study and the text description included in the pathology report, and a review diagnosis was obtained classifying each ovarian carcinoma as LGSC, HGSC, using a modification of the criteria reported by Malpica et al. [26], serous borderline ovarian tumors (SBT) or other types of tumor. The Malpica et al. criteria [26] for grading ovarian serous carcinoma use nuclear features as a primary feature with mitotic index as a secondary feature. Thus, the emphasis on this classification is nuclear features. In our application of these criteria, we employed the same morphologic findings to assess nuclear features as specified by Malpica et al. [26]. However, our modification was only minor in that we did not use a specific mitotic index [28]. Instead, we assessed mitotic activity by simply estimating whether mitotic figures were sparse (which is more common in low-grade tumors) or easily identifiable (which is more common in high-grade tumors). In all other respects, our grading per this system (low versus high) was essentially similar to that of Malpica et al. [26].
Statistical analysis
Survival was measured from the date of the ovarian serous carcinoma diagnosis until date of death, emigration or end of follow-up, whichever came first. Survival times were calculated for stage at diagnosis and histologic grade, respectively, and for histologic grade stratified by stage using Kaplan-Meier curves, and the survival curves were compared using the Log-Rank test.
We used Cox proportional hazards regression analysis with follow-up time as timescale to evaluate the prognostic value on survival of age, year and stage at diagnosis and histologic grade. The effect on risk of death was estimated by hazard ratios (HR) and 95% confidence intervals (95% CI). The following clinical and pathologic parameters were evaluated: age at diagnosis (continuous), year of diagnosis (continuous), stage at diagnosis (localized disease, regional spread and distant metastases), and histologic grade (LGSC, HGSC and unknown grade). In addition, survival of women with an original diagnosis of well-differentiated carcinoma in 1997–2006 who after expert pathology review was classified as LGSC, HGSC or SBT was evaluated. Statistical modeling was performed using the SAS/STAT version 8.2 (SAS Institute, Cary, NC, USA). The study was approved by the Danish Protection Agency and the Danish Scientific Ethical Committee.
Results
Baseline characteristics
We identified 4,592 women with ovarian serous carcinoma in the Pathology Data Bank in 1978–2006. Thirty two women with ovarian cancer detected at autopsy only, cases in which the diagnosis was made prior to the beginning of our study or had an unknown date of the primary diagnosis were excluded, leaving 4,560 women. In the Cancer Registry and National Patient Registry, we identified 243 women with unknown stage at diagnosis whom we excluded from our analyses, leaving 4,317 women consisting of 636 women with localized disease, 2,626 women with regional spread and 1,055 women with distant metastases.
During the process of going through the pathology reports from the 4,317 women, we identified 279 women with LGSC, 2,029 women with HGSC and 2,009 women with unknown grade (Table 1). The mean age at diagnosis was 62.3 years, ranging from 18.7 to 94.7 years. Women with LGSC were slightly younger at diagnosis (mean=56.4 years) compared with women with unknown grade (mean=61.9 years) and HGSC (mean=63.4 years). More women with LGSC were diagnosed with localized disease (37.3%) compared with women with unknown grade (16.1%) and HGSC (10.3%), whereas more women with unknown grade (27.1%) and HGSC (23.9%) were diagnosed with distant metastases compared with women with LGSC (9.3%). Linkage with the Civil Registration System showed that 3,524 women died during follow-up (81.6%). Among these, 25.8% died within the first year. This number was similar for women with LGSC (22.2%), unknown grade (24.8%) and HGSC (27.0%).
Table 1.
