Abstract
The diverse and independently-varying signs of Parkinson’s disease (PD) are often attributed to one simple mechanism: degeneration of the dopaminergic innervation of the posterolateral striatum. However, growing recognition of the dopamine (DA) loss and other pathology in extra-striatal brain regions has led to uncertainty whether loss of DA in the striatum is sufficient to cause parkinsonian signs. We tested this hypothesis by infusing cis-flupenthixol (cis-flu; a broad-spectrum D1/D2 receptor antagonist) into different regions of the macaque putamen (3 hemispheres of 2 monkeys) while the animal performed a visually-cued choice reaction time task in which visual cues indicated the arm to reach with and the peripheral target to contact to obtain food reward. Following reward delivery, the animal was required to self-initiate release of the peripheral target and return of the chosen hand to its home position (i.e., without the benefit of external sensory cues or immediate rewards). Infusions of cis-flu at 15 of 26 sites induced prolongations of reaction time (9 of 15 cases), movement duration (6 cases), and/or dwell time of the hand at the peripheral target (8 cases). Dwell times were affected more severely (+95%) than visually-triggered reaction times or movement durations (+25% and +15%, respectively). Specifically, the animal’s hand often ‘froze’ at the peripheral target for up to 25-s, similar to the akinetic freezing episodes observed in PD patients. Across injections, slowing of self-initiation did not correlate in severity with prolongations of visually-triggered reaction time or movement duration, although the latter two were correlated with each other. Episodes of slowed self-initiation appeared primarily in the arm contralateral to the injected hemisphere and were not associated with increased muscle co-contraction or global alterations in behavioral state (i.e., inattention or reduced motivation), consistent with idea that these episodes reflected a fundamental impairment of movement initiation. We found no evidence for an anatomic topography within the putamen for the effects elicited. We conclude that acute focal blockade of DA transmission in the putamen is sufficient to induce marked akinesia-like impairments. Furthermore, different classes of impairments can be induced independently, suggesting that specific parkinsonian signs have unique pathophysiologic substrates.
Keywords: macaque, bradykinesia, akinesia, microinjection, cis-flupenthixol
INTRODUCTION
Given the spectacular therapeutic efficacy of dopamine-replacement therapies (Hornykiewicz and Kish, 1987), there is little doubt that the core motor signs of Parkinson’s disease (akinesia, bradykinesia, rigidity and tremor) arise from a loss of dopamine (DA) in the central nervous system. Historically, loss of DA from the posterolateral striatum (i.e., the skeletomotor region of the putamen) has been thought to be the primary factor that leads to parkinsonism (Damier et al., 1999; Forno, 1996). Indeed, the largest loss of DA in absolute terms is from the posterior putamen (Ehringer and Hornykiewicz, 1960; Kish et al., 1988) and symptom severity does correlate with the level of putamenal DA depletion (Bernheimer et al., 1973; Morrish et al., 1996; Nandhagopal et al., 2009; Seibyl et al., 1995). Putamenal DA depletion, however, is not a perfect predictor of symptom severity or symptom progression in individual patients (Gallagher et al., 2011; Pavese et al., 2011; Pirker, 2003), suggesting that factors other than putamenal DA influence the genesis of parkinsonian symptoms. Dopamine loss in PD is not restricted to the striatum (reviewed by Rommelfanger and Wichmann, 2010), but has also been observed in the subthalamic nucleus, thalamus, globus pallidus, and cortex (Francois et al., 2000; Freeman et al., 2001; Jan et al., 2000; Scatton et al., 1983; Scatton et al., 1982). Thus, the minimal pathologic defect necessary to induce parkinsonian signs remains unknown.
The diverse topography of DA loss in PD is mirrored by a variety of motor signs, which can be broken down into relatively independent groupings (Parkinson, 1817). Akinesia is a multi-component sign, characterized by a poverty of willed movement, slowness to initiate sensory-triggered movement (i.e., lengthened reaction times, RTs), and particular difficulty initiating movements in the absence of external sensory cues (Flowers, 1976; Morris et al., 1996; Oliveira et al., 1997). Freezing episodes, a facet of akinesia characterized by a temporary inability to initiate movement (Fahn, 1995; Jankovic, 2008; Nieuwboer et al., 2009) are difficult to treat, but can often be overcome with the help of external sensory cues (Arias and Cudeiro, 2008; Dietz et al., 1990; Marchese et al., 2000; Praamstra et al., 1998). Bradykinesia refers exclusively to slowed execution of movement (measurable as prolonged movement durations, MDs, Hallett and Khoshbin, 1980), while rigidity and tremor manifest as increased muscular resistance to passive joint movement and involuntary 4–6 Hz tremulous movements of one or more body part, respectively (Jankovic, 2008). Each of these parkinsonian signs varies independently in severity, rate of progression, and response to therapy (Espay et al., 2009; Evarts et al., 1981; Jankovic, 2008; Jordan et al., 1992; Kimber et al., 1999; Kishore et al., 2007; Meyer, 1982; Nieuwboer et al., 1998; Selikhova et al., 2009; Temperli et al., 2003; Zetusky and Jankovic, 1985), implying that different parkinsonian signs may have unique pathophysiologic substrates. Similarly, the fact that anatomically-segregated regions of the striatum are devoted to skeletomotor, associative and limbic functions (Alexander et al., 1990; Kelly and Strick, 2004; Worbe et al., 2009) has prompted proposals that dissociable symptoms of PD arise from loss of DA from separate functional regions of the striatum (Alexander et al., 1986; Joel and Weiner, 1994; Wichmann et al., 2011). It is therefore important to determine whether DA loss in specific striatal regions elicits separate parkinsonian signs.
Here, we sought to establish whether acute focal blockade of striatal DA neurotransmission is sufficient to induce behavioral changes that reflect parkinsonian signs. This question is not amenable to current DA-targeted neurotoxin approaches (i.e., using 6-OHDA or MPTP, Emborg, 2007). Administration of these agents leads to degeneration of dopaminergic somata in the substantia nigra compacta (Oiwa et al., 2003; Sauer and Oertel, 1994) and of their extensive multi-nuclear axonal arborizations (Debeir et al., 2005; Freeman et al., 2001; Pifl et al., 1991) even when the neurotoxin is infused directly into the striatum (Debeir et al., 2005; Freeman et al., 2001). Neurotoxin models, therefore, cannot rule out the potential roles of extra-striatal DA loss, degeneration of the somata of dopaminergic neurons, or chronic DA depletion in the development of parkinsonian signs.
An alternative approach involves the selective blockade of DA receptors in the striatum. Infusions of DA receptor antagonists into the striatum are known to elicit catalepsy in rodents (Amalric and Koob, 1987; Ellenbroek et al., 1985; Hauber et al., 2001; Kaur et al., 1997; Salamone et al., 1993; Yoshida et al., 1994). It is difficult, however, to relate the cataleptic state, a nonspecific combination of abnormal posturing and immobility, to specific signs of human parkinsonism. To our knowledge, only one previous study examined the behavioral effects of intra-striatal DA blockade in non-human primates (Hikosaka et al., 2006). In that study, small intra-striatal infusions of D1- or D2-specific antagonists disrupted the normal relationship between oculomotor reaction times and size of rewards (Hikosaka et al., 2006), but overt signs of parkinsonism were not noted. By infusing large volumes of a non-specific D1/D2-receptor antagonist at various sites in the putamen, we tested whether acute blockade of striatal DA transmission is sufficient to cause changes in motor performance reflective of parkinsonian signs.
