Abstract
Sublingual (SL) delivery, a non-invasive immunization method that bypasses the intestinal tract for direct entry into the circulation, was evaluated with an adenovirus (Ad5)-based vaccine for Ebola. Mice and Guinea pigs were immunized via the intramuscular (IM), nasal (IN), oral (PO) and SL routes. SL immunization elicited strong transgene expression in and attracted CD11c(+) antigen presenting cells to the mucosa. A SL dose of 1 × 108 infectious particles induced Ebola Zaire glycoprotein (ZGP)-specific IFN-γ+ T cells in spleen, bronchoalveolar lavage, mesenteric lymph nodes and submandibular lymph nodes (SMLN) of naïve mice in a manner similar to the same dose given IN. Ex vivo CFSE and in vivo cytotoxic T lymphocyte (CTL) assays confirmed that SL immunization elicits a notable population of effector memory CD8+ T cells and strong CTL responses in spleen and SMLN. SL immunization induced significant ZGP-specific Th1 and Th2 type responses unaffected by pre-existing immunity (PEI) that protected mice and Guinea pigs from lethal challenge. SL delivery protected more mice with PEI to Ad5 than IM injection. SL immunization also reduced systemic anti-Ad5 T and B cell responses in naïve mice and those with PEI, suggesting that secondary immunizations could be highly effective for both populations.
Keywords: adenovirus 5, Ebola Zaire, sublingual, vaccine, mouse, Guinea pig, pre-existing immunity, CD4 T cell, memory response, toxicity
Introduction
Ebola hemorrhagic fever is a fatal disease in humans and non-human primates caused by Ebola, a single stranded RNA virus of the Filoviridae family. Four of the five species of Ebola are infectious to humans, with Zaire and Sudan having fatality rates of ~90 and 55% respectively 1. Although Bundigbugyo, first identified in 2007, has the lowest reported fatality rate (25%), only a single, non-lethal infection in an individual working on an infected chimpanzee can be attributed to Cote d’Ivoire 2, 3. Ebola-Reston has primarily caused disease in non-human primates and pigs, with IgG antibodies detected in the absence of clinical infection in individuals working close to sick animals in the Philippines 4. Clinical symptoms develop within 2–21 days after exposure. Initial, non-specific, flu-like symptoms (malaise, chills, fever) rapidly progress to severe nausea, diarrhea, shortness of breath, hypotension, bleeding and coma 5. Because there are currently no licensed vaccines or medicinal agents available for preventing or managing Ebola, supportive measures to maintain blood volume and electrolyte balance are the only therapeutic options for infected patients 6.
The scarcity of medicinal remedies, unpredictability of outbreaks and its possible use as a bioweapon highlight the necessity for an effective immunization strategy for prevention of Ebola infection and limiting its spread once it is recognized. Current vaccine platforms employ recombinant vector systems to deliver genetic sequences for Ebola proteins. Although plasmids have had limited success alone, they have been effective when given with recombinant adenoviruses in prime-boost regimens 7. Virus-like particles that contain key Ebola surface proteins within their capsids require several doses to confer protective immunity 8. While adenovirus and human parainfluenza virus type 3 (HIPV3) vectors confer protection after one dose, they are common pathogens and may be ineffective in those who have been exposed to these viruses by natural means 9. Vesicular stomatitis virus (VSV) and adenoviral vectors have been developed to protect against several species of Ebola with a single dose in both pre- and post-exposure scenarios 10, 11. The VSV platform, much like HIPV3, utilizes replication competent virus, which may pose a significant risk for certain patients 5. Until recently, these vaccines were given only by direct injection.
In this report, we assess the utility of the sublingual mucosa as a site for immunization against Ebola using a recombinant adenovirus-based vaccine. In humans, the sublingual mucosa consists of immobile smooth muscle that supports 40–50 layers of actively dividing squamous, non-keratinized cells, providing a large surface area for antigen delivery 12. Cell turnover is relatively slow (4–14 days), allowing for sustained release of antigen 13. Below the epithelial layer is a dense vascular network, allowing the antigen to bypass the harsh environment of the gastrointestinal tract and directly enter the systemic circulation. Antigen presenting cells (APCs) and T lymphocytes also reside within the mucosa, with direct access to mucosa-associated lymphoid tissues 14. These studies were designed to evaluate a mucosal immunization strategy, largely unexplored with adenovirus-based vaccines, and to address two issues hindering the clinical development of this vaccine platform: a) limited potency and b) side effects in those with pre-existing immunity (PEI). Adenovirus serotype 5 infects humans frequently, making PEI a global phenomenon 15. We first describe adenovirus transduction efficiency in the SL mucosa and its ability to recruit APCs during immunization. Systemic and mucosal T and B cell responses to Ebola Zaire glycoprotein after SL immunization were then characterized in naive mice and those with PEI. Survival rates of mice and Guinea pigs after challenge were then compared to animals immunized by IM injection and the oral and nasal routes.
Experimental Section
Adenovirus Production
The E1/E3-deleted adenovirus serotype 5 vector containing the codon optimized full-length Ebola Zaire glycoprotein sequence under the control of the chicken-β-actin promoter (Ad-CAGoptZGP) was amplified in HEK 293 cells (ATCC CRL-1573) and purified according to established methods 16. The number of virus particles present in each preparation was determined by measuring the optical density at 260 nm. Infectious titer was determined by serial dilution of each preparation, infection of 293 cells and immunodetection of the hexon protein using the Adeno-X Rapid Titer Kit (Clontech, Mountain View, CA) according to the manufacturer’s instructions. Preparations with infectious to physical particle ratios < 1:200 were used in this study.
Animal Studies
All procedures were approved by Institutional Animal Care and Use Committees at The University of Texas at Austin and The University of Texas Medical Branch (UTMB) in Galveston and are in accordance with the guidelines established by the National Institutes of Health for the humane treatment of animals.
Immunization
Six-week-old male B10.Br mice (MHC H-2k) were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in a temperature-controlled, light-cycled facility at the Animal Research Center of The University of Texas at Austin with free access to standard rodent chow (Harlan Teklad, Indianapolis, IN) and tap water. Hartley Guinea pigs (male, 200g) were purchased from Charles River Laboratories (Raleigh, NC) and housed under the same conditions. Animals were anesthetized by a single intra-peritoneal injection of a 3.9:1 (v:v) mixture of ketamine (100 mg/ml, Fort Dodge, Animal Health, Fort Dodge, IA) and xylazine (100 mg/ml, Sigma Aldrich, St. Louis, MO). Once deep plane anesthesia was achieved, mice were immunized with 1×108 and Guinea pigs 1×109 infectious particles of Ad-CAGoptZGP regardless of immunization route. IM injections were divided between each gastrocnemius muscle (50 µl/muscle mouse, 100 µl/muscle Guinea pig) located on the hind limb. IN immunization (mice) was performed by dripping 10 µl of the preparation in each nostril with a micropipette (Gilson, Middleton, WI). For SL immunization, sterile forceps were placed under the tongue of the animal and 10 µl (mouse)/40 µl (Guinea pig) slowly dispensed onto the exposed area with a micropipette. Animals immunized by the IN and SL routes were maintained in an upright position for 30 minutes after treatment to minimize accidental swallowing of the vaccine. Mice were immunized orally with tuberculin syringes attached to feeding needles (18G, Popper & Sons, Inc, New Hyde Park, NY).
Establishment of Pre-Existing Immunity to Adenovirus
Pre-existing immunity was established in 3-week-old mice by injecting 2.5 × 1011 particles of first generation adenovirus expressing beta-galactosidase under the control of a CMV promoter (AdlacZ) in the muscle of each hind limb 30 days prior to vaccination. Blood was collected 24 days later from the saphenous vein and serum screened for anti-adenovirus neutralizing antibodies (NABs). The average anti-adenovirus NAB titer in mice prior to immunization was 1:165 ± 22.
Challenge with Ebola Zaire
All challenge experiments were performed under biosafety level 4 (BSL-4) conditions in an AAALAC accredited animal facility at the Robert E. Shope BSL-4 Laboratory at UTMB in Galveston, Texas. Twenty-one days post-immunization, mice were transferred to UTMB where they were challenged on day 28 by intraperitoneal injection with 1,000 pfu of either mouse- (MA-ZEBOV) or Guinea pig- (GP-ZEBOV) adapted Ebola Zaire. Animals were monitored for clinical signs of disease and weighed daily for 14 days. At the end of the study, survivors were bled and serum γ-irradiated (5 mrad) prior to removal from the BSL-4 lab for analysis.
Localization and Characterization of Transduced Cells in the Sublingual Epithelium
Sublingual tissues were harvested and immersed in disposable peel-away molds containing Tissue-Tek® O.C.T compound (Sakura Finetek, Torrance, CA) and stored at −80 °C prior to analysis. Sections were fixed and stained for beta-galactosidase 17. APCs were identified near the site of immunization with rat anti-mouse MHC II, rat anti-mouse CD11b or hamster anti-mouse CD11c antibodies (Abcam, Cambridge, MA) and with horseradish-peroxidase (HRP)-conjugated IgG antibodies (Abcam), developed with 3-amino-9-ethylcarbazole and H2O2 substrate buffer (Sigma) and counterstained with hematoxylin (Sigma). Sections were examined with a Lecia DM LB microscope (Leica Microsystems Inc., Buffalo Grove, IL) and photographed using a Leica DFC 320 camera.