Baseline characteristics of 4,317 women with ovarian serous carcinoma in Denmark 1978–2006
| Overall (n=4,317) |
LGSC (n=279) |
HGSC (n=2,029) |
Unknown grade (n=2,009) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Year of diagnosis | ||||||||
| 1978–1982 | 316 | (7.3) | 6 | (2.2) | 15 | (0.7) | 295 | (14.7) |
| 1983–1987 | 585 | (13.5) | 15 | (5.4) | 107 | (5.3) | 463 | (23.0) |
| 1988–1992 | 742 | (17.2) | 38 | (13.6) | 212 | (10.4) | 492 | (24.5) |
| 1993–1997 | 798 | (18.5) | 55 | (19.7) | 369 | (18.2) | 374 | (18.6) |
| 1998–2002 | 1,070 | (24.8) | 100 | (35.8) | 701 | (34.6) | 269 | (13.4) |
| 2003–2006 | 806 | (18.7) | 65 | (23.3) | 625 | (30.8) | 116 | (5.8) |
| Age at diagnosis (years) | ||||||||
| <50 | 712 | (16.5) | 97 | (34.8) | 257 | (12.7) | 358 | (17.8) |
| 50–59 | 1,015 | (23.5) | 60 | (21.5) | 509 | (25.1) | 446 | (22.2) |
| 60–69 | 1,241 | (28.7) | 53 | (19.0) | 603 | (29.7) | 585 | (29.1) |
| 70+ | 1,349 | (31.3) | 69 | (24.7) | 660 | (32.5) | 620 | (30.9) |
| Mean age at diagnosis | 62.3 | years | 56.4 | years | 63.4 | years | 61.9 | years |
| Stage at diagnosis | ||||||||
| Localized | 636 | (14.7) | 104 | (37.3) | 208 | (10.3) | 324 | (16.1) |
| Regional spread | 2,626 | (60.8) | 149 | (53.4) | 1,336 | (65.8) | 1,141 | (56.8) |
| Distant metastases | 1,055 | (24.5) | 26 | (9.3) | 485 | (23.9) | 544 | (27.1) |
| Vital status (end of follow-up) | ||||||||
| Alive | 787 | (18.2) | 153 | (54.8) | 372 | (18.3) | 262 | (13.0) |
| Emigrated | 6 | (0.2) | 0 | (0.0) | 4 | (0.2) | 2 | (0.1) |
| Dead | 3,524 | (81.6) | 126 | (45.2) | 1,653 | (81.5) | 1,745 | (86.9) |
| Survival time (years)* | ||||||||
| <1 | 908 | (25.8) | 28 | (22.2) | 447 | (27.0) | 433 | (24.8) |
| 1–4 | 1,657 | (47.0) | 45 | (35.7) | 885 | (53.5) | 727 | (41.7) |
| 5–9 | 540 | (15.3) | 35 | (27.8) | 252 | (15.3) | 253 | (14.5) |
| 10+ | 419 | (11.9) | 18 | (14.3) | 69 | (4.2) | 332 | (19.0) |
Based on 3,524 women with ovarian serous carcinoma who died during follow-up
Women diagnosed with ovarian serous carcinoma during 1978–2006
The 5- and 10-year survival rates were 69% and 52% in women with localized disease; 31% and 19% in women with regional spread; and 25% and 19% in women with distant metastases. Furthermore, 5- and 10-year survival rates were 69% and 56% for LGSC; 38% and 28% for unknown grade; and 28% and 15% for HGSC. We found similar patterns for women diagnosed in the two time periods 1978–1996 and 1997–2006, respectively (data not shown).
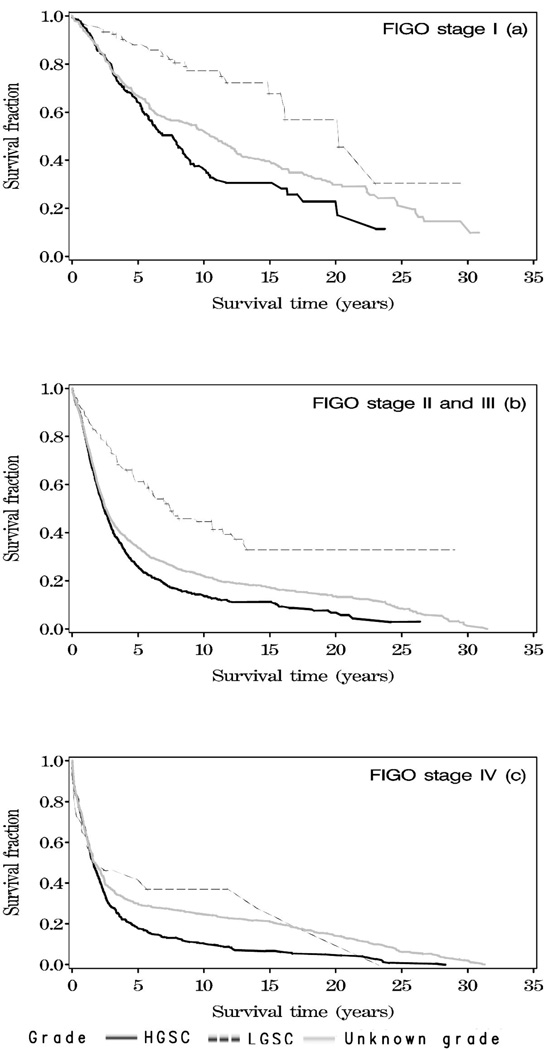
The survival of women with LGSC, unknown grade and HGSC in relation to stage at diagnosis is shown in Figure 1a–c. Women with LGSC had a significantly better overall survival compared with women with unknown grade and HGSC within each stage of disease (p<0.0001).