METHODS
Animals and task
Two monkeys (Macaca mulatta; D, male ~7.5 kg; E, female ~6 kg) were used in the study. All aspects of animal care were in accord with the “Guide for the Care and Use of Laboratory Animals” (National Academy Press, 1996), and all procedures were approved by the institutional animal care and use committee. An animal was seated in a primate chair facing a vertically-mounted response panel ~31 cm in front of the animal’s sternum (Fig. 1A). The panel contained two identical bi-colored LED “targets”, aligned with the animals’ left and right shoulders. Infrared proximity sensors (Takex, GS20N) were mounted above each LED to detect hand contact with the colored LEDs. Two metal rods (“homekeys”, also equipped with proximity sensors) were mounted on the left and right sides of the primate chair ~7 cm lateral to the animal’s body. The vertical distance from the animals’ shoulder to the homekeys was ~26 cm. The linear distance between each homekey and the ipsilateral and contralateral panel-mounted LED was ~34 and ~40 cm, respectively.
Figure 1.
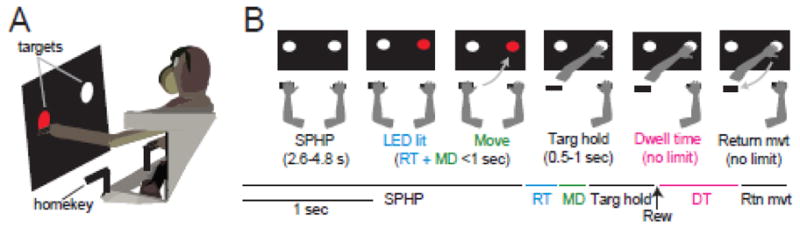
Figure 1A) Schematic of the reaching task. B) Each trial began with a mandatory hold period (SPHP) during which the animal was required to hold right and left homekeys for a variable period of time. One of the target LEDs was then lit and the animal was required to move to the correct target with the correct hand and hold the hand at the target for a variable time interval (Targ hold) to receive a reward (Rew). After the reward, the animal was allowed to initiate a return movement (Return mvt) to the homekey whenever the animal wanted. There were no cues, time limits, or rewards for the return movement. Each segment of one example trial is depicted along a timeline, color-coded by the key time intervals. RT: the period of time between cue presentation and movement initiation. MD: the duration of movement execution. DT: dwell time – the time interval during which the animal’s hand remained at the target after reward delivery and before initiation of a return movement.
The bimanual reaching task required an animal to reach to the lit LED target with the left or right hand to obtain a food reward (Fig. 1). Which hand to reach with (left or right) was signaled by the color of the LED target (red and yellow, respectively). During an initial start position hold period (SPHP, 2.6–4.8 second duration, randomized trial-to-trial following a uniform distribution) left and right hands remained stationary on their respective homekeys (Fig. 1B). At the end of the SPHP, one of the target LEDs was illuminated [four trial types (left or right target position red or yellow LED) selected at random]. Cross-body and same-side reaches were generated to targets that were contralateral and ipsilateral to the reaching arm, respectively.
The animal was required to lift the appropriate hand from the homekey and move it to the correct target in ≤ 1 second. The animal was then required to hold its hand at the correct target for 0.5–1 second (randomized) to receive a drop of liquid food reward delivered via a sipper tube. The animal was then allowed to return the hand to the homekey with no external cues and no time requirements. On 3/26 injection days, the animal performed the task with only the arm contralateral to the infusion site while the ipsilateral arm was loosely restrained in a padded splint. This was done while one of the animals was being trained to accept external EMG leads on the contralateral arm. On other days, the animal performed the task bimanually. Use of a bimanual reaching task allowed us to determine if behavioral effects were restricted to the contralateral side of the body, thus resembling specific parkinsonian-like motor impairments, or affected both arms equally, thereby more likely reflecting a global dysfunction of cognition, attention or motivation.
Structural MRI
Prior to implantation surgery, MRI images were obtained using a Magnetom Allegra 3 Tesla MR scanner (Siemens Medical Solutions USA, Malvern, PA). The animal was anesthetized with Isoflurane and placed into a MR-compatible stereotactic holder (Crist Instrument Co., Hagerstown, MD) built into an MR transmit/receive coil (Nova Medical Inc., Wilmington, MA). The birdcage-style coil (16 cm I.D.) operated in quadrature mode. Three-dimensional T1 images of the whole head were acquired using a MPRAGE sequence with 0.6 mm isotropic voxels. Four successive scans were averaged to improve the signal-to-noise ratio. The resulting images were used for stereotaxic targeting in the subsequent surgery and for later reconstruction of injection locations (see below).
Surgical procedures and targeting
Many of the methods have been described previously (Desmurget and Turner, 2008). Animals were prepared surgically using aseptic technique under Isoflurane anesthesia. Two cylindrical titanium recording chambers (18 mm ID) were affixed to the skull over craniotomies at stereotaxic coordinates to allow transdural access to the right and left putamen from a coronal approach. The chamber was fixed to the skull with bone screws and dental acrylic. Bolts embedded in the acrylic allowed fixation of the head.
After the animal recovered from the implantation surgery, the boundaries of cortex, putamen, and the globus pallidus were located with respect to chamber coordinates using standard microelectrode mapping techniques (Turner and DeLong, 2000). The resulting 3-dimensional information regarding the locations of nuclear boundaries was overlaid onto each monkey’s MRI using Cicerone, a purpose-designed neurophysiologic mapping software (Miocinovic et al., 2007). Microelectrode mapping continued until putamenal and globus pallidus borders were localized with a high degree of confidence (Fig. 2A). The skeletomotor region of the putamen was located as the region where microstimulation (40–60 μA constant current, 10 biphasic pulses 200 μsec duration at 300–400 Hz) evoked reliable muscle contractions in the leg, arm, or face. The neurophysiologic mapping information was brought into register in 3-dimensions with the animal’s own structural MRI and atlas-derived nuclear boundaries (Martin and Bowden, 1996) using Cicerone. Additional microelectrode mapping tracks performed on days following an injection yielded nearly identical locations for the nuclear boundaries, reinforcing the conclusion that our recording system and the Cicerone software allowed reliable targeting of sites within the putamen.
Figure 2.
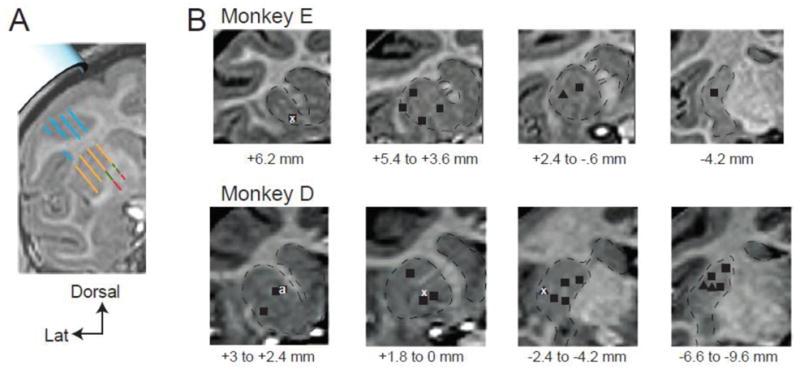
Anatomic localization of injection locations using microelectrode mapping and structural MRI. A) Representative coronal plane of MRI and corresponding microelectrode mapping results. Sites along parallel recording tracks are color-coded based on the characteristic neurophysiologic activity observed at those locations (blue = cortex, orange = striatum, green = external globus pallidus, red = internal globus pallidus). Estimation of the exact position of the implanted recording chamber (light blue cylinder, upper left) relative to the striatum was determined by finding the best fit between microelectrode mapping results and MRI-defined structural landmarks integrated across multiple coronal planes (not shown). As seen for this exemplar plane, microelectrode mapping results aligned very well with structural boundaries visible in the animal’s MRI. B) Localization of injection sites. Mapping-based alignment of the recording chamber with an animal’s MRI allowed estimation of intracerebral location of injections. For monkey E and monkey D, each injection site is marked by a black square (cis-flu), black triangle (cis-flu into microexcitable arm area of the putamen) or white x (saline). Injection sites are collapsed across coronal slices (≤ 3 mm per section). The distance ranges below each figure represent the AP planes collapsed into that figure (in mm relative to the anterior commissure). For monkey D, injection sites from right and left hemispheres are collapsed onto one hemisphere.