The T Cell Response
Intracellular Cytokine Staining
Splenocytes were isolated by grinding tissue through strainers (BD Falcon, Franklin Lakes, NJ) into sterile 50 ml conical tubes containing Leibovitz's L-15 Medium (Mediatech, Inc., Herndon, VA). Cells were pelleted by centrifugation and red blood cells removed by resuspending the pellet in ACK lysis buffer (Quality Biological, Inc., Gaithersburg, MD). Cells were washed and the concentration adjusted to 2×106 cells/well in complete Dulbecco’s Modified Eagle′s Medium (DMEM, Mediatech) containing 50 µM beta-mercaptoethanol (Sigma), penicillin (10,000 I.U./ml)/streptomycin (10,000 µg/ml) (Gibco, Invitrogen, Grand Island, NY), L-glutamine (1 mM, Hyclone, Salt Lake City, UT), mouse interleukin-2 (50 U/ml, R & D Systems, Minneapolis, MN), sodium pyruvate (1 mM, Lonza, Walkersville, MD), non-essential amino acids (1 mM, Lonza), and Brefeldin A (1 µg/ml, Sigma). Cells were cultured for 5 hours at 37°C in 5% CO2 with TELRTFSI peptide (5 µg/ml, New England Peptide, Gardner, MA) that carries the Ebola Zaire glycoprotein immunodominant MHC class I epitope for mice with the H-2k haplotype (B10.Br) 18. Cells stimulated with an irrelevant peptide, containing a binding sequence for influenza hemagglutinin (YPYDVPDYA, 5 µg/ml, GenScript, Piscataway, NJ) served as negative controls. Samples were incubated with PerCP-Cy5.5-labeled anti-CD3ε and fluorescein isothiocyanate (FITC)-labeled CD8α antibodies (BD Pharmingen, San Diego, CA) for 30 minutes at 4 °C. Cells were then fixed and permeabilized with Cyto-fix/Cytoperm (BD Pharmingen) for 20 minutes at 4 °C. For intracellular cytokine staining, cells were washed and stained with phycoerytherin (PE)-labeled anti-mouse IFN-γ antibody (BD Pharmingen) for 30 minutes at 4 °C. Positive cells were counted using three-color flow cytometry (FACS Calibur, BD Biosciences, Palo Alto, CA). Over 500,000 events were captured per sample. Data were analyzed by FCS Express (Version 3, De Novo Software, Los Angeles, CA). A response was considered to be positive when the frequency of IFN-γ+ CD8+ T cells in samples stimulated with the relevant peptide was more than 5 times that obtained from cells stimulated with the irrelevant peptide.
ELISPOT
ELISPOT assays were performed using the ELISPOT Mouse Set (BD Pharmingen). ELISPOT plates were pre-coated with anti-mouse IFN-γ capture antibody overnight at 4 °C and blocked for 2 hours at room temperature. Cells from the spleen, bronchoalveolar lavage fluid, MLNs and SMLNs in complete DMEM were added to each well (5×105 cells/well) with TELRTFSI peptide. Negative control cells were incubated with an irrelevant peptide (YPYDVPDYA). Plates were placed at 37°C for 20 hours, washed and incubated with biotinylated anti-mouse IFNγ antibody for 2 hours at room temperature. Plates were then washed, incubated with horseradish peroxidase (HRP)-conjugated streptavidin antibody for an additional hour and developed with AEC substrate (Sigma). Spots were counted using an automated ELISPOT reader (CTL-ImmunoSpot® S5 Micro Analyzer, Cellular Technology Ltd., Shaker Heights, OH).
CFSE Assay
Splenocytes were isolated 42 days post-vaccination and stained using the Vybrant CFDA SE Cell Tracer kit (Invitrogen, Carlsbad, CA). Mononuclear cells were extensively washed and then stained with 5 µM of CFDA SE for 10 minutes at 37 °C and 5% CO2. Cells were then cultured (5×106 cells/well) for 5 days at 37 °C in 5% CO2 with TELRTFSI peptide. Cell surface markers were identified with a cocktail of antibodies (perCPCy5.5 labeled anti-CD8, PE labeled anti-CD44, and allophycocyanin (APC) labeled–anti-CD62L, BD Pharmingen). For evaluation of adenovirus serotype 5-specific memory CD4 T cell proliferation, CFSE staining was performed as described except that 5×106 CFSE labeled mononuclear cells were incubated with 5×1010 particles of a recombinant adenovirus serotype 5 that does not contain a transgene cassette (AdNull) 17 for 5 days at 37 °C. These cells were then stained with PE-anti-CD3ε and PerCP-Cy5.5-CD8α antibodies (1:150, BD Pharmingen) for 30 minutes at 4 °C and analyzed by flow cytometry. Over 1,000,000 events were captured per sample.
In Vivo CTL
Splencoytes were isolated from naïve mice and divided in half. According to established methods 19, 20, half were stained with 5 µM CFSE (CFSEHI), the other with 0.5 µM (CFSELOW). CFSEHI cells were pulsed with 5 µM TELRTFSI peptide. CFSELOW cells were not stimulated. CFSEHI and CFSELOW cells were mixed (1:1) and 2×107 cells given to naïve and immunized mice via tail vein injection. Twenty-four hours later, cells from the spleen and SMLNs were isolated. CFSE intensities from each population were assessed by flow cytometry. Lysis of target (CFSEHI) cells was calculated as: percent lysis = [1 – (ratio control mice/ratio vaccinated mice)] × 100 ; ratio = percent CFSELOW/percent CFSEHI
The B Cell Response
Full length Ebola Zaire glycoprotein was produced using the Zaire GP33–637ΔTM-HA construct and purified according to published methods 21. Plates were coated with purified glycoprotein (0.5 µg/well). Serum and BAL were serially diluted in two-fold increments with sterile PBS. Dilutions (100 µl) were added to plates prior to the addition of HRP-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgG2b, IgA and IgM (Southern Biotechnology Associates, Birmingham, AL) antibodies. Plates were developed with substrate (0.4 mg/ml o-phenylenediamine (Sigma)) and optical densities read at 450 nm. Endpoint titers are expressed as reciprocal log2 titers of the last dilution reading of 0.1 units above background according to the method of Frey et al. 22. Samples used to detect anti-adenovirus antibodies were diluted in the same manner and incubated with recombinant adenovirus for one hour and then used to infect HeLa cells 16. Dilutions that reduced transgene expression by 50% were calculated using the method of Reed and Muench 23. The absence of neutralization in samples containing medium only (negative control) or FBS (serum control) and an average titer of 1:1,280 ± 210 from an internal positive control stock serum were the criteria for qualification of each assay.
Serum Cytokines and Transaminases
Cytokines (IL-6, IL-12, TNF-α, IL-2 and IL-10) were quantitated with commercially available ELISA kits (Invitrogen, BioSource International, Camarillo CA). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were determined using Vitros AST/SGOT and ALT/SGPT DT slides on a Vitros DTSC autoanalyzer (Ortho-Clinical Diagnostics, Rochester, NY).
Statistical Analysis
Data were analyzed for statistical significance using SigmaStat (Systat Software Inc., San Jose, CA) by performing a one-way analysis of variance (ANOVA) between control and experimental groups, followed by a Bonferroni/Dunn post-hoc test when appropriate. Differences in the raw values among treatment groups were considered statistically significant when p<0.05.
Results
Immunohistochemical Analysis of Transgene Expression and Accumulation of Antigen Presenting Cells Induced by SL Administration of an Adenovirus-Based Vaccine
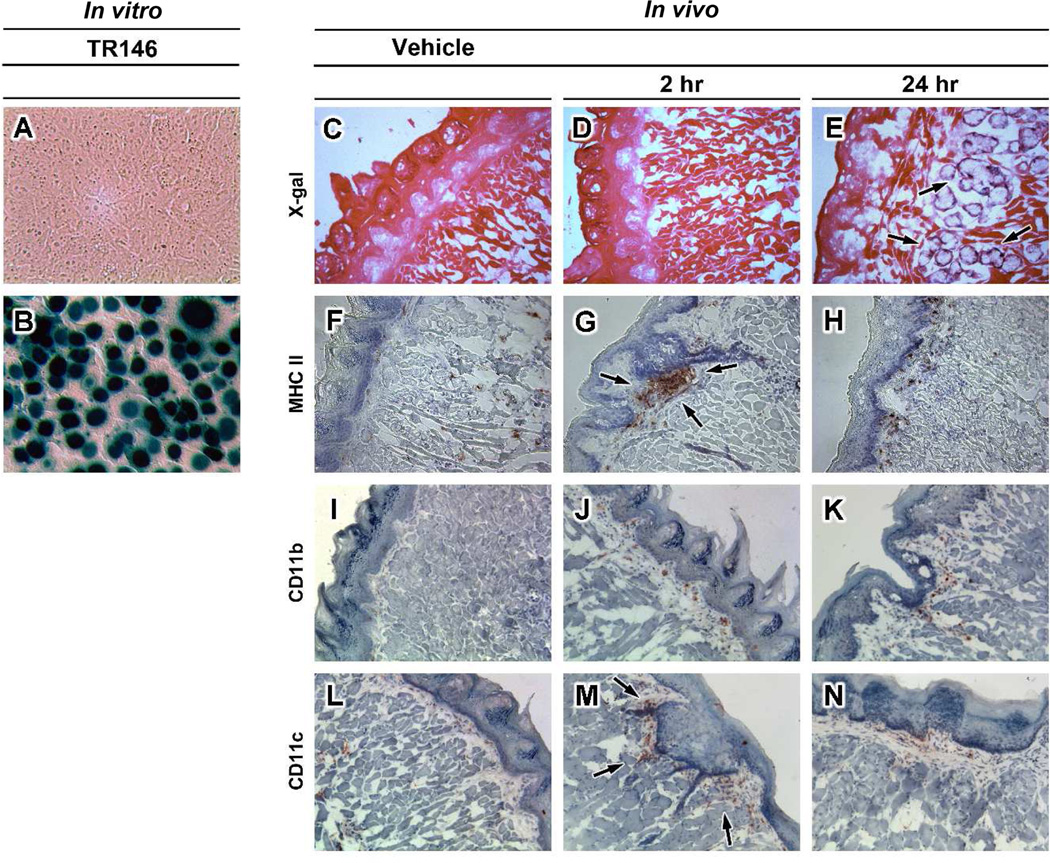
In order to evaluate the sublingual mucosa as a potential target for adenovirus-based vaccines, we first examined transgene expression in TR146 cells, an established model of human oral mucosa 24, and in mice. Adenovirus at a low concentration of 30 particles per cell easily infected human oral epithelial cells, with more than 90% of the monolayer expressing beta-galactosidase within 24 hours (Figure 1B). Transverse sections obtained mice given saline clearly illustrated the thick, keratinized, epithelial layers that cover the murine sublingual mucosa, the lamina propria and numerous fibroblasts (Figure 1C). Acinar cells in the lamina propria stained positive for beta-galactosidase 24 hours after treatment (black arrows, Figure 1E). Transgene expression was not detected in sections from animals given saline (Figure 1C) and those necropsied 2 hours after immunization (Figure 1D). MHC class II+ cells were widely disseminated in the epithelium and lamina propria of untreated animals (Figure 1F). Large clusters of these cells were noted within 2 hours after immunization at the delivery site (Figure 1G). Sparse amounts of CD11b (+) cells and dense areas of CD11c (+) cells were observed at the same timepoint (Figure 1J and 1M). Similar amounts of CD11b and CD11c (+) cells were noted in the epithelium 24 hours after treatment (Figure 1K and 1N).