Figure 1.
a–c. Survival of 4,317 women with ovarian serous carcinoma in Denmark 1978–2006 by histologic grade at the time of diagnosis (low-grade serous carcinomas (LGSC), high-grade serous carcinomas (HGSC) and unknown grade).
We also analyzed the effect of grade on survival after adjusting for age and stage in a multivariate Cox model (Table 2) and found that women with regional spread (HR=2.5; 95% CI: 2.2–3.8) and distant metastases (HR=5.0; 95% CI: 4.4–5.6), respectively, had a significantly increased risk of dying compared with women with localized disease while adjusting for age and histologic grade. In addition, women with HGSC (HR=1.9; 95% CI: 1.6–2.3) had a significantly increased risk of dying compared with women with LGSC while adjusting for age and stage. We found the same results when classifying stage using localized disease (including FIGO stage I), regional spread (including FIGO stage II) and distant metastases (including FIGO stage III and IV) (data not shown). In addition, similar results were reached when repeating the analyses for women with FIGO stages I, II, III and IV instead of using extent of disease in three groups (localized disease (including FIGO stage I), regional spread (including FIGO stage II and III) and distant metastases (including FIGO stage IV)) (data not shown). Finally, similar findings were seen for women diagnosed in the two time periods 1978–1996 and 1997–2006, respectively, the estimates being slightly stronger for women diagnosed in 1997–2006 (data not shown).
Table 2.
Survival analysis using Cox proportional hazards regression model of 4,317 women diagnosed with ovarian serous carcinoma in Denmark 1978–2006
| No. of women (n=4,317) |
No. of deaths (n=3,524) |
Age-adjusted | Mutually adjusted* | |||||
|---|---|---|---|---|---|---|---|---|
| n | n | HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| Age at diagnosis (years) | (continuous - per year) | 1.04 | (1.04–1.04) | <0.0001 | ||||
| Stage at diagnosis | ||||||||
| Localized | 636 | 364 | 1.0 | - | - | 1.0 | - | - |
| Regional spread | 2,626 | 2,178 | 2.6 | (2.3–2.9) | <0.0001 | 2.5 | (2.2–3.8) | <0.0001 |
| Distant metastases | 1,055 | 982 | 5.3 | (4.7–6.0) | <0.0001 | 5.0 | (4.4–5.6) | <0.0001 |
| Histologic grade | ||||||||
| LGSC | 279 | 126 | 1.0 | - | - | 1.0 | - | - |
| Unknown | 2,009 | 1,745 | 2.6 | (2.2–3.2) | <0.0001 | 2.1 | (1.8–2.5) | <0.0001 |
| HGSC | 2,029 | 1,653 | 2.5 | (2.1–3.0) | <0.0001 | 1.9 | (1.6–2.3) | <0.0001 |
Adjusted for age and stage at diagnosis and histologic grade
Women with well-differentiated serous carcinoma during 1997–2006
We identified 179 women with a diagnosis of well-differentiated serous carcinoma during 1997–2006 which were reviewed by two expert gynecologic pathologists (R.J.K and R.V). Eight women were excluded due to missing slides. At the expert review of the remaining 171 women, 51 women had LGSC (29.8%), 21 had HGSC (12.3%), 86 had SBT (50.3%), 12 women had another type of tumor (7.0%) (clear cell carcinoma (n=7), endometrioid carcinoma (n=1), unclassified high-grade adenocarcinoma (n=1), suspected primary peritoneal tumor (n=1), borderline endometrioid tumor (n=1) and endometrioma (n=1)), and 1 woman had insufficient tissue (0.6%).