Electromyographic (EMG) data were collected from multiple arm muscles in both monkeys. For monkey D, surface EMG was collected from biceps and triceps muscles using gold cup electrodes (Grass, Inc.) attached to the upper arm. These electrodes were removed after each experimental session. In monkey E, pairs of Teflon-insulated multi-stranded stainless steel wires were implanted chronically into four muscles of the arm (biceps, triceps, latissimus dorsi, and deltoid muscles) in a separate surgery. The wires were led subcutaneously to a connector fixed to the skull implant. Accurate placement of the wires was verified post-surgically by: 1) determining that each electrode pair provided independent EMG-like signals (thereby ruling out cross talk); and 2) observing palpable contraction of the appropriate muscle when electrical stimulation was applied to each electrode. Following both surgeries, animals were given prophylactic antibiotics and analgesics. After recovery from surgery, animals resumed behavioral testing.
Injection procedures
Injection Apparatus
An evenly-distributed set of putamenal injection sites was selected using microelectrode mapping results, each monkey’s MRI, and the Cicerone software system. The custom-built microinjection cannula consisted of a piece of fused silica tubing 140 cm in length (99 μm ID, 196 μm OD, Polymicro Technologies, L.L.C.) glued to a Leur-lock fitting, allowing attachment of a 25 μl Hamilton syringe for drug delivery. The end of the fused silica to be lowered into the brain was glued inside of a nested series of stainless steel cannulae, leaving 3–5 cm of bare silica tubing exposed at the end. Using this design, only silica tubing entered the brain. The tip of the injection apparatus was lowered into the brain through a dura-piercing guide tube held in a 1-mm resolution grid mounted in a microdrive (MT, Alpha Omega Co. USA). The Hamilton syringe (Hamilton Co.) was placed in a motorized syringe pump (Harvard Apparatus) which controlled the rate and volume of solution infused.
Injection Protocol
Infusions of either cis-(Z)-Flupenthixol dihydrochloride (cis-flu; antagonist to D1- and D2-families of dopamine receptors, Sigma-Aldrich) or 0.9% sterile saline were performed at various sites (Fig. 2B) using convection-enhanced delivery, a safe and effective way to deliver large volumes of solution into the primate brain at a slow constant rate using a motorized syringe pump (Bankiewicz et al., 2000; Krauze et al., 2005; Saito et al., 2004). On the day of an infusion, cis-flu was dissolved in 0.9% sterile saline (5–10 μg/μl) and filter-sterilized (0.22 micron Milipore filter) before infusion. After filling the injection cannula with cis-flu and attaching it to a Hamilton syringe loaded with cis-flu, the Hamilton syringe was placed into the syringe pump. One-half microliter of sterile saline was then drawn into the tip of the cannula so that any leakage from the tip into neuronal tissue during cannula placement would contain only saline. The tip of the injection cannula was then lowered manually into the brain through a dura-piercing guide tube mounted on a microdrive. Once the cannula was at the appropriate depth, behavioral data from the task were collected before (15–20 min), during (10.5 min), and after each injection. The monkey was allowed to perform the behavioral task until satiation (total duration of data collection ~90–120 min). The rate (1 μL/min) and volume (10.5 μL) of the infusion was controlled by the syringe pump. The infusion cannula was left in place at the injection site throughout the post-injection recording period.
We infused a large volume of solution in order to block DA transmission in relatively large regions of the striatum. Based on previous studies that used similar techniques (Vogelbaum, 2005), this method of infusion affects a sphere of tissue around the injection site ~4.6 mm in diameter. On saline injection days, 10.5 μl of sterile saline was infused using the same apparatus and identical methods. At the end of each experiment session, the injection cannula and microdrive were removed. Injections were performed, at minimum, 7 days apart. To reduce inhomogeneous spread of injectate, due to recent nearby penetrations for example (Alexander et al., 2009), injection locations alternated between anterior and posterior regions of the striatum to maximize the distance between consecutive injections.
Data collection
The timing of behavioral events was stored with 40 sec accuracy. EMG signals were amplified (×10k), band-pass filtered 100–5000 Hz, digitized at 6104 Hz, rectified, then low-pass filtered (500 Hz cutoff) and down-sampled to 1017 Hz. All of the data were collected using a real-time processor (RZ2, Tucker-Davis Technologies). A separate video capture system (Nuvico EV-4000) recorded the animal’s general behavior and movement while performing the task.
Data analyses
Measures of task performance reflective of the speed of movement initiation included reaction time (RT; the time interval between illumination of a target LED and lifting of the appropriate hand from the homekey; Fig. 1) and dwell time (DT; the time interval the animal’s hand remained at a peripheral target following reward delivery and before onset of a return movement to the homekey). It is important to differentiate between RTs and DTs. RTs reflect the time needed to initiate an externally-triggered and rewarded movement to a visible target location. DTs are a measure of the time needed to initiate a movement without the benefit of external sensory cues, promised rewards, or a visible target location. Movement durations (MDs; the time required to transport the hand from the homekey to the appropriate peripheral target) were used to quantify the degree of bradykinesia. The durations of return movements (moving the hand from the target to the homekey) were highly variable, even on non-injection days, and thus were not analyzed.
Significant effects of injections were detected using a chi-square analysis. The first half of the ~10.5 minute injection period was considered part of the “pre-injection” period. The beginning of the “post-injection” period included the last half of the injection period. While this approach could eliminate the possibility of detecting an effect that occurred during the first half of the injection period, preliminary analyses found no evidence that behavioral effects occurred this early.
The chi-square analysis compared the number of trials pre- versus post-injection in which a behavioral measure deviated ≥ 3.5 standard deviations from the pre-injection mean. A chi-square analysis was performed on trial-by-trial measures of task performance (RT, DT, MD) separately for each arm (contralateral and ipsilateral to the injection site) and each injection. Behavioral effects were tested for significance using one-tailed chi-square tests (p < 0.05).
We measured the mean magnitude (i.e., severity) of injection-induced changes in behavior using a moving average (20 consecutive trials of the same trial type) stepped trial-by-trial through the post-injection data. The resulting four moving averages (one for each trial type: left-arm left-target, left-arm right-target, right-arm right-target, right-arm left-target) were normalized to reflect percent change from that measure’s pre-injection mean such that 0% indicated no change from pre-injection. Based on our prediction that DTs, RTs, and MDs would increase following injections, the maximum increase across the four post-injection moving averages was taken as the magnitude of the injection-related behavioral change. Results are reported as means ±SEM unless noted otherwise.
We tested for co-variations in the severity of different injection-induced impairments. Specifically, we identified the maximum change in each behavioral measure for each injection using the same moving average approach. The magnitudes of injection-related changes in DTs, RTs, and MDs were then correlated with each other across all injections using Spearman correlation analyses.
To test for a topography of injection effects, we categorized each injection experiment according to its location relative to striatal functional territories. The locations of anatomic boundaries between the general skeletomotor, associative, and limbic territories of the striatum were taken from reports of consistent boundary locations in recent physiologic mapping studies (Worbe et al., 2009; Worbe et al., 2011), in tract tracing studies of the corticostriatal projections from motor, associative and limbic cortical regions (Flaherty and Graybiel, 1991; Fudge et al., 2004; Kunzle, 1975; Selemon and Goldman-Rakic, 1985; Yeterian and Van Hoesen, 1978), and in studies of variations in the density of calbindin-immunoreactivity across striatal territories (Francois et al., 1994; Parent et al., 1996). [Note: we did not attempt to address the finer-grained functional topographies known to exist within each of the general functional territories (Inase et al., 1999; Takada et al., 2001).] Individual injections were categorized according to whether they were placed in skeletomotor or non-skeletomotor (i.e., associative or limbic) territories. Injections located close to a boundary between skeletomotor and non-skeletomotor territories were excluded from the functional topography analysis.