Figure 1. Adenovirus Serotype 5 Efficiently Transduces TR146 Cells and the Murine Oral Mucosa after Sublingual Administration and Stimulates Migration of CD11c(+) and CD11b(+) Antigen Presenting Cells to the Delivery Site.
A) Uninfected TR146 cells. B) TR146 cells infected with first generation adenovirus 5 expressing beta-galactosidase (AdlacZ, moi 30) for 24 hours prior to histochemical staining for transgene expression. C) Representative longitudinal section of murine sublingual mucosa obtained from an animal 2 hours after administration of saline (Vehicle) stained for endogenous beta-galactosidase expression. D) Section of sublingual mucosa obtained 2 hours after sublingual administration of 1 × 108 infectious particles of AdlacZ stained for beta-galactosidase expression. E) Beta-galactosidase expression in submandibular glands 24 hours after treatment (black arrows). F) Cryosection from mouse given saline and stained for cells expressing MHC class II surface antigens (brown dots). G) Cryosection taken 2 hours after administration of adenovirus and stained for the presence of MHC class II+ cells. A significant number of cells were visualized at the delivery site (arrows). H) Spotted pattern of MHC class II+ cells in the oral mucosa 24 hours after sublingual administration of adenovirus. Additional staining revealed that cells positive for MHC II antigens were also positive for CD11b (Panels i–k) and CD11c surface molecules (Panels l–n). Magnification: Panels a–f, i and l 200×; Panel g, h, j, k, m, and n 100×.
Systemic and Mucosal T Cell Responses after SL Immunization of Naïve Mice and Those with PEI
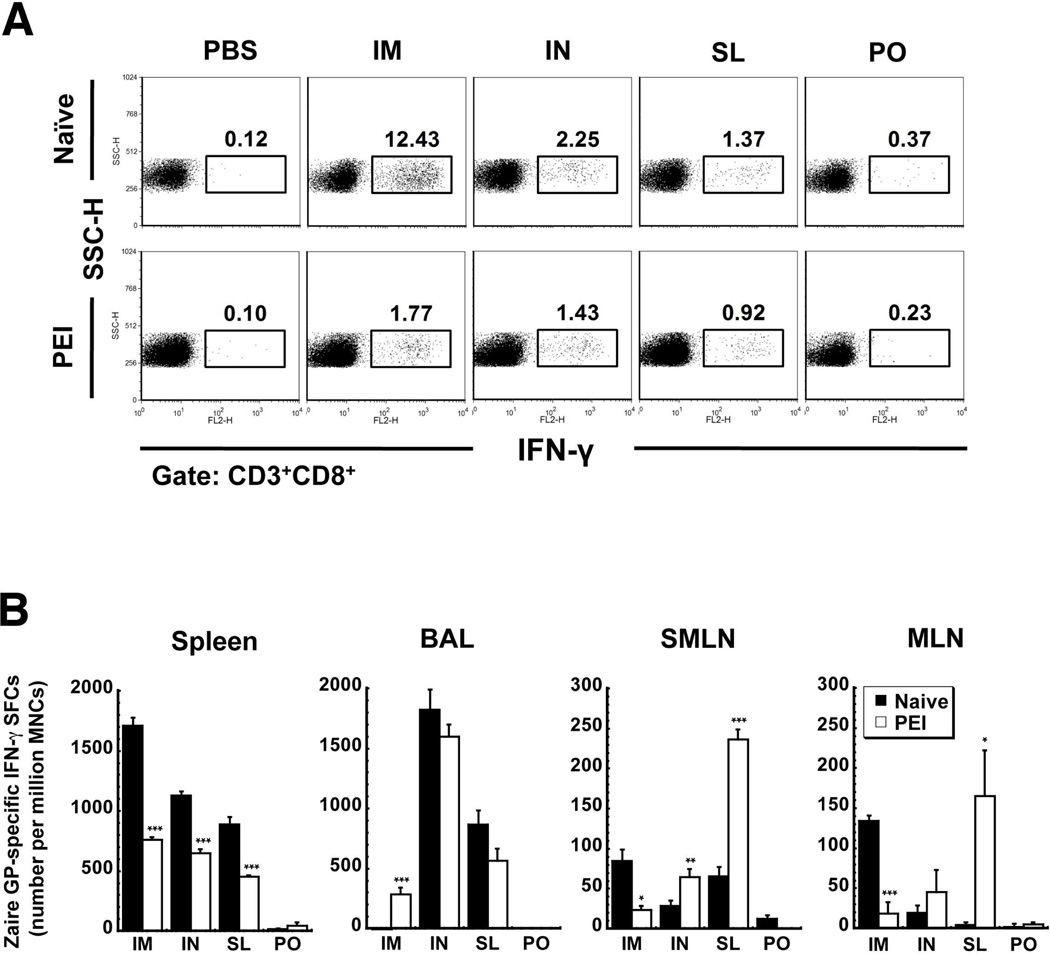
Ten days after administration of 1×108 infectious particles of the vaccine by the IM, IN, SL and PO routes, animals were sacrificed, splenocytes harvested and the proportion of ZGP-specific IFN-γ producing CD8+ T cells evaluated by flow cytometry. IM administration induced the strongest response in naïve mice (Figure 2A). This was reduced by 85.7% in mice with PEI. Although the frequency of IFN-γ producing CD8+ T cells was lower in mice vaccinated by the IN and SL routes (2.25 and 1.37% respectively), PEI suppressed the response to a lesser degree (36% IN and 33% SL). Oral immunization failed to produce many IFN-γ producing CD8+ T cells in naïve mice and those with PEI. Similar trends were noted in ELISPOT data (Figure 2B).
Figure 2. Pre-Existing Immunity to Adenovirus Strengthens the CD8+ T cell Response against Ebola Zaire Glycoprotein (ZGP) in Mice Immunized by the Sublingual Route in Local Compartments.
B10.Br mice (10/group) were given 1 × 108 infectious particles of recombinant adenovirus expressing ZGP by various routes. A subset of mice from each group (n=5) were given 2.5 × 1011 particles of adenovirus containing the beta-galactosidase transgene by intramuscular injection 28 days prior to vaccination to establish pre-existing immunity (PEI). Mice treated in this manner had an average neutralizing antibody titer of 1:165 ± 22 prior to vaccination. Panel A. Systemic CD8+ T Cell Response in Naïve Mice and Those with Pre-Existing Immunity. Ten days after vaccination, splenocytes were harvested, pooled according to treatment, stimulated with a ZGP-specific peptide (TELRTFSI) and stained with antibodies against CD8 surface proteins and intracellular interferon gamma (IFN-γ). Positive cells were identified by flow cytometry. Numbers in each box represent the proportion of each cell population that was activated by the antigen-specific peptide. Panel B. Mucosal Response. Mononuclear cells were harvested from various compartments of individual mice and analyzed for production of IFN-γ in response to the antigen-specific peptide by ELISPOT 10 days after immunization. Results are reported as the mean ± the standard error of the mean. *, p<0.05, **, p<0.01, ***, p<0.001, one-way ANOVA, Bonferroni/Dunn post-hoc analysis. I.M. – intramuscular, I.N. – intranasal, S.L. – sublingual, P.O. – oral, PEI – pre-existing immunity, BAL - bronchioalveolar lavage fluid, SMLN – submandibular lymph nodes, MLN – mesenteric lymph nodes.
The mucosal T cell-mediated immune response was also evaluated by quantifying the number of IFN-γ secreting mononuclear cells harvested from various compartments. In contrast to what was observed systemically, PEI significantly increased the number of IFN-γ+ cells in BAL of mice immunized by IM injection (p<0.001, Figure 2B). Prior exposure to the virus, however, significantly reduced the number of activated cells in SMLNs (85 ± 13.9 spot-forming cells (SFCs)/million mononuclear cells (MNCs), naïve vs. 23.8 ± 4.7 SFCs/million MNCs, (PEI), p<0.001) and MLNs (134.5 ± 6.6 SFCs/million MNCs, naïve vs. 18.5 ± 14.1 SFC/million MNCs, PEI p<0.05) (IM, Figure 2B). PEI did not compromise the number of IFN-γ secreting cells in BAL and MLN of mice immunized by the IN route. A significant rise in the number of these cells in the SMLNs was noted (28.5 ± 6.8 SFCs/million MNCs naïve vs. 64.8 ± 10.2 SFC/million MNCs, PEI p<0.01). The T cell response elicited by SL immunization was enhanced in the SMLNs and MLNs of mice with PEI (p<0.001 and p<0.05 respectively). The response in BAL was also not compromised by PEI in this group (Figure 2B).