Among the 158 women with well-differentiated serous carcinoma and an expert review diagnosis of LGSC, HSCG and SBT, 63 women had localized disease (39.9%), 84 had regional spread (53.1%) and 11 women had distant metastases (7.0%). The 5- and 10-year survival rates were 91% and 83% for women with localized disease; 69% and 56% for women with regional spread; and 41% and 41% for women with distant metastases. In addition, 5- and 10-year survival rates were 69% and 48% for women with LGSC; 49% and 16% for HGSC; and 87% and 85% for SBT.
Table 3 shows that women with regional spread (HR=2.8; 95% CI: 1.3–6.0) and distant metastases (HR=3.4; 95% CI: 1.1–10.1), respectively, had a significantly increased risk of dying compared with women with localized disease while adjusting for age and review diagnosis. In addition, women with a review diagnosis of SBT (HR=0.5; 95% CI: 0.22–0.99) had a significantly lower risk of dying, and women with a review diagnosis of HGSC (HR=1.6; 95% CI: 0.74–3.4) had a non-significantly increased risk of dying compared with women with a confirmed review diagnosis of LGSC. Similar results were found while repeating the analyses using localized disease (including FIGO stage I), regional spread (including FIGO stage II) and distant metastases (including FIGO stage III and IV) and the FIGO classification instead of stage in the three groups (data not shown).
Table 3.
Survival analysis using Cox proportional hazards regression model of 158 women with a diagnosis of well-differentiated serous carcinoma in Denmark 1997–2006
| No. of women (n=161) |
No. of deaths (n=49) |
Age-adjusted | Mutually adjusted* | |||||
|---|---|---|---|---|---|---|---|---|
| n | n | HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| Age at diagnosis (years) | (continuous - per year) | 1.05 | (1.03–1.08) | <0.0001 | ||||
| Stage at diagnosis | ||||||||
| Localized | 63 | 9 | 1.0 | - | - | 1.0 | - | - |
| Regional spread | 84 | 33 | 3.3 | (1.6–6.9) | 0.0016 | 2.8 | (1.3–6.0) | 0.0075 |
| Distant metastases | 11 | 6 | 4.0 | (1.4–11.4) | 0.0096 | 3.4 | (1.1–10.1) | 0.0300 |
| Review diagnosis | ||||||||
| SBT | 86 | 13 | 0.4 | (0.19–0.80) | 0.0108 | 0.5 | (0.22–0.99) | 0.0460 |
| LGSC | 51 | 22 | 1.0 | - | - | 1.0 | - | - |
| HGSC | 21 | 13 | 1.6 | (0.79–3.2) | 0.1912 | 1.6 | (0.74–3.4) | 0.2418 |
Adjusted for age and stage at diagnosis and review diagnosis
Discussion
FIGO staging and the amount of residual tumor following cytoreductive surgery are the most important prognostic factors in ovarian serous carcinoma [8,9]. The importance of histologic grade has, however, been questioned [26]. In the present study, we found that histologic grade routinely made by Danish gynecologic pathologists or expert gynecologic pathologists was a significant prognostic factor in ovarian carcinoma as women with LGSC had a significantly longer survival than women with HGSC even after adjusting for age and stage.
Our 69% 5-year survival rate for LGSC is longer than the 56% reported by Seidman et al. [7] and the 40% reported by Malpica et al. [26] but this may be explained by the fact that 50% of women with an original diagnosis of LGSC in 1997–2006 in our study were judged as SBT after expert review. Accordingly, it is likely that this also occurred in 1978–1996. However, our 10-year survival rate of 56% for women with LGSC is lower as compared with the 71% reported by Motohara et al. [4]. Furthermore, our 5-year survival rate for HGSC of 49% is higher than the 34% reported by Seidman et al. [7] and the 9% rate reported by Malpica et al. [26]. In addition, our 10-year survival rate of 16% for HGSC is similar to the 13% reported by Motohara et al. [4]. Finally, Bodurka et al. [32] found 3-year survival rates of 90.5% and 67.6%, respectively, for LGSC and HGSC.
Eighty eight percent of women in our study had HGSC whereas only 12% had LGSC. This is in line with the other studies [4,7,32,33] examining survival of ovarian serous carcinomas using the 2-tier system [26]. Women with LGSC in our study were younger at diagnosis (mean=56 years) than women with HGSC (mean=63 years). Similar results were found in the studies by Malpica et al. [26], Bodurka et al. [32] and Seidman et al. [7] for women with LGSC (42, 51 and 57 years, respectively) and HGSC (55, 57 and 65 years, respectively). In addtion, Gershenson et al. [31] found a mean age of 45 years for women with LGSC. Thus, LGSC seem to occur in women around 6–13 years earlier than HGSC.