RESULTS
A total of 26 cis-flu injections were performed in three hemispheres across two monkeys (18 injections across two hemispheres in monkey D, 8 injections in one hemisphere in monkey E; Fig. 2B). Three saline injections into the putamen were also performed, one in each hemisphere (Table 1).
Table 1.
Summary of all injections. Each injection is listed in the temporal order in which it was performed. Mnk: the animal in which the injection was performed. AC plane: distance from the injection site (in mm) anterior (+) or posterior (−) to the anterior commissure.
| Effects | ||||||||
|---|---|---|---|---|---|---|---|---|
| # | Mnk | AC plane | Func. territory | drug | μg/μl | Hem | Contralateral arm (%, p) | Ipsilateral arm (%, p) |
| 1 | D | −4.2* | SM | cis-flu | 5 | R | DT (52, .035), RT (28, .04) | - |
| 2 | D | −6.6 | SM | cis-flu | 10 | R | DT (25, .02), RT (19, .038), MD (13, .029) | - |
| 3 | D | −4.2 | SM | saline | 10 | R | - | - |
| 4 | D | −4.2* | SM | cis-flu | 10 | R | - | - |
| 5 | D | 2.4† | Assoc | cis-flu | 10 | R | DT (313, .0009), RT (20, .018), MD (24, <.0001) | NT |
| 6 | D | −2.4 | SM | cis-flu | 10 | R | DT (184, .005) | NT |
| 7 | D | 2.4† | Assoc | cis-flu | 10 | R | - | - |
| 8 | D | −2.4 | SM | cis-flu | 10 | R | - | - |
| 9 | D | 2.7 | Limb | cis-flu | 10 | R | RT (25, .048) | NT |
| 10 | D | 3 | Assoc | cis-flu | 10 | R | RT (27, .018), MD (16, .0005) | - |
| 11 | D | −9.6 | SM | cis-flu | 10 | R | - | - |
| 12 | D | 1.8 | Limb | cis-flu | 10 | L | - | RT (31, < .0001) |
| 13 | D | −9.6 | SM | cis-flu | 10 | L | MD (9, .027) | - |
| 14 | D | −7.2 | SM | cis-flu | 10 | L | RT (14, .04) | - |
| 15 | D | 1.2 | - | cis-flu | 10 | L | - | RT (71, .018) |
| 16 | D | −3 | SM | cis-flu | 10 | L | - | - |
| 17 | D | −3 | SM | cis-flu | 10 | L | RT (37, .038) | DT(31, .0003), RT (20, .03), MD(13, .02) |
| 18 | D | −7.2 | - | cis-flu | 10 | L | - | - |
| 19 | D | 3 | Assoc | cis-flu | 10 | L | - | - |
| 20 | D | 0 | Assoc | saline | 10 | L | - | MD (14, .03) |
| 21 | E | 5.4 | Assoc | cis-flu | 10 | R | - | - |
| 22 | E | 3.6 | Assoc | cis-flu | 10 | R | - | - |
| 23 | E | −0.6 | SM | cis-flu | 10 | R | DT (37, .012) | - |
| 24 | E | 6.6 | Assoc | cis-flu | 10 | R | - | - |
| 25 | E | 2.4 | Assoc | cis-flu | 10 | R | DT (33, .015) | DT (26, .047) |
| 26 | E | 6.6 | Assoc | saline | 10 | R | - | - |
| 27 | E | 3.6 | Assoc | cis-flu | 10 | R | DT (21, .01) | - |
| 28 | E | −4.2 | SM | cis-flu | 10 | R | MD (10, .013) | - |
| 29 | E | 5.4 | Assoc | cis-flu | 10 | R | - | - |
symbols identify repeat injections at the same location.
Underlines: injection sites where microstimulation elicited movements of the arm during previous microelectrode mapping.
Func. territory: SM=skeletomotor; Assoc=associative; Limb=limbic. Contralateral arm effects and Ipsilateral arm effects identified by type (DT = prolonged dwell times, RT = prolonged reaction times, MD = prolonged movement duration), magnitude (as percent increase relative to pre-injection mean), and significance level (p value). NT in the Ipsilateral arm column indicates experiments in which the animal performed the task with the contralateral arm only.
Pre-injection task performance
Both animals performed the bimanual reaching task reliably prior to injections. Mean DTs were relatively brief and consistent across trials (503±33 and 527±20 msec for monkeys D and E, respectively) suggesting that the animals initiated these non-cued non-rewarded movements efficiently in order to proceed to the next rewarded trial. Mean RTs (320±7 and 343±5 msec) and MDs (204±4 and 207±3 msec) were within ranges that are common for choice reaction time tasks such as this one (Gardinier et al., 2006).
On non-injection days, the two animals performed an average of 854 (±24) trials and 1169 (±60) trials per day over the course of approximately 1–2 hours. Day-to-day variability in the number of trials and the overall duration of task performance was due to: 1) gradual improvements in task performance over the several months these experiments required; and 2) daily variations in an animal’s satiety.
General effects of intra-striatal cis-flu injections
Following intra-striatal injections of cis-flu, animals continued to perform the behavioral task and displayed no gross abnormalities in behavioral state (e.g., no signs of catalepsy, agitation, or intrusion of grossly interfering movements or behaviors). However, marked changes in task performance were often noted. Figure 3 shows results from one cis-flu injection (injection site “a” in Fig. 2B) that induced significant increases in DT, RT, and MD for the arm contralateral to the injected hemisphere. Soon after injection onset (~10 minutes), the animal exhibited prolonged DTs (p < 0.05; χ2 = 9.7), which were more pronounced when the hand was held at the target ipsilateral to the moving arm (i.e., the “same-side” target) as compared with the cross-body target (454% and 173% increase for same-side and cross-body trials respectively, p < 0.05; Fig. 3A). During each prolonged DT, the animal’s arm appeared to be “frozen” in an elevated and extended posture with the animal’s hand touching the peripheral target. The animal continued to perform normal chewing, licking, and eye movements during these freezing events, so it is unlikely they reflected a global impairment such as general catalepsy. In this example, the prolongation of DTs peaked ~20 minutes following injection onset, was no longer evident ~37 minutes after injection onset, and only appeared in a subset of trials (i.e., of the 20 same-side movements that exhibited the maximal change in DTs during the post-injection period, only 45% were significantly prolonged).
Figure 3.