Effect of SL Immunization on Antibody Production
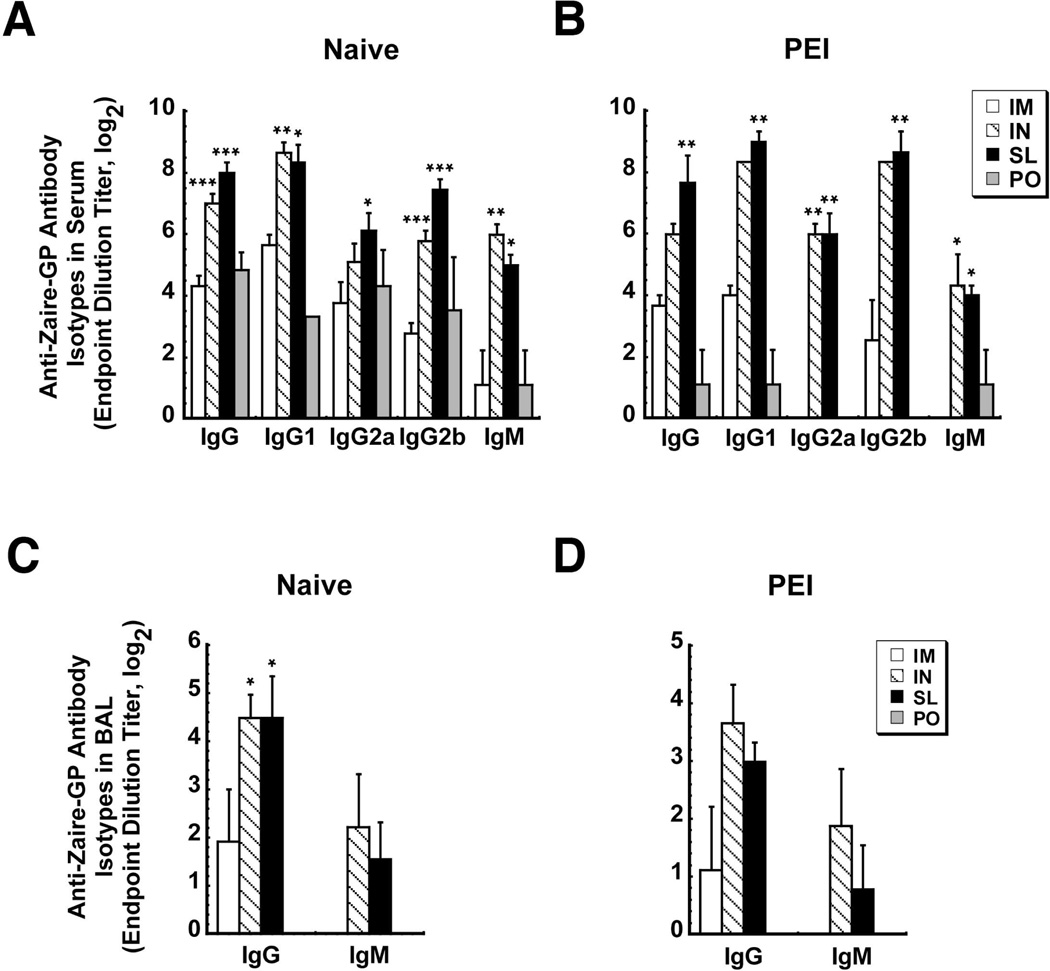
SL immunization significantly increased anti-ZGP IgG1, IgG2 and IgM responses in naive mice with respect to the same dose given IM (Figure 3A). There was no significant difference between IgG1 and IgG2 levels in naïve mice immunized via the SL route and those with PEI (Figure 3A and 3B). Similar trends were noted after IN administration. PEI significantly reduced total IgG and IgG1 levels in mice vaccinated by IM injection by a factor of 1.5 and hampered production of 1gG2a and IgM (Figure 3A and 3B). PEI also significantly compromised total anti-ZGP IgG, IgG1 and IgM of mice vaccinated orally. IgG2a and Ig2b could not be detected in this group (Figure 3B). SL and IN delivery elicited notable anti-ZGP IgG and IgM levels in BAL that were not compromised by PEI (p<0.05, Figure 3D). Anti-ZGP IgG and IgM were not detected in BAL from animals immunized orally. IgA antibodies were not detected in any samples.
Figure 3. Sublingual Immunization Induces Higher Levels of Ebola Zaire-Specific IgG and IgM Antibodies in Serum and Mucosal Secretions than Intramuscular Immunization that are not Compromised by Pre-Existing Immunity.
Serum (Panels A and B) and bronchoalveolar lavage fluid (BAL, Panels C and D) were collected from naïve mice and those with pre-existing immunity 42 days after immunization with 1 × 108 infectious particles of a recombinant adenovirus expressing ZGP by various routes. Samples from individual mice were evaluated for the presence of ZGP-specific IgG subclasses, IgM and IgA by ELISA. End point titers are expressed as the reciprocal log2 titer of the last dilution giving an OD at 450 nm of 0.1 units higher than background. Results are expressed as average values ± the standard error of the means and are representative of two separate experiments each containing 4 mice per immunization route. *, p<0.05, **, p<0.01, ***, p<0.001, one-way ANOVA, Bonferroni/Dunn post-hoc analysis. I.M. – intramuscular, I.N. – intranasal, S.L. – sublingual, P.O. – oral, PEI – pre-existing immunity, BAL - bronchioalveolar lavage fluid.
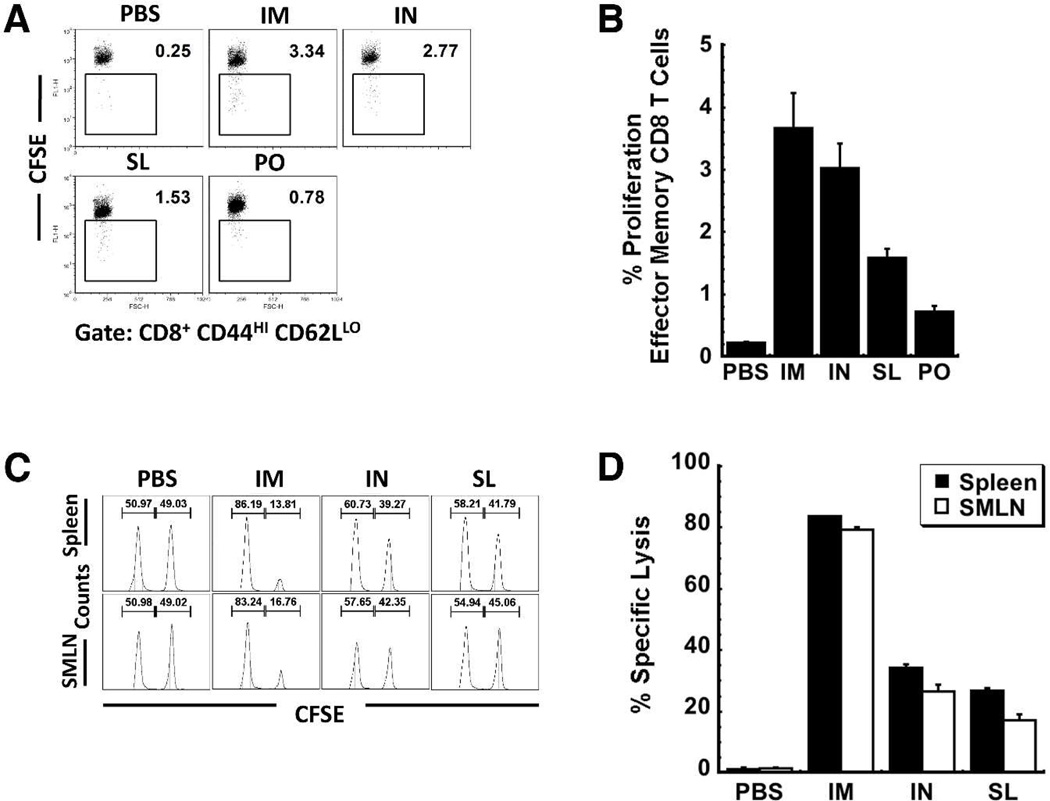
Effect of SL Immunization on the Local and Systemic Effector Memory and Cytotoxic T Cell Responses
The antigen-specific proliferative response of splenocytes harvested 42 days after immunization was first evaluated in an in vitro CFSE assay (Figure 4A and 4B). Five days after restimulation, 3.7 ± 0.6% effector memory CD8+ T cells of mice immunized by IM injection had undergone proliferation in response to the antigen-specific peptide (Figure 4B). This was quite similar to the response of mice immunized by the IN route (3.02 ± 0.4%), approximately twice that of mice immunized by the SL route (1.6 ± 0.1%) and 4 times that of mice immunized orally (0.72 ± 0.08%).
Figure 4. Sublingual Immunization Can Induce Long-Lasting Antigen-Specific T Cell-Mediated Immune Responses.