In the present study, we were able to perform histologic slide review by expert gynecologic pathologists for all tumors with an original diagnosis of well-differentiated carcinoma in 1997–2006. Based on this review, a large proportion of these women were judged as having SBT (50%) or HGSC (12%). It was therefore not surprising, that women with well-differentiated carcinoma that on expert review were classified as SBT had a significantly longer survival than women in whom the original diagnosis was well-differentiated carcinoma and confirmed by the expert review as LGSC. It should be mentioned that in the majority (65%) of well-differentiated carcinomas that were reclassified as SBT, the pathology description revealed that the Danish routine pathologists had been in doubt as to whether the tumor was SBT or well-differentiated carcinoma; however, it was not reflected in the final diagnosis. Thus, expert slide review further confirms the utility of the binary grading system.
Our study has several strengths. Data were collected from a population-based, nationwide registry in Denmark covering a period of nearly 30 years. We included 4,317 women of which 2,308 had LGSC or HGSC which is by far the largest study examining survival of serous carcinoma using the 2-tier grading system [26]. Previous studies have all been limited by relatively small number of cases (44–241 cases) [4,7,26,32,33]. In the present study, we took advantage of the existence of the PIN, which insures accurate linkage between health registries and indicates that it is possible to do follow-up studies with virtually no loss to follow-up. In addition, the use of nationwide registries reduces the risk of patient referral bias that may occur in case-control studies. However, our study also has some limitations. Since our nationwide study was based on pathology reports and pathologic slide review, we did not have information on clinical factors such as residual disease which is also an important independent prognostic factor in ovarian serous carcinoma [8,26]. In addtion, we were not able to calculate disease-specific survival. However, since ovarian cancer-related deaths are high among women with this disease [39], we may assume that most women who died during follow-up in our study were ovarian cancer-related deaths. Furthermore, we found that the survival for reviewed LGSC and HGSC was not significantly different; however, this analysis might be limited by having too few cases of HGSC. In addition, we had a large number of tumors with unknown grade in the first half of our study period (1978–1996). However, we observed the same trends in survival in the latter study period (1997–2006) where we had fewer cases with unknown grade. Finally, we were unable to perform expert review of well-differentiated serous carcinomas diagnosed in the first half of the study period 1978–1996 because a lot of the histologic material from that period is no longer available. In addtion, the Pathology Data Bank is not complete before 1997. Thus, the survival of LGSC in the first half of the study period may be slightly overestimated as they likely contain a number of SBT which was the case for the period 1997–2006. However, we found similar trends in survival of ovarian serous carcinomas regardless of whether the histologic diagnosis was routinely made by the Danish pathologists or an expert gynecologic pathologist review was performed. Thus, the review by our expert gynecologic pathologists further added information to our results found by the routine pathologists, but the findings were similar. In addition, we did not perform expert review of the 1,425 cases with HGSC in 1997–2006, but we do not believe that the extent of misclassification that was found in LGSC exists among HGSC since the differentiation between LGSC and SBT is the most complicated differentiation.
In conclusion, we have examined a binary histologic grading system as a prognostic factor in ovarian serous carcinoma based on a large cohort in Denmark over nearly 30 years, and our findings suggest that histologic grade (low-grade versus high-grade), based on the diagnosis of either Danish gynecologic pathologists in the everyday clinic or expert gynecologic pathologists, is a significant independent predictor of survival.
Highlights.
-
▪
The histologic diagnosis based on a binary grading system is an independent predictor of survival following ovarian serous carcinoma.
-
▪
Expert gynecologic pathologists’ review of the histologic diagnosis further confirms the utility of the binary grading system.
Acknowledgements
We are grateful to Pernille Clausen and Peter Bo Aarslev for assistance with data management and analysis.