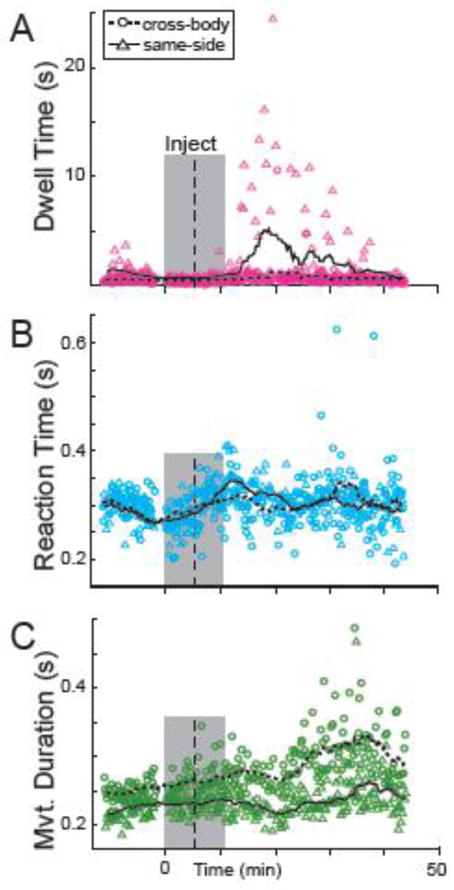
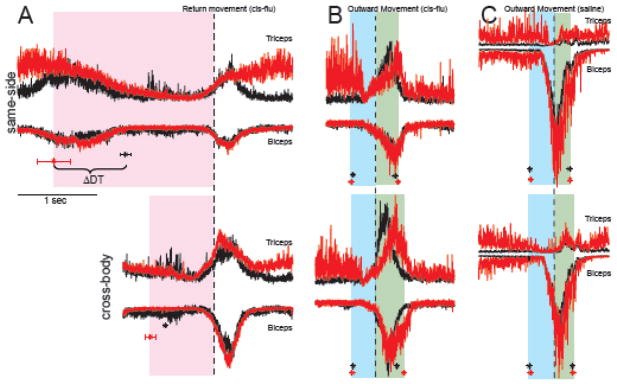
Exemplar data from one cis-flu injection experiment. Dwell times at the peripheral target (DTs) were prolonged markedly following this injection (A) while reaction times (RTs; B) and movement durations (MDs, C) showed smaller changes. During this experiment, the animal performed the task with the left arm only (contralateral to the infusion site). Data points represent the DTs (pink), RTs (blue), and MDs (green) of individual trials. Circles and triangles denote data from reaches to cross-body (right) and same-side (left) targets. Shaded box: period during which cis-flu was injected. Vertical dashed line: delineation between “pre-“ and “post-injection” periods used for all behavioral analyses. Dotted and continuous lines: moving averages (20 trials of the same trial type) for cross-body and same-side reaches, respectively.
The animal’s RTs and MDs began to slow as well starting ~20 minutes following injection onset (p < 0.05, χ2 = 4.4, Fig. 3B; p < 0.05, χ2 = 27.7, Fig. 3C, respectively). Injection-related increases in RT were comparable for cross-body (18%) and same-side (21%) movements. Increases in MD were greater for cross-body movements (32% increase) than for same-side reaches (17%; p < 0.05). Note that the increases in RT and MD appeared later and were of smaller magnitude than the increase in DT. Also note that for this injection, the animal was performing the task only with the arm contralateral to the infusion site.
Electromyographic (EMG) activity collected during the same experiment suggested that the prolongation of DTs was not attributable to exaggerated co-contraction of antagonist muscle pairs or a simple loss of muscle tone (Fig. 4A). EMG activity during the DT period (pink shading in Fig. 4A) changed very little following this and other cis-flu injections. More specifically, triceps and biceps EMG were not elevated tonically throughout prolonged DTs, contrary to what would be expected if co-contraction was a major contributor to DT prolongation. The initial ramp up of EMG leading to the onset of return movements (vertical dashed line in Fig. 4A) was also unchanged following this and other injections. Triceps activity was tonically elevated during the SPHP following completion of return movements (Fig. 4A) and prior to the onset of outward reaches (Fig. 4B). This elevation reflected the animal’s idiosyncratic tendency to push down on the homekey with more force later in a data collection session. Similar tonic elevations in triceps activity were also observed on non-injection days and following saline injections (Fig. 4C).
Figure 4.
Peri-event EMG activity from one cis-flu injection (same experiment as in Fig. 3). A) Mean “peri-return” EMG activity (mean rectified EMG averaged around the onsets of return movements) during pre- (black) and post- (red) injection periods. There was no evidence of increased co-contraction or loss of muscle tone to explain the lengthened DTs of the post-injection period. ΔDT: Difference in mean DTs from pre- and post-injection periods (black and red asterisks, respectively). Triceps EMG was elevated post-injection during the start position hold period, i.e., following return movements (A) and before outward movements (B). B) Mean EMG averaged around onsets of outward movements before (black) and after (red) the cis-flu injection. The peri-movement burst of triceps EMG showed a slowed rate of rise following the injection, particularly for cross-body movements (bottom row). Data in B were taken from the 20-trial epoch during which a maximal increase in MDs was observed. C) Peri-movement EMG activity before (black) and after (red) a saline infusion. Asterisks and horizontal error bars indicate means ± SEMs for DTs (panel A), and RTs and MDs (panels B and C). Shaded boxes indicate mean post-injection DT (pink), RT (blue) and MD (green). These EMG data were from the same cis-flu injection day as illustrated in Figure 3.
Injection-related increases in MD were accompanied by changes in peri-movement EMG activity. Figure 4B illustrates mean EMG averaged around the onset of reach movements to the peripheral targets. As compared with EMG from the pre-injection period, triceps activity during post-injection trials was characterized by a slower rate of rise and a later peak in activity during movement (Fig. 4B, green shading). Consistent with the effects of cis-flu on MDs (Fig. 3C), these EMG changes were more prominent for cross-body movements (bottom row, Fig. 4B) than for same-side movements.
Qualitative assessments indicated that striatal DA blockade increased limb rigidity, but did not elicit tremor. Specifically, experimenter-imposed passive flexions and extensions of the arm contralateral to the injection site revealed subjective evidence of increased rigidity following a subset of injections. Tremor was not observed after any injection.
Frequency and magnitude of behavioral effects
Fifteen of the twenty-six cis-flu injections induced some behavioral effect (prolonged DTs, RTs, and/or MDs, according to the χ2 tests; Table 1). Of the 15 injections that induced behavioral effects, 8 injections prolonged DTs, 9 injections prolonged RTs, and 6 prolonged MDs (Fig. 5A). The majority of the behavioral effects (13/15 cases, 87%) consisted of changes in DTs and/or RTs. Only rarely (2/15 cases, 13%) did an injection lengthen MDs alone. Injections of saline never prolonged DTs or RTs, although one saline injection did prolong MDs. Overall, impairments in movement initiation (lengthened DTs and/or RTs) were observed more than twice as frequently as bradykinesia-like effects (lengthened MDs; p < 0.05, χ2 = 4.1). RT and MD effects combined did not occur any more or less often than DT effects (p > 0.05, χ2 = 0.75).
Figure 5.
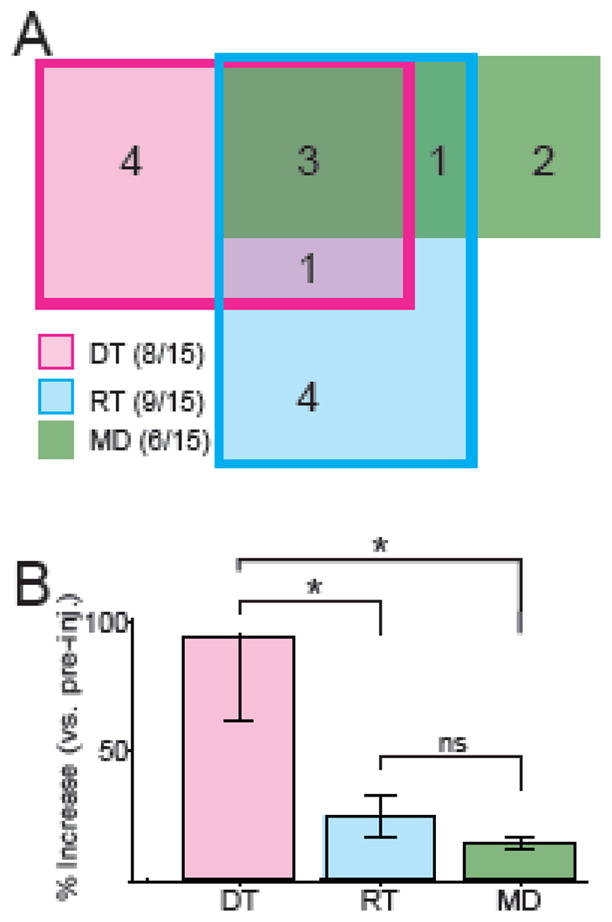
Summary of the behavioral effects following intra-striatal DA receptor blockade. A) Venn diagram of numbers of injections that induced one or multiple effects. RT (blue rectangle) and DT (pink rectangle) effects frequently occurred alone. In comparison, MD effects (green rectangle) did not typically occur alone. B) Mean (±SEM) magnitude of significant effects of cis-flu on DT, RT, and MD. Prolongations of DTs were significantly greater in magnitude than prolongations of RTs and MDs. * = p<0.05; ns = not significant; Mann-Whitney U test.