The presence of immunological memory to Ebola Zaire GP was assessed in mice immunized with recombinant adenovirus by various routes 42 days after treatment by two separate assays. Panel A. Scatter Dot Plot Illustrating the CD8 Effector Memory T Cell Response to an Ebola Glycoprotein-Specific Peptide as Determined In Vitro. Splenocytes were stained with CFSE and stimulated with the TELRTFSI peptide for 5 days. Cells positive for CD8+, CD44HI and CD62LLOW were then evaluated for CFSE by four-color flow cytometry. A decrease in CFSE staining denotes cell division/expansion. Panel B. Quantitative Analysis of the Effector Memory T Cell Response: CFSE Assay. Data was generated from scatter plots shown in Panel A and represent the average values obtained from two separate experiments containing 4 mice per treatment. Error bars reflect the standard error of the data. Panel C. Representative Histograms Illustrating the In Vivo Ebola Glycoprotein-Specific Cytolytic T Cell Response. An equal mixture of TELRTFSI peptide pulsed CFSEHI and unpulsed CFSELOW splenocytes (2 × 107 cells total) were adoptively transferred to immunized mice by tail vein injection. Twenty-four hours later, splenocytes and mononuclear cells from submandibular lymph nodes (SMLN) were harvested and analyzed using flow cytometry. The number above each peak in the histogram plot denotes the percentage of gated CFSEHI (right peak) and CFSELOW (left peak) cells for each subpopulation. Panel D. Quantitative Analysis of the Effector Cytotoxic T Cell Response: In Vivo CTL Assay. Data represent the average values obtained from two separate experiments each containing 4 mice per treatment. Error bars reflect the standard error of the data. I.M. – intramuscular, I.N. – intranasal, S.L. – sublingual, P.O. – oral
In order to determine if a single SL dose of vaccine could induce functional anti-Ebola Zaire GP CTL responses, syngeneic splenocytes harvested from naïve mice labeled with CFSE and pulsed with antigen-specific peptide were transferred to vaccinated mice 42 days after treatment. As an internal control, the same number of non-pulsed splenocytes labeled with a lower concentration of CFSE was co-injected with the pulsed cells. The shift in the ratios of the two populations provoked by elimination of the pulsed cells allows for quantitative measurement of cell lysis in response to the Ebola glycoprotein antigen. Significant elimination of pulsed CFSEHI cells was noted in the spleen (83.6 ± 0.2%) and SMLNs (79.2 ± 0.8%) of mice vaccinated by IM injection (Figure 4D). Cytolysis induced by IN and SL immunization was lower in the spleen (34.4 ± 1.1%, IN, 26.9 ± 0.8% SL) and SMLNs (26.5 ± 2.2%, IN, 17.2 ± 2.0% SL).
Toxicology of SL Vaccine
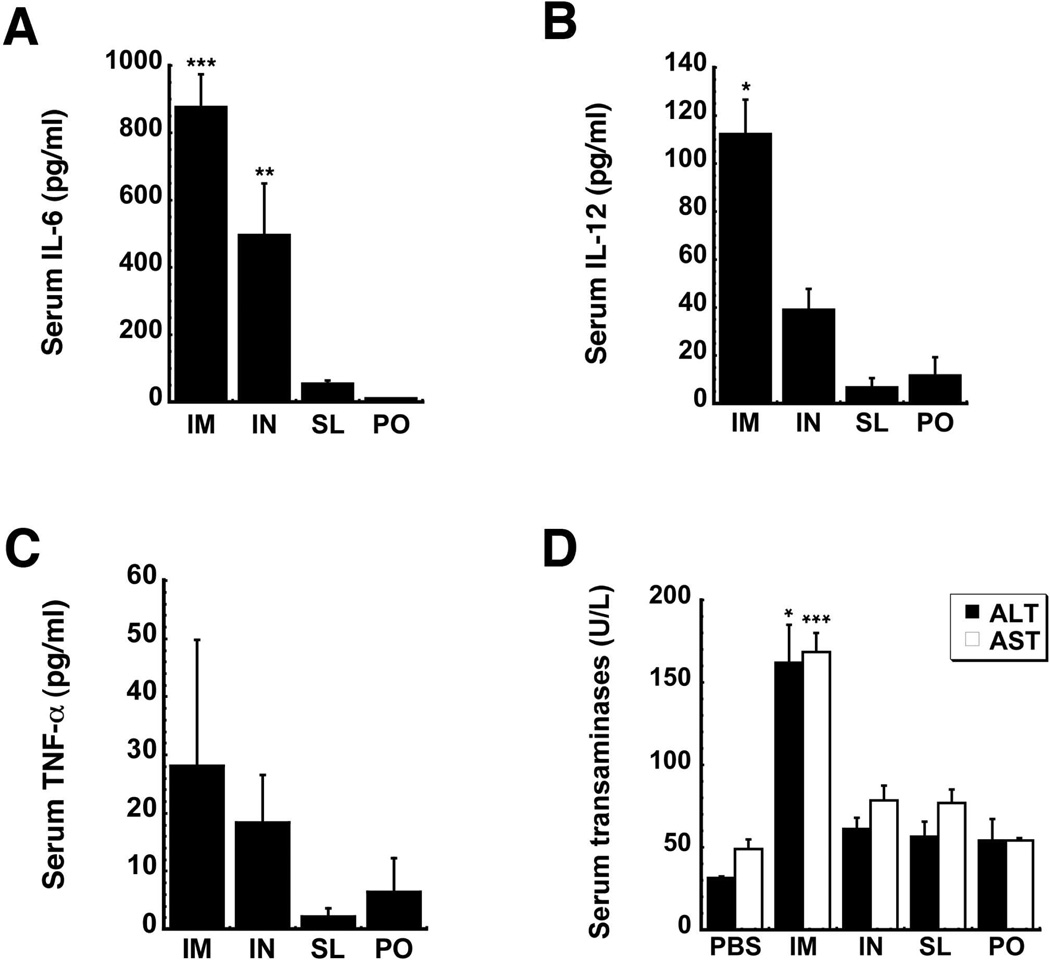
IL-6 was significantly elevated 6 hours after immunization by the IM (876.5 ± 114.5 pg/ml, p<0.001) and IN (498.3 ± 169.4 pg/ml, p<0.01) routes with respect to saline controls (23 ± 16 pg/ml, data not shown). Although IL-6 was detected in mice vaccinated by the SL (55.6 ± 25.4 pg/ml) and PO (14.9 ± 8.0 pg/ml) routes, it was not statistically higher than baseline (Figure 5A). IL-12 (112.2 ± 35.8 pg/ml) and TNF-α (28.1 ± 21.7 pg/ml) levels of mice immunized by the IM injection were 1.5 times that of baseline (Figures 5B and 5C). These cytokines were also not above baseline in mice immunized by the IN, PO and SL routes. Serum transaminase levels followed a similar pattern with alanine (ALT) and aspartate (AST) aminotransferases spiking to 3 times baseline 4 days after IM administration (Figure 5D). Samples from animals immunized by other routes were within baseline values (ALT: 31.5 ± 0.95 U/L, AST: 49 ± 5.8 U/L). AST and ALT levels of those vaccinated by IM injection returned to baseline by day 7 (data not shown).
Figure 5. Sublingual Immunization Significantly Reduces Production of IL-6 in Response to the Adenovirus Vector and Minimizes Toxicity Associated with Adenovirus-Based Vaccines.
Serum IL-6 (A), IL-12 (B) and TNF-α (C) were assessed from samples taken from B10.Br mice 6 hours after administration of a single dose of 1 × 108 infectious particles of a first generation adenovirus expressing Ebola Zaire glycoprotein. (D) Serum alanine (ALT) and aspartate (AST) aminotransferase levels were evaluated 4 days after immunization. Data reflect average values ± the standard error of the mean for four mice from each group. *, p<0.05, **, p<0.01, ***, p<0.001, one-way ANOVA, Bonferroni/Dunn post-hoc analysis. I.M. – intramuscular, I.N. – intranasal, S.L. – sublingual, P.O. – oral
Effect of SL Immunization on Survival after Lethal Challenge
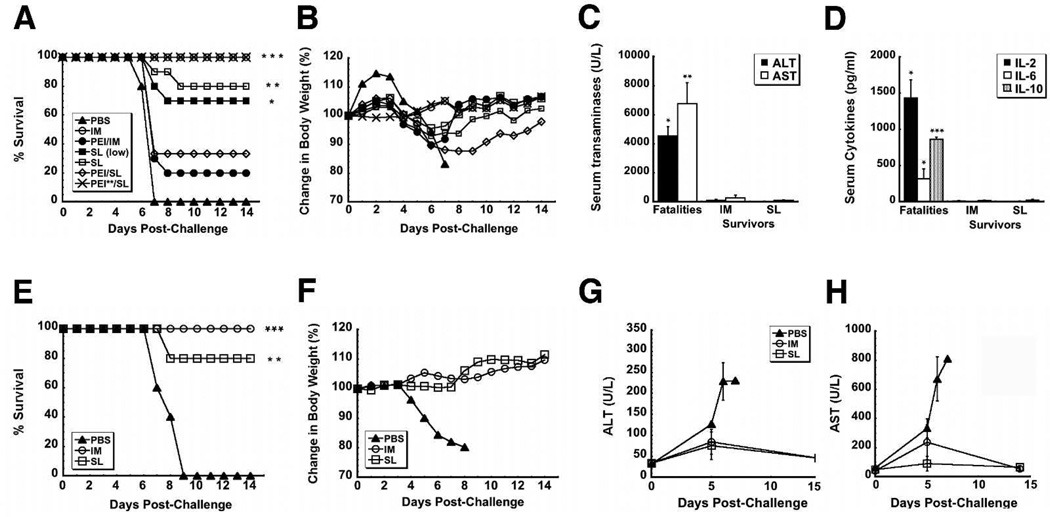
To fully evaluate the utility of SL immunization, naïve mice were given either 1×108 infectious particles or a lower dose, 1×107 infectious particles (SL (low), Figure 6A) of the vaccine. These mice, others given 1×108 infectious particles by IM injection and those given saline were challenged with mouse-adapted Ebola Zaire (1,000 pfu ⋍ 30,000 × LD50). The challenge was uniformly lethal in mice given saline. Mice vaccinated by IM injection survived without notable loss of body weight (Figure 6B). While 80% of mice given the same dose of vaccine by the SL route, 70% survived after treatment with the lower dose (1 × 107 infectious virus particles). Survival rates and weight loss profiles for Guinea pigs immunized by the IM and SL routes and challenged with a dose of 1,000 pfu (~1,000 × LD50) Guinea pig-adapted Ebola Zaire followed the same trend (Figure 6E and 6F).