The study has been funded by the Danish Cancer Society (22806059) and the National Cancer Institute (NCI) (RO1 CA116184).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009 Sep 15;80(6):609–616. [PubMed] [Google Scholar]
- 3.Vergote I, De BJ, Fyles A, Bertelsen K, Einhorn N, Sevelda P, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001 Jan 20;357(9251):176–182. doi: 10.1016/S0140-6736(00)03590-X. [DOI] [PubMed] [Google Scholar]
- 4.Motohara T, Tashiro H, Miyahara Y, Sakaguchi I, Ohtake H, Katabuchi H. Long-term oncological outcomes of ovarian serous carcinomas with psammoma bodies: a novel insight into the molecular pathogenesis of ovarian epithelial carcinoma. Cancer Sci. 2010 Jun;101(6):1550–1556. doi: 10.1111/j.1349-7006.2010.01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidman JD, Kurman RJ. Pathology of ovarian carcinoma. Hematol Oncol Clin North Am. 2003 Aug;17(4):909–925. doi: 10.1016/s0889-8588(03)00061-3. vii. [DOI] [PubMed] [Google Scholar]
- 6.Gershenson DM. The heterogeneity of epithelial ovarian cancer: getting it right. Cancer. 2010 Mar 15;116(6):1400–1402. doi: 10.1002/cncr.24926. [DOI] [PubMed] [Google Scholar]
- 7.Seidman JD, Horkayne-Szakaly I, Cosin JA, Ryu HS, Haiba M, Boice CR, et al. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol. 2006 Nov;103(2):703–708. doi: 10.1016/j.ygyno.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Mayr D, Diebold J. Grading of ovarian carcinomas. Int J Gynecol Pathol. 2000 Oct;19(4):348–353. doi: 10.1097/00004347-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Blair AR, Casas CM. Gynecologic cancers. Prim Care. 2009 Mar;36(1):115–130. doi: 10.1016/j.pop.2008.10.001. ix. [DOI] [PubMed] [Google Scholar]
- 10.Brugghe J, Baak JP, Wiltshaw E, Brinkhuis M, Meijer GA, Fisher C. Quantitative prognostic features in FIGO I ovarian cancer patients without postoperative treatment. Gynecol Oncol. 1998 Jan;68(1):47–53. doi: 10.1006/gyno.1997.4884. [DOI] [PubMed] [Google Scholar]
- 11.Bertelsen K, Holund B, Andersen E. Reproducibility and prognostic value of histologic type and grade in early epithelial ovarian cancer. Int J Gynecol Cancer. 1993 Mar;3(2):72–79. doi: 10.1046/j.1525-1438.1993.03020072.x. [DOI] [PubMed] [Google Scholar]
- 12.Sevelda P, Vavra N, Schemper M, Salzer H. Prognostic factors for survival in stage I epithelial ovarian carcinoma. Cancer. 1990 May 15;65(10):2349–2352. doi: 10.1002/1097-0142(19900515)65:10<2349::aid-cncr2820651031>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Young RC, Walton LA, Ellenberg SS, Homesley HD, Wilbanks GD, Decker DG, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med. 1990 Apr 12;322(15):1021–1027. doi: 10.1056/NEJM199004123221501. [DOI] [PubMed] [Google Scholar]
- 14.Bertelsen K, Holund B, Andersen JE, Nielsen K, Stroyer I, Ladehoff P. Prognostic factors and adjuvant treatment in early epithelial ovarian cancer. Int J Gynecol Cancer. 1993 Jul;3(4):211–218. doi: 10.1046/j.1525-1438.1993.03040211.x. [DOI] [PubMed] [Google Scholar]
- 15.Doyle EM, Foley M, Kelehan P, Mooney EE. Histological grading of epithelial ovarian carcinomas. J Obstet Gynaecol. 2007 Jan;27(1):71–74. doi: 10.1080/01443610601056434. [DOI] [PubMed] [Google Scholar]
- 16.Ishioka S, Sagae S, Terasawa K, Sugimura M, Nishioka Y, Tsukada K, et al. Comparison of the usefulness between a new universal grading system for epithelial ovarian cancer and the FIGO grading system. Gynecol Oncol. 2003 Jun;89(3):447–452. doi: 10.1016/s0090-8258(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 17.Sato Y, Shimamoto T, Amada S, Asada Y, Hayashi T. Prognostic value of histologic grading of ovarian carcinomas. Int J Gynecol Pathol. 2003 Jan;22(1):52–56. doi: 10.1097/00004347-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Trope C, Kaern J, Hogberg T, Abeler V, Hagen B, Kristensen G, et al. Randomized study on adjuvant chemotherapy in stage I high-risk ovarian cancer with evaluation of DNA-ploidy as prognostic instrument. Ann Oncol. 2000 Mar;11(3):281–288. doi: 10.1023/a:1008399414923. [DOI] [PubMed] [Google Scholar]