On average, maximal effects of cis-flu on DTs amounted to a near doubling of dwell time duration for the contralateral arm (95±33% increase; mean ±SEM of moving average maxima; Fig. 5B). Maximal effects of cis-flu on contralateral RTs (25±8% increase) and MDs (15±2%) were small in comparison to those on DTs (both p’s < 0.05, Mann-Whitney test; Fig. 5B). Across all effects, there was no difference in the magnitude of effects on same side movements and cross-body movements (paired t-tests, all p’s > 0.05). This suggests that the effects were more specific to the contralateral arm itself rather than for a specific reaching direction.
In summary, intra-striatal cis-flu injections slowed movement initiation (DTs and RTs) more frequently than movement execution (MDs). Furthermore, the initiation of non-cued non-rewarded movements (measured as a lengthening of DTs) was affected more severely than the initiation of externally-cued movements (measured as prolongation of RTs).
Behavioral abnormalities were induced independently
On many occasions, an injection affected one of the three measures of task performance without affecting the other two (Fig. 5A). In other cases, when multiple effects were induced by the same infusion, they often emerged with different latencies and severities (e.g., compare Fig. 3A–C). Thus, qualitative observations suggested that cis-flu injections could affect DTs, RTs, and MDs independently.
Consistent with that idea, we found no correlation between the effects of injections on DTs and RTs or between effects on DTs and MDs (p > 0.05, Spearman R values < 0.25; Table 2). Interestingly, injection-induced changes in RTs and MDs were well correlated across injections (p < 0.05, Spearman R = 0.56; Table 2) but only for the arm contralateral to the site of infusion, not for the ipsilateral arm (p = 0.56, Spearman R = 0.13).
Table 2.
Correlations between injection-induced changes in specific behaviors. The severity of injection-related changes in RTs and MDs was correlated for movements of the contralateral arm (Spearman correlations). The magnitude of cis-flu related changes were not correlated for any other behavioral measure.
| HT/RT | HT/MD | RT/MD | |
|---|---|---|---|
| Contralateral arm | p = 0.3, R = 0.2 | p = 0.27, R= 0.22 | p = 0.003, R = 0.56* |
| Ipsilateral arm | p = 0.9, R = −0.03 | p = 0.75, R = 0.07 | p = 0.56, R = 0.13 |
Topography of behavioral effects
We found no clear relationship between the location of an injection in the putamen and the pattern of behavioral deficits induced (Fig. 6). Contrary to predictions that depletion of the posterolateral skeletomotor region contributes most directly to motor impairments (Alexander et al., 1990; Kelly and Strick, 2004), many of the strongest effects on task performance were elicited by injections into pre-commissural regions of the putamen (Fig. 6A). Injections into areas of the putamen where arm movements were evoked by microstimulation (underlined letters and dots, Fig. 6B) did not elicit consistent behavioral effects.
Figure 6.
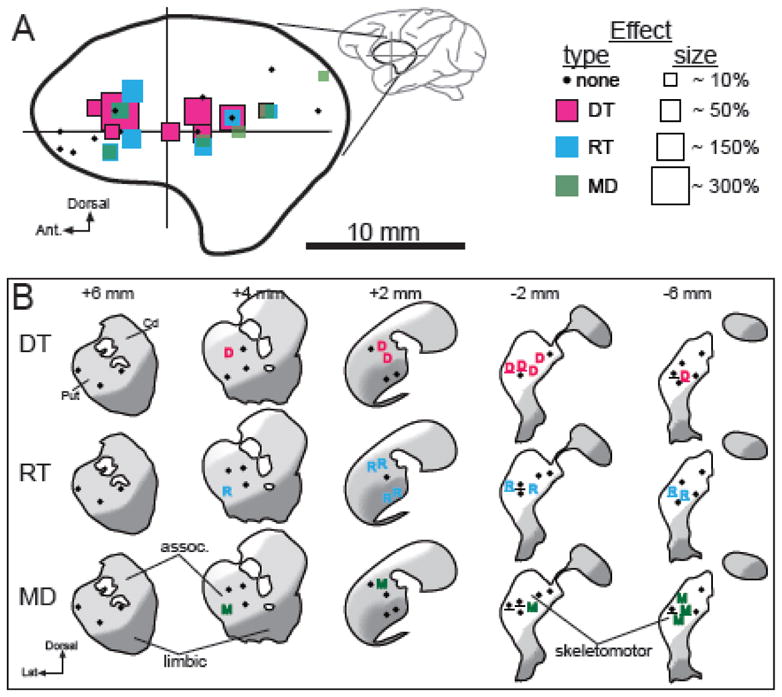
Anatomical reconstruction of injection sites and their effects. A) Each injection site is represented by a square color-coded by the type of impairment elicited and scaled in size according to the severity of the effect. Results are collapsed across the sagittal plane. Boundaries of the putamen in sagittal view (thick line) were taken from Francois et al. (1996). Crossed lines indicate the position of the anterior commissure. B) The locations of significant DT, RT, and MD effects are plotted onto standard coronal planes of the striatum adapted from (Martin and Bowden, 1996). Black dots: injection sites that elicited no effect. Underlines: injections performed at sites in the putamen where microstimulation evoked movement of the arm. Distances (top) indicate distance of each coronal plane anterior (+) or posterior (−) to the anterior commissure. Cd = caudate and Put = putamen. Approximate locations of the general skeletomotor, associative, and limbic functional territories of the striatum are indicated by white, light gray, and dark gray shading, respectively (see Methods).
Of the 26 cis-flu injections, 12 injections were located well within established boundaries for the skeletomotor territory (SM in Table 1 and white regions in Fig. 6B) and 12 injections were placed in associative or limbic regions of the putamen (light and dark gray regions in Fig. 6B). Two injections were located close to the boundary between skeletomotor and non-skeletomotor territories and thus were excluded from the analysis of functional topography. Injections of cis-flu into the skeletomotor region prolonged MDs in 33% of cases (4 of 12 injections) compared with MD effects in 17% of cases for injections into associative and limbic regions (2 of 12 injections; p=0.35, χ2 = 2.1). Significant effects on DTs and RTs appeared at approximately equal frequencies for injections into skeletomotor and associative or limbic striatal regions (both p’s>0.38, χ2 < 1.8). Similarly, the mean magnitudes of significant effects on DTs, RTs and MDs did not differ significantly for injections into skeletomotor and non-skeletomotor territories (all p’s>0.25, Mann Whitney test).