Figure 6. Sublingual Vaccination Performs in a Manner Similar to that of Traditional Intramuscular Vaccination with Respect to Survival after Lethal Challenge in Mice and Guinea Pigs.
Naïve mice and those with prior exposure to adenovirus serotype 5 (indicated by PEI, n=10) were challenged with a lethal dose of 1,000 pfu mouse-adapted Ebola Zaire (30,000 × LD50) by intraperitoneal injection 28 days after immunization with a single dose of 1 ×108 infectious particles of vaccine. A separate group of naïve mice was given 1×107 infectious particles of the vaccine (SL (low)). Two groups of mice were given 2.5 ×1011 particles of adenovirus to establish pre-existing immunity (indicated by PEI) while another was given 5×1010 particles (indicated by PEI**) A) Mouse Kaplan-Meier survival curve. *indicates a significant difference with respect to the PEI/IM treatment group. B) Mouse Body Weight Profile After Challenge. No significant changes in body weight were noted in animals that survived challenge. C) Serum alanine (ALT) and aspartate (AST) aminotransferase levels Post-Challenge (mouse). Samples from non-survivors were taken at time of death. Samples from survivors were taken 14 days post-challenge. D) Serum Cytokines Post-Challenge. For panels c and d, data reflect average values ± the standard error of the mean for five mice per group. *indicates a significant difference between values from survivors and those that did not survive challenge (fatalities). E) Kaplan-Meier survival curve (Guinea Pig). Naïve Guinea pigs were challenged with a uniformly lethal dose of Guinea pig-adapted Ebola Zaire (1,000 pfu, ~1,000 × LD50) by intraperitoneal injection 28 days after immunization. *indicates a significant difference with respect to saline controls (PBS). F) Change in Body Weight After Challenge (Guinea Pig). No significant changes in body weight were noted in animals that survived challenge. G) Serum alanine (ALT) and H) aspartate (AST) aminotransferase levels Post-Challenge (Guinea Pig). Samples were taken from survivors at days 0, 5, 7 and 14 post-challenge. Samples were taken from non-survivors at the described times and at time of death. In all panels, * p<0.05, ** p<0.01, ***p<0.001, one-way ANOVA, Bonferroni/Dunn post-hoc analysis.
To fully define the limitations associated with SL administration of adenovirus-based vaccines, some mice were also given 2.5 × 1011 particles of adenovirus containing the beta-galactosidase transgene 28 days prior to immunization. This dose, five times that used in previous studies 16, significantly compromised the efficacy of the vaccine given by IM injection with only 20% survival observed (PEI/IM, Figure 6A). The immune response elicited after SL immunization was less effected by PEI with 33.3% survival noted. When pre-existing immunity was established with the dose of virus used in prior studies (5 × 1010 particles), 100% survival was observed in mice immunized by the SL route (PEI**/SL, Figure 6A).
Samples taken from mice post-mortem revealed sharp elevations in ALT (4,536.7 ± 617.7 U/L) and AST (6,737.5 ± 1,469.9 U/L), indicative of severe liver damage from infection (Figure 6C). A similar trend was also noted in Guinea pigs with AST and ALT rising above baseline by a factor of 10 and 7 respectively by day 6 (Figure 6G and 6H). Transaminases also rose around this time in survivors, but to a lesser degree (6 times baseline, IM, 2 times baseline SL). Samples taken from mice during challenge also contained elevated levels of cytokines (IL-2, 1,433.3 ± 251.8 pg/ml, IL-6, 318.3 ± 139.8 pg/ml, IL-10, 862.1 ± 34.2 pg/ml, Figure 6D), while samples from survivors contained trace amounts of each compound.
Effect of SL Immunization on the Immune Response against Adenovirus
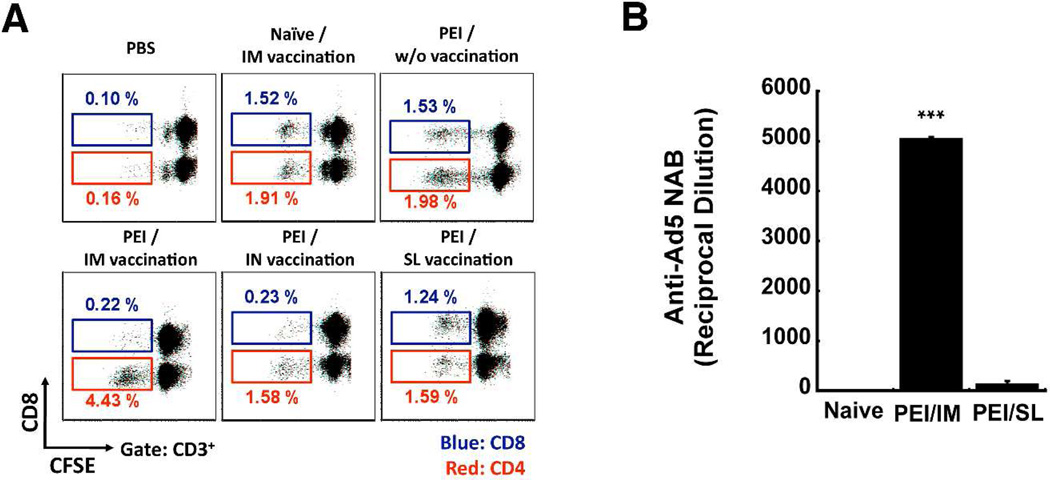
To understand the immune response elicited against the adenoviral vector after SL vaccination, the proliferative response of adenovirus-specific memory CD8+ and CD4+ T cells from mice with PEI and immunized by the IM, IN, and SL routes was evaluated (Figure 7). An even distribution of CD8+ (1.5%) and CD4+ (1.9%) T cells was noted in samples isolated from naïve mice immunized by IM injection (Figure 7A). A similar response was observed in mice with PEI immunized by the SL route (CD8+ (1.2%) and CD4+ (1.6%)). Preferential expansion of CD4+ (4.4%) with respect to CD8+ (0.22%) T cells in response to adenovirus was noted in mice with PEI immunized by IM injection. A similar trend was found in mice immunized nasally. PEI exponentially increased the amount of circulating anti-adenovirus antibodies after IM immunization as levels rose from an average of 1:165 ± 22 prior to vaccination to 1:5,038 ± 44 after treatment (Figure 7B). Anti-adenovirus antibodies in mice with PEI and vaccinated by SL delivery were not significantly different from those prior to vaccination (1:120 ± 75, p = 0.08).
Figure 7. Sublingual Immunization Does Not Facilitate Preferential Expansion of Anti-Adenovirus CD4+ Memory T Cells and Curtails Production of Anti-Adenovirus Neutralizing Antibodies in Mice with Pre-Existing Immunity.
Pre-existing immunity was established by a single intramuscular injection of 2.5 × 1011 particles of adenovirus containing the beta-galactosidase transgene 28 days prior to vaccination by various routes. a) Adenovirus-Specific Memory T Cell Proliferation. Splenocytes were harvested, labeled with CFSE and co-cultured with a recombinant adenovirus serotype 5 vector without a transgene cassette (AdNull) for 5 days. The number in each dot plot denotes the percentage of gated CFSE-negative cells for each subpopulation. Numbers and gates in red indicate the CD4+ population. Numbers and gates in blue indicate the CD8+ population. Dot plots were generated with samples pooled from 5 mice/treatment. b) Anti-Adenovirus Neutralizing Antibodies Produced after Immunization of Mice with Pre-Existing Immunity. Serum was collected from mice prior to immunization and 28 days after treatment. Neutralization was assessed by serial dilution of each sample, incubation with a set amount of adenovirus expressing beta-galactosidase and assessment of transduction efficiency on HeLa cells as described in the Materials and Methods section. Data reflect average values ± the standard error of the mean for samples collected from eight mice per group over 2 separate experiments. *indicates a significant difference with respect to immunization route. ***p<0.001, one-way ANOVA, Bonferroni/Dunn post-hoc analysis. Naïve – samples obtained from mice given saline (negative control).
Discussion
Although an oral wild type adenovirus vaccine has been effectively used for over 25 years 25, successful oral immunization with replication deficient adenoviruses has been challenging. Oral immunization with recombinant adenovirus serotype 5 encoding antigens from rabies, influenza, and other viruses has afforded some protection against infection in animals 26–28. However, oral immunization requires a much higher dose 29 to elicit much lower systemic immune responses 30. Prior to this work, few reports have described the transduction efficiency of recombinant adenoviral vectors in the SL mucosa 31, 32. We have found that SL immunization can reduce the amount of virus necessary for protection with respect to oral immunization. In previous studies, a single oral dose of 1 × 109 infectious particles of our vaccine induced markedly lower T and B cell responses than those achieved by IM and IN delivery, but was fully protective against a challenge dose of 200 × LD50 of mouse-adapted Ebola Zaire 16, 18. In this study, SL administration of a dose that is 1 log lower (1 × 108 infectious particles) generated systemic and mucosal responses superior to those induced by oral immunization and conferred 80% protection against a larger challenge dose (30,000 × LD50). Although mice immunized orally were not challenged in this study, they would not have survived given the overall poor immune response achieved by this delivery route (Figures 1–3).