- 19.Sjovall K, Nilsson B, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994 Sep;4(5):333–336. doi: 10.1046/j.1525-1438.1994.04050333.x. [DOI] [PubMed] [Google Scholar]
- 20.Reles AE, Gee C, Schellschmidt I, Schmider A, Unger M, Friedmann W, et al. Prognostic significance of DNA content and S-phase fraction in epithelial ovarian carcinomas analyzed by image cytometry. Gynecol Oncol. 1998 Oct;71(1):3–13. doi: 10.1006/gyno.1998.5156. [DOI] [PubMed] [Google Scholar]
- 21.Eltabbakh GH, Belinson JL, Kennedy AW, Biscotti CV, Casey G, Tubbs RR, et al. p53 overexpression is not an independent prognostic factor for patients with primary ovarian epithelial cancer. Cancer. 1997 Sep 1;80(5):892–898. [PubMed] [Google Scholar]
- 22.Finn CB, Dunn J, Buxton EJ, Luesley DM, Shafi M. Can we predict a high risk group in stage I epithelial ovarian cancer? Int J Gynecol Cancer. 1993 Jul;3(4):226–230. doi: 10.1046/j.1525-1438.1993.03040226.x. [DOI] [PubMed] [Google Scholar]
- 23.Carey MS, Dembo AJ, Simm JE, Fyles AW, Treger T, Bush RS. Testing the validity of a prognostic classification in patients with surgically optimal ovarian carcinoma: a 15-year review. Int J Gynecol Cancer. 1993 Jan;3(1):24–35. doi: 10.1046/j.1525-1438.1993.03010024.x. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu Y, Kamoi S, Amada S, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998 Mar 1;82(5):893–901. doi: 10.1002/(sici)1097-0142(19980301)82:5<893::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu Y, Kamoi S, Amada S, Hasumi K, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma. I. Prognostic significance of histopathologic features--problems involved in the architectural grading system. Gynecol Oncol. 1998 Jul;70(1):2–12. doi: 10.1006/gyno.1998.5051. [DOI] [PubMed] [Google Scholar]
- 26.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004 Apr;28(4):496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Vang R, Shih I, Salani R, Sugar E, Ayhan A, Kurman RJ. Subdividing ovarian and peritoneal serous carcinoma into moderately differentiated and poorly differentiated does not have biologic validity based on molecular genetic and in vitro drug resistance data. Am J Surg Pathol. 2008 Nov;32(11):1667–1674. doi: 10.1097/PAS.0b013e31816fd555. [DOI] [PubMed] [Google Scholar]
- 28.Vang R, Shih I, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009 Sep;16(5):267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih I, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004 May;164(5):1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurman RJ, Shih I. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer-Shifting the paradigm. Hum Pathol. 2011 Jul;42(7):918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershenson DM, Sun CC, Lu KH, Coleman RL, Sood AK, Malpica A, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006 Aug;108(2):361–368. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 32.Bodurka DC, Deavers MT, Tian C, Sun CC, Malpica A, Coleman RL, et al. Reclassification of serous ovarian carcinoma by a 2-tier system: A Gynecologic Oncology Group Study. Cancer. 2011 Nov 9; doi: 10.1002/cncr.26618. [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobel M, Kalloger SE, Santos JL, Huntsman DG, Gilks CB, Swenerton KD. Tumor type and substage predict survival in stage I and II ovarian carcinoma: insights and implications. Gynecol Oncol. 2010 Jan;116(1):50–56. doi: 10.1016/j.ygyno.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 34.The Danish Pathology Data Bank . available in 2011 at http://www.patobank.dk.
- 35.Kjaerbye-Thygesen A, Huusom LD, Frederiksen K, Kjaer SK. [Primary ovarian cancer. A comparison of registrations in the Danish Cancer Registry and the Pathology Data Bank] Ugeskr Laeger. 2007 Jan 1;169(1):50–54. [PubMed] [Google Scholar]
- 36.The Danish National Board of Health. available in 2011 at http://www.sst.dk.
- 37.The Danish Cancer Registry. available in 2011 at http://www.sst.dk.
- 38.The Danish Civil Registration System. available in 2011 at http://www.cpr.dk.
- 39.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–437. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]