No evidence of general impairment in motivation or attention
Next, we tested whether intra-striatal infusions of cis-flu selectively impaired food-motivated operant responding, as predicted by a previous report (Beninger et al., 1993). We found no evidence that cis-flu injections reduced the rewarding nature of food or reduced an animal’s willingness or motivation to perform the operant task. When cis-flu was injected into monkey D’s left putamen, the monkey performed an average of 714±41 trials before reaching satiation. When saline was infused into the same hemisphere, the monkey performed only 519 trials. He therefore completed more trials after infusions of cis-flu than after saline. The same monkey performed 1093±92 trials when cis-flu was injected into the right hemisphere, similar to the 1094 trials he performed when saline was infused into the same hemisphere. Similarly, monkey E worked an average of 1227±29 trials on cis-flu injection days, equivalent to the 1230 trials she completed when saline was infused into the same hemisphere. In summary, both animals performed comparable numbers of trials on saline and cis-flu infusion days, providing no evidence that intra-putamenal cis-flu injections reduced an animal’s general motivation, attentiveness, or willingness to perform an operant task for food reward.
The laterality of cis-flu effects was also inconsistent with the idea that injections affected global motivation, cognition, or attention. Such effects would impair task performance equally for both arms, ipsilateral and contralateral to the injected hemisphere. Instead, the preponderance of behavioral effects appeared for reaches with the arm contralateral to the site of infusion (p < 0.05, χ2 = 11; Table 1). Specifically, 11 of the 15 injections that induced significant effects selectively affected performance of the contralateral arm, while only 2 of 15 selectively affected the ipsilateral arm and 2 of 15 induced bilateral effects.
DISCUSSION
For decades, the pathophysiology of PD has been routinely attributed to loss of DA from the skeletomotor region of the posterior putamen. It has become increasingly unclear, however, whether simple under-activation of DA receptors in this region is sufficient to induce parkinsonian signs. Recent studies have suggested possible roles for loss of DA from non-skeletomotor striatal regions, chronic striatal DA depletion, and extra-striatal DA loss in the emergence of parkinsonian signs (Francois et al., 2000; Freeman et al., 2001; Jan et al., 2000; Scatton et al., 1983; Scatton et al., 1982). Furthermore, some studies that blocked DA receptors in the striatum reported no evidence of parkinsonian signs (Beninger et al., 1993; Nakamura and Hikosaka, 2006). Given current efforts to develop genetic and transplant therapies to restore DA levels at selected sites in the CNS (Forsayeth et al., 2010; Wakeman et al., 2011), it is all the more important to establish the specific roles of striatal DA loss in the genesis of parkinsonian signs.
Intra-striatal DA receptor blockade is sufficient to induce parkinsonian signs
Intracerebral infusion of DA antagonists is one of few methods available that can block DA function at one CNS location in relative isolation. We used the DA antagonist approach to determine if acute focal blockade of striatal DA function can, by itself, generate the cardinal signs of PD. Although numerous studies in rodents have shown that intra-striatal infusions of DA receptor antagonists elicit a cataleptic state (Amalric and Koob, 1987; Ellenbroek et al., 1985; Hauber et al., 2001; Kaur et al., 1997; Salamone et al., 1993; Yoshida et al., 1994), it is unclear how those observations relate to the specific clinical signs of PD. Furthermore, not all studies of intra-striatal DA receptor blockade have observed catalepsy. When Beninger et al. (1993) infused cis-flu into the rat striatum, they observed only a time-dependent decline in operant responding that resembled extinction, with no evidence of parkinsonian signs. We know of only one previous report on the behavioral effects of DA antagonists infused into the striatum of monkeys (Nakamura and Hikosaka, 2006). In that study, intra-caudate infusions altered the normal variation of oculomotor RTs with the size of a reward, but did not elicit overt signs of parkinsonism. Because those infusions were performed in the oculomotor territory of the striatum, however, they may have induced other impairments that were not tested for (Chan et al., 2005; Shibasaki et al., 1979; White et al., 1983).
In contrast to those studies, we found that intra-putamenal infusions of cis-flu induced specific motor impairments reminiscent of parkinsonian signs. We found no evidence that cis-flu injections had a global impact on the rewarding nature of food or on an animal’s general attentiveness, willingness, or motivation to perform the operant task. Both animals performed comparable numbers of trials on saline and cis-flu infusion days. Furthermore, cis-flu infusions seldom (2 of 15 significant effects) affected task performance in both arms (i.e., contralateral and ipsilateral to the site of injection) as would be expected if those effects were mediated by a general impairment of cognition, motivation, reward processing or attention. Rather, cis-flu injections primarily affected performance of the contralateral arm, consistent with the idea that these were specific impairments of motor control.
Our results may diverge from previous reports because of differences in injection location [i.e., putamen here versus caudate and nucleus accumbens in previous studies (Beninger et al., 1993; Nakamura and Hikosaka, 2006)], agents used, species, tasks, and behavioral measures. Unlike the intra-caudate infusions performed previously in macaques (Nakamura and Hikosaka, 2006), we infused large quantities of the active agent, affected both major classes of DA receptors with the same infusion, and we targeted the putamen. Furthermore, our animals performed a task designed to measure motor deficits relevant to the cardinal skeletomotor signs of PD rather than eye movements specifically.
Intra-striatal cis-flu impaired movement initiation
Movement initiation was affected more frequently and more severely by intra-striatal DA receptor blockade than movement execution. Interestingly, movement initiation was affected more severely in the absence of direct exteroceptive cues of when to move and the promise of immediate future rewards. Similarly, PD patients are particularly impaired at initiating and executing internally-generated movements (Cooke et al., 1978; Flowers, 1976; Morris et al., 1996; Oliveira et al., 1997). The importance of this specific impairment in the overall disability of PD is highlighted by the success of assistive technologies that provide external sensory cues during activities that would otherwise require self-initiated movements (Arias and Cudeiro, 2010; Bachlin et al., 2010; Cunningham et al., 2009).
It is likely that intra-striatal infusions of cis-flu triggered pathophysiologic mechanisms similar to those explicated previously for other models of PD. Loss of striatal DA induces an imbalance in activity between striatal D1-receptor bearing direct pathway neurons and D2-receptor bearing indirect pathway neurons (Albin et al., 1989; Kravitz et al., 2010; Shen et al., 2008; Wichmann and DeLong, 1996), which in turn induces pathologic disturbances in neuronal signaling in whole basal ganglia-thalamocortical circuits (DeLong and Wichmann, 2007; McIntyre and Hahn, 2010). Why intra-striatal infusions of cis-flu induced the specific pattern of behavioral impairments observed here remains a matter of speculation. One potential explanation is that striatal DA, or more generally, the BG, plays a particularly important role in the generation of self-initiated movements (Cunnington et al., 2002; van Donkelaar et al., 1999; van Donkelaar et al., 2000). An alternative explanation is that neural systems that mediate self-initiated movement may be more susceptible to disruption because of an absence of redundant and compensatory mechanisms for that type of motor control. Irrespective of the specific mechanisms involved, our results indicate that initiation of movement in the absence of immediate sensory cues is especially susceptible to disruption by transient blockade of putamenal DA transmission.
In addition to prolonged DTs and RTs, we observed prolongation of MDs following a subset of cis-flu injections. Changes in MDs, however, were fewer in number and milder in severity than changes in DTs and RTs. This result raises the possibility that development of severe bradykinesia, as observed in many PD patients, depends on loss of DA at sites outside of the putamen (Rommelfanger and Wichmann, 2010) or perhaps chronic DA deficiency (Mallet et al., 2008).
Specific parkinsonian signs may have distinct pathophysiologies
A related implication of our results is that different parkinsonian signs are a product of different abnormalities in neuronal function. We were able to elicit one form of akinesia (prolongation of DTs) independent of other signs (prolongation of RTs and MDs). Following many injections, only one of the three measures of task performance showed significant prolongation (DTs, RTs, or MDs). Even when multiple effects were detected after an injection, the timing and severity of effects often varied considerably (Fig. 3). These observations are consistent with the general idea that different parkinsonian signs have separable pathophysiologic substrates.