In 1998, the World Health Organization officially recognized SL delivery as a valid method for treatment of allergic rhinitis, asthma and hymenoptera venom allergy 33. More recently, this approach has been evaluated as a viable means for immunization against bacterial, parasitic and viral pathogens 19, 34–38. Results from these studies indicate that the submandibular lymph nodes are primary inductive sites for immune responses elicited by SL immunization. They house Langerhans, dendritic and other migratory APCs that travel to the central lymph nodes where they establish protective cellular and humoral immune responses 39, 40. SL delivery of our adenovirus-based vaccine rapidly attracted a significant number of MHC II+ and CD11c(+) APCs (Figure 1). Recombinant adenoviruses can efficiently transduce CD11c(+) migratory dendritic cells and those with a Langerhans phenotype in vivo 41, 42. Further characterization of APCs that collect in the submucosa and their transduction and migration patterns after sublingual administration are warranted to determine if MHC II+/CD11c(+) cells played a key role in eliciting the protective Ebola Zaire GP-specific CD8+ T cell responses observed in these studies.
Although SL immunization elicited antigen-specific T and B cell responses similar to IN immunization in naïve mice, protection against challenge was significant but not complete (Figure 6A and 6E). While this was initially concerning, one must note that, unlike the single dose of unformulated vaccine used in our experiments, most published SL immunization strategies require several doses of vaccine with adjuvant for protection 19, 34–38. One must also realize that, unlike humans, the SL mucosa of mice and Guinea pigs is covered with a thick, keratinized layer that impedes virus uptake 43. Studies with preparations containing excipients to further augment antigen production and adjuvant to foster recruitment of APCs in rodents and studies in primates with non-keratinized mucosal linings will provide additional support for SL immunization using adenovirus-based vaccines against Ebola and other pathogens 44.
Prior exposure to adenovirus serotype 5 remains a significant issue and continues to limit the utility of this vector in clinical immunization protocols. This is further compounded by variation in anti-adenovirus 5 neutralizing antibody levels within the general population according to geographical location with lowest levels found in the United States (30–60% of the population positive), moderate levels in Europe and Asia (40–80% positive) and highest levels in sub-Saharan Africa (80–100% positive) where many vaccines are needed 45. One of the primary objectives of these studies was to develop formulations and delivery methods for recombinant adenovirus-based vaccines that could overcome the inherent immune response against the viral vector and promote strong immune responses against the encoded Ebola Zaire glycoprotein. We also hoped that by establishing this immunological state prior to administration of our vaccine candidates, we would be able to identify the types of immune responses generated by adenovirus-based vaccines that are essential for protection against Ebola challenge.
Despite the excellent protective efficacy of several recombinant virus-based vaccines currently under development for Ebola, correlates and mechanisms of protection have not been well defined in animal models of infection 46–48. This is partly due to the fact that several factors can contribute to protective immune responses such as the route of challenge, size of the inoculum, cell types initially infected, heterologous immunity and MHC status, making cross-comparisons between individual studies sometimes difficult 49. According to the current literature, data from the assays we used to evaluate the antibody response, T cell proliferation and cytotoxic T cell lymphocyte responses suggest that cell-mediated immunity, antibody production, and T cell memory are essential for protection against lethal Ebola infection 46, 47, 50. Several observations made during our studies support the notion that strong response from both arms of the immune response are required for protection in the mouse. Despite the fact that naïve animals immunized by the SL route produced anti-Ebola Zaire GP antibodies at a level that was higher than naïve animals given the vaccine by IM injection, protection was not complete (Figures 3 and 6). This suggests that some antibodies produced by the vaccine when given via the SL route may not be fully neutralizing. Proliferative, cytotoxic and memory T cell responses elicited by SL immunization, however, were lower than that observed with IM and IN delivery, suggesting that there is a threshold level of T cell-mediated immunity necessary for full protection against Ebola (Figures 2 and 4). This is in line with a recent study highlighting the necessity of the Ebola Zaire-specific T cell response elicited by adenovirus-based vaccines for protection against Ebola challenge in primates 50. Our results in the mouse model of pre-existing immunity also do not favor one arm of the immune response over the other with respect to protective efficacy. The T and B cell responses were both significantly compromised in mice with prior exposure to adenovirus and immunized by IM injection. Only 20% of this group survived challenge. In contrast, pre-existing immunity did not compromise T and B cell responses in animals immunized by the IN and SL routes and full protection was achieved16 (Figures 2 and 3). Since there is a limited number of reagents available to characterize T and B cell-mediated immune responses in Guinea pigs, there is an extremely limited amount of data in the literature for immune correlates in this model of Ebola infection with only a single reference describing a variable protective response after administration of a human monoclonal antibody that could neutralize the virus in in vitro assays 51. Although we did characterize the anti-Ebola Zaire GP-specific antibody isotypes present in immunized animals (data not shown), responses were equally variable and solid conclusions about the role of the antibody response in immune protection in this animal model cannot be made at this time.
Our study also illustrates that pre-existing immunity can significantly alter the outcome of a vaccine regimen in a manner very different from what may be initially realized. A dose of 5×1010 particles produced a neutralizing antibody level of 1:171.5 ± 48 prior to immunization. This was not significantly different from that generated by 2.5 ×1011 particles (1:165 ± 22), suggesting that neutralization is not the primary reason for differences in protective efficacy between these groups. Although the nature of this response is not clear at this time, it is apparent that PEI enhanced infection of local APCs and recruited more cells to the immunization site, increasing the number of regional antigen-specific CD8+ T cells and limited the amount of virus/activated APCs in the circulation to support a robust systemic response (Figure 2). Studies in which PEI is induced by the nasal route and characterization of transduction and activation patterns of regional and systemic APCs in this model are underway.
Although reduction of the potency of an adenovirus-based vaccine in those with PEI is a legitimate concern, the toxicity profile must also be evaluated. SL administration has been successfully used for allergen-specific desensitization for decades and its safety and ease of use well established 52. We have shown that SL delivery reduces the cytokine response and the sharp rise in transaminases observed with IM injection (Figure 5). Recent results from a phase IIb clinical trial illustrate that PEI to adenovirus can influence immunological and toxicological responses to a vaccine that may be inappropriate in some disease models. It was initially thought that adenovirus-antibody complexes triggered expansion of adenovirus-specific memory CD4+ cells, increasing the targets available for HIV infection 53, 54. Although this was found to not be the primary cause of the outcome of that trial 55, we demonstrate that SL immunization minimizes expansion of CD4+ T cells in mice with PEI and could be useful in mitigating untoward effects in special patient populations (Figure 7).
To our knowledge, this is the first report in which SL immunization against Ebola is described. Unlike current platforms, SL delivery promotes compliance since it does not require medical personnel and is easier to administer than nasal preparations. Nasal vaccination can induce unwanted effects in the nervous system. Reports of Bell’s palsy caused by an influenza vaccine containing a toxin-based adjuvant 56 and adenovirus translocation to the olfactory bulb of mice have limited acceptance of this immunization route 57. Our results indicate that SL administration significantly reduced anti-viral immune responses. Inclusion of reagents that foster antigen expression and target specific APCs will further improve this immunization strategy.
Acknowledgements
The authors thank Erica Ollmann Saphire and Marnie Fucso of The Scripps Research Institute for providing Zaire Ebola GP33–637ΔTM-HA plasmid and assistance with glycoprotein purification. This work was funded by the National Institutes of Health NIAID Grant U01AI078045 (MAC). The content and the views expressed in this manuscript do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
References
- 1.Richardson JS, Dekker JD, Croyle MA, Kobinger GP. Recent Advances in Ebolavirus Vaccine Development. Hum. Vaccine. 2010;6(6):439–449. doi: 10.4161/hv.6.6.11097. [DOI] [PubMed] [Google Scholar]
- 2.Towner JS, Sealy TK, Khristova ML, Albariño CG, Conlan S, Reeder SA, Quan PL, Lipkin WI, Downing R, Tappero JW, Okware S, Lutwama J, Bakamutumaho B, Kayiwa J, Comer JA, Rollin PE, Ksiazek TG, Nichol ST. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4(11):e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Formenty P, Hatz C, Le Guenno B, Stoll A, Rogenmoser P, Widmer A. Human infection due to Ebola virus, subtype Côte d'Ivoire: clinical and biologic presentation. J. Infect. Dis. 1999;179(Suppl. 1):S48–S53. doi: 10.1086/514285. [DOI] [PubMed] [Google Scholar]
- 4.Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, Rollin PE, Towner JS, Shieh WJ, Batten B, Sealy TK, Carrillo C, Moran KE, Bracht AJ, Mayr GA, Sirios-Cruz M, Catbagan DP, Lautner EA, Ksiazek TG, White WR, McIntosh MT. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325(5397):204–206. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- 5.Hartman AL, Towner JS, Nichol ST. Ebola and Marburg Hemorrhagic Fever. Clin. Lab. Med. 2010;30(1):161–177. doi: 10.1016/j.cll.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Olinger GG, Biggins JE, Melanson VR, Wahl-Jensen V, Geisbert TW, Hensley LE. Drug targets in infections with Ebola and Marburg viruses. Infect. Disord. Drug Targets. 2009;9(2):191–200. doi: 10.2174/187152609787847730. [DOI] [PubMed] [Google Scholar]
- 7.Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, Bailer RT, Chakrabarti BK, Bailey MA, Gomez PL, Andrews CA, Moodie Z, Gu L, Stein JA, Nabel GJ, Graham BS. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin. Vaccine Immunol. 2006;13(11):1267–1277. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, Ye L, Compans RW. Protection against filovirus infection: virus-like particle vaccines. Expert Rev. Vaccines. 2008;7(3):333–344. doi: 10.1586/14760584.7.3.333. [DOI] [PubMed] [Google Scholar]
- 9.Geisbert T, Bausch DG, Feldmann H. Prospects for immunisation against Marburg and Ebola viruses. Rev. Med. Virol. 2010;20(6):344–357. doi: 10.1002/rmv.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt WD, Wang D, Nichols DK, Luo M, Woraratanadharm J, Dye JM, Holman DH, Dong JY. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin. Vaccine Immunol. 2010;17(4):572–581. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Feldmann H. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J. Virol. 2009;83(14):7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wertz PW, Squier CA. Cellular and molecular basis of barrier function in oral epithelium. Crit. Rev. Ther. Drug Carrier Syst. 1991;8(3):237–269. [PubMed] [Google Scholar]
- 13.Hill MW. Cell Renewal in Oral Epithelia. In: Meyer J, Squier CA, Gerson SJ, editors. The Structure and Function of Oral Mucosa. New York: Pergamon; 1984. 'Ed.'^'Eds.' 'Vol.' p^pp. [Google Scholar]
- 14.Desvignes C, Estèves F, Etchart N, Bella C, Czerkinsky C, Kaiserlian D. The murine buccal mucosa is an inductive site for priming class I-restricted CD8+ effector T cells in vivo. Clin. Exp. Immunol. 1998;113(3):386–393. doi: 10.1046/j.1365-2249.1998.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Amezaga M, Casimiro DR, Coplan P, Straus WL, Shiver JW. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28(4):950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 16.Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008;3(10):e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callahan SM, Wonganan P, Croyle MA. Molecular and macromolecular alterations of recombinant adenoviral vectors do not resolve changes in hepatic drug metabolism during infection. Virol. J. 2008;5(1):111. doi: 10.1186/1743-422X-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel A, Zhang Y, Croyle M, Tran K, Gray M, Strong J, Feldmann H, Wilson JM, Kobinger GP. Mucosal delivery of adenovirus-based vaccine protects against Ebola virus infection in mice. J Infect Dis. 2007;196(Suppl 2):S413–S420. doi: 10.1086/520603. [DOI] [PubMed] [Google Scholar]
- 19.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, Stadler K, Schiller JT, Anjuere F, Czerkinsky C. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol. 2009;183(12):7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuere F, Czerkinsky C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25(51):8598–8610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 21.Lee JE, Fusco ML, Ollman-Saphire E. An efficient platform for screening expression and crystallization of glycoproteins produced in human cells. Nat. Protoc. 2009;4(4):592–604. doi: 10.1038/nprot.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods. 1998;221(1–2):35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 23.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 24.Rupniak HT, Rowlatt C, Lane EB, Steele JG, Trejdosiewicz LK, Laskiewicz B, Povey S, Hill BT. Characteristics of four new human cell lines derived from squamous cell carcinomas of the head and neck. J. Natl. Cancer Inst. 1985;75(4):621–635. [PubMed] [Google Scholar]
- 25.Tucker SN, Tingley DW, Scallan CD. Oral adenoviral-based vaccines: historical perspective and future opportunity. Expert Rev. Vaccines. 2008;7(1):25–31. doi: 10.1586/14760584.7.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Vemula SV, Mittal SK. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin. Biol. Ther. 2010;10(10):1469–1487. doi: 10.1517/14712598.2010.519332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Cheng C, Ko SY, Kong WP, Kanekiyo M, Einfeld D, Schwartz RM, King CR, Gall JG, Nabel GJ. Delivery of human immunodeficiency virus vaccine vectors to the intestine induces enhanced mucosal cellular immunity. J. Virol. 2009;83(14):7166–7175. doi: 10.1128/JVI.00374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson H, Jackson F, Bean K, Panasuk B, Niezgoda M, Slate D, Li J, Dietzschold B, Mattis J, Rupprecht CE. Oral immunization of raccoons and skunks with a canine adenovirus recombinant rabies vaccine. Vaccine. 2009;27(51):7194–7197. doi: 10.1016/j.vaccine.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 29.Mercier GT, Nehete PN, Passeri MF, Nehete BN, Weaver EA, Templeton NS, Schluns K, Buchl SS, Sastry KJ, Barry MA. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine. 2007;25(52):8687–8701. doi: 10.1016/j.vaccine.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe S, Fooks A, Lee J, Hayes K, Clegg C, Cranage M. Single oral immunization with replication deficient recombinant adenovirus elicits long-lived transgene-specific cellular and humoral immune responses. Virology. 2002;293(2):210–216. doi: 10.1006/viro.2001.1281. [DOI] [PubMed] [Google Scholar]
- 31.Adriaansen J, Zheng C, Perez P, Baum BJ. Production and sorting of transgenic, modified human parathyroid hormone in vivo in rat salivary glands. Biochem. Biophys. Res. Commun. 2010;391(1):768–772. doi: 10.1016/j.bbrc.2009.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Zeeburg HJ, van Beusechem VW, Huizenga A, Haisma HJ, Korokhov N, Gibbs S, Leemans CR, Brakenhoff RH. Adenovirus retargeting to surface expressed antigens on oral mucosa. J. Gene Med. 2010;12(4):365–376. doi: 10.1002/jgm.1447. [DOI] [PubMed] [Google Scholar]
- 33.Compalati E, Rogkakou A, Villa E, Passalacqua G, Canonica GW. Emerging sublingual immunotherapy drugs. Expert Opin. Pharmacother. 2010;11(18):2963–2972. doi: 10.1517/14656566.2010.512420. [DOI] [PubMed] [Google Scholar]
- 34.Hervouet C, Luci C, Cuburu N, Cremel M, Bekri S, Vimeux L, Marañon C, Czerkinsky C, Hosmalin A, Anjuère F. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine. 2010;28(34):5582–5590. doi: 10.1016/j.vaccine.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Raghavan S, Ostberg AK, Flach CF, Ekman A, Blomquist M, Czerkinsky C, Holmgren J. Sublingual immunization protects against Helicobacter pylori infection and induces T and B cell responses in the stomach. Infect. Immun. 2010;78(10):4251–4260. doi: 10.1128/IAI.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ralli-Jain P, Tifrea D, Cheng C, Pal S, de la Maza LM. Enhancement of the protective efficacy of a Chlamydia trachomatis recombinant vaccine by combining systemic and mucosal routes for immunization. Vaccine. 2010;28(48):7659–7666. doi: 10.1016/j.vaccine.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho HJ, Kim JY, Lee Y, Kim JM, Kim YB, Chun T, Oh YK. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine. 2010;28(14):2598–2606. doi: 10.1016/j.vaccine.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008;105(5):1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat. Immunol. 2010;11(8):647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 40.Mascarell L, Lombardi V, Zimmer A, Louise A, Tourdot S, Van Overtvelt L, Moingeon P. Mapping of the lingual immune system reveals the presence of both regulatory and effector CD4+ T cells. Clin. Exp. Allergy. 2009;39(12):1910–1919. doi: 10.1111/j.1365-2222.2009.03337.x. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay RW, Darrah PA, Quinn KM, Wille-Reece U, Mattei LM, Iwasaki A, Kasturi SP, Pulendran B, Gall JG, Spies AG, Seder RA. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol. 2010;185(3):1513–1521. doi: 10.4049/jimmunol.1000338. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31(12):446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathbone MJ, Purves R, Ghazali FA, Ho PC. In vivo techniques for studying the oral mucosal absorption characteristics of drugs in animals and humans. In: Rathbone MJ, editor. Oral Mucosal Delivery. New York: Marcel Dekker; 1996. pp. 121–156. 'Ed.'^'Eds.' 'Vol.' p^pp. [Google Scholar]
- 44.Hassan N, Ahad A, Ali M, Ali J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin. Drug Deliv. 2010;7(1):97–112. doi: 10.1517/17425240903338758. [DOI] [PubMed] [Google Scholar]
- 45.Pilankatta R, Chawla T, Khanna N, Swaminathan S. The prevalence of antibodies to adenovirus serotype 5 in an adult Indian population and implications for adenovirus vector vaccines. J. Med. Virol. 2010;82(3):407–414. doi: 10.1002/jmv.21721. [DOI] [PubMed] [Google Scholar]
- 46.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377(9768):849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leroy EM, Gonzalez JP, Baize S. Ebola and Marburg haemorrhagic fever viruses: major scientific advances, but a relatively minor public health threat for Africa. Clin Microbiol Infect. 2011;17(7):964–976. doi: 10.1111/j.1469-0691.2011.03535.x. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7(5):393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, Honko A, Olinger G, Bailey M, Geisbert JB, Reimann KA, Bao S, Rao S, Roederer M, Jahrling PB, Koup RA, Nabel GJ. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17(9):1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 51.Parren PW, Geisbert TW, Maruyama T, Jahrling PB, Burton DR. Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J. Virol. 2002;76(12):6408–6412. doi: 10.1128/JVI.76.12.6408-6412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toskala E. A contemporary review of sublingual immunotherapy. Laryngoscope. 2009;119(11):2178–2181. doi: 10.1002/lary.20693. [DOI] [PubMed] [Google Scholar]
- 53.Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 2008;205(12):2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benlahrech A, Harris J, Meiser A, Papagatsias T, Hornig J, Hayes P, Lieber A, Athanasopoulos T, Bachy V, Csomor E, Daniels R, Fisher K, Gotch F, Seymour L, Logan K, Barbagallo R, Klavinskis L, Dickson G, Patterson S. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl. Acad. Sci. U.S.A. 2009;106(47):19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Brien KL, Liu J, King SL, Sun YH, Schmitz JE, Lifton MA, Hutnick NA, Betts MR, Dubey SA, Goudsmit J, Shiver JW, Robertson MN, Casimiro DR, Barouch DH. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 2009;15(8):873–875. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4(9):e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damjanovic D, Zhang X, Mu J, Medina MF, Xing Z. Organ distribution of transgene expression following intranasal mucosal delivery of recombinant replication-defective adenovirus gene transfer vector. Genet. Vaccines Ther. 2008;6(5) doi: 10.1186/1479-0556-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]