The magnitude of cis-flu-related changes in RTs and MDs were correlated with each other, but neither was correlated with the magnitude of change in DTs. The correlation between RTs and MDs was observed only for movements of the arm contralateral to the infusion site, suggesting that increased RTs and MDs arose from one lateralized pathophysiologic mechanism. The independence of effects on DTs from those on RTs and MDs suggests that impairments of self-initiation and impairments of visually-triggered movement arise from different pathophysiologic substrates. Clearly, this is a topic for future study.
More generally, our findings are consistent with the many studies showing that subgroups of parkinsonian signs vary independently. It is now firmly established that patients with PD can be classified into subtypes characterized by postural instability/gait difficulty versus tremor dominant symptoms (Zetusky and Jankovic, 1985). The severity and rate of progression of these symptom subtypes varies independently between patients (Nieuwboer et al., 1998; Selikhova et al., 2009; Temperli et al., 2003; Zetusky and Jankovic, 1985). Our data support the idea that impairments in self-initiation and in the control of sensory-cued movement also vary independently in parkinsonism and that each may arise from different pathophysiologic substrates. While acute striatal DA loss appears to be an adequate trigger to produce akinetic signs, other defects such as extra-striatal DA loss or chronic striatal DA may be necessary to produce the profound impairments in sensory-cued movement observed in idiopathic PD.
Lack of Topographic organization of effects
The striatum is divided into anatomically segregated regions devoted to skeletomotor, associative, and limbic functions (Alexander et al., 1990; Kelly and Strick, 2004; Worbe et al., 2009). Loss of DA from the skeletomotor region is traditionally assumed to be the primary factor that leads to parkinsonian motor signs (Damier et al., 1999; Forno, 1996) while DA loss from associative and limbic regions has been proposed to underpin impairments of cognition and motivation (Redgrave et al., 2010; Salamone et al., 2003).
Our results are not consistent with that view. DA receptor blockade in posterior skeletomotor regions of the striatum was not more likely to elicit motor impairments than that into associative and limbic regions nor did it elicit more severe impairments (Fig. 6). Our observation of severe behavioral effects following injections into anterior/medial regions of the putamen is consistent with several radioligand imaging studies in human subjects showing that measures of symptom progression (e.g., akinesia measured as prolonged reaction times) correlate with the degree of DA loss from medial regions of the striatum, but not with DA loss in the dorsolateral “skeletomotor” region of putamen (Gallagher et al., 2011; Morrish et al., 1996; Pirker, 2003). These results also fit well with the recent finding that injections of bicuculline into the anterior-medial putamen induce hypoactivity in non-human primates (Worbe et al., 2009). This striatal location may correspond to the rostral ventromedial striatal site strongly associated with catalepsy in rodent microinjection studies (Yoshida et al., 1994).
Several factors may explain the apparent absence of an effect topography. From previous studies (Vogelbaum, 2005), we estimate that the drug diffused into a roughly spherical volume ~4.6 mm in diameter around the injection site, which amounts to ~11% of the total volume of the macaque putamen (Harman and Carpenter, 1950). Given this volume, it is likely that some injections affected more than one striatal functional region, especially when the injections were performed near functional boundaries (see Fig. 6). Cross-territory spread, however, is unlikely to account completely for the jumbled topography in our results. One possible explanation is that our results agree with the general idea that the striatum is organized as a patchy functional mosaic (Gerfen, 1992; Goldman and Nauta, 1977; Malach and Graybiel, 1986). Recent studies performed in rodents describe similar heterogeneous functional topographies (Aragona et al., 2009; Mahler et al., 2007). The limited number of injections performed in the current study prevent firm conclusions regarding this idea, however (see below). Taken together, our results indicate that components of the parkinsonian state can be elicited by transient interruption of DA signaling at a variety of putamenal locations, including sites in motor, associative, and limbic regions.
Limitations and Potential Confounds
Many infusions (42%) elicited no apparent behavioral effect. Some of these infusions may have altered behaviors that were not assayed by the arm movement task [e.g., changes in facial or leg motor control or in higher cognitive functions (Cooper et al., 1991; Dubois and Pillon, 1997; Lees and Smith, 1983; Owen et al., 1992; Pessiglione et al., 2003)]. Consistent with previous studies, however (Beninger et al., 1993; Costall et al., 1972; Nakamura and Hikosaka, 2006), we found that acute infusions of cis-flu can induce robust behavioral effects within sixty minutes or less. Additional variability may arise from cumulative tissue damage and the resulting low resistance paths for injectate reflux (Alexander et al., 2009). Importantly, failure to produce detectable behavioral effects in a large fraction of experiments is a common feature of intra-striatal pharmacologic manipulations in macaques (Bronfeld et al., 2011; McCairn et al., 2009; Worbe et al., 2009).
It is possible that larger areas of DA receptor blockade or longer periods of blockade would have yielded additional or more severe behavioral effects. Bilateral intra-striatal infusions also might have yielded more severe behavioral effects, although the strongly lateralized output organization of the basal ganglia (Hoover and Strick, 1993; Kelly and Strick, 2004) argues against that idea. Also, our experiments did not include the ventral-most portion of the posterior putamen, a region that appears to relay limbic cortical inputs to skeletomotor cortical regions (Kelly and Strick, 2004).
The pharmacodynamics of cis-flupenthixol must also be considered. While cis-flu binds with strong affinity to both the D1 and D2 receptors, the drug exhibits a slightly greater affinity for D2 over D1 receptors (Reimold et al., 2007). Cis-flu also binds with low affinity to 5-HT2 receptors, alpha 1-adrenergic receptors, and some cholinergic and histamine receptors (Hyttel et al., 1985; Reimold et al., 2007). It is therefore possible that some of the behavioral effects we observed were mediated by blockade of non-dopaminergic receptors, or preferential blockade of D2 receptors over D1 receptors. Further studies are needed to exclude these possibilities.
One additional point that may be seen as a limitation is that we were unable to elicit tremor. This is not surprising, however, because resting tremor is rarely observed in macaque models of parkinsonism, even following near total loss of putamenal DA (Bergman et al., 1994; Eberling et al., 2000). Furthermore, tremor is an outlier among the cardinal signs in that it’s severity does not correlate with the degree of striatal DA loss (Pirker, 2003) and some therapies (i.e., thalamotomy and thalamic stimulation) are effective only for tremor (Lyons and Pahwa, 2008; Walter and Vitek, 2004). It is difficult to interpret the effects of intra-striatal dopamine receptor blockade on rigidity, as the measurements of rigidity obtained here were unblinded and subjective. Importantly, we were able to elicit other cardinal signs of parkinsonism independently and with differing degrees of severity.
Conclusions
Our results indicate that acute, focal blockade of striatal DA receptors is sufficient to induce motor deficits reflective of specific parkinsonian signs. Intra-striatal infusion of a D1- and D2-receptor antagonist in awake, behaving macaques was found to slow the initiation of movement more than it’s execution. Paralleling observations in PD patients, the initiation of self-generated movements was impaired more severely than initiation of externally-cued movements. Additionally, motor deficits reminiscent of specific parkinsonian signs were often induced independently, thereby suggesting the existence of distinct pathophysiologic substrates for different signs. Finally, the less-frequent and weak effects of striatal cis-flu on movement speed (reflecting bradykinesia) accentuate the potential importance of extra-striatal DA loss in the genesis of that specific sign.
Highlights.
Is focal striatal dopamine loss sufficient to elicit parkinsonian signs in primates?
D1/D2 antagonist infusion into putamen variably elicited akinesia and bradykinesia.
Akinesia was most severe for self-initiated non-rewarded reaching movements.
Acute focal blockade of putamenal dopamine is sufficient to induce akinesia.
Elicting individual signs in isolation suggests they have distinct pathophysiologies.
Acknowledgments
We wish to thank Mary Watach and Angela Cowan for expert assistance with animal care and surgery. Dr. Benjamin Pasquereau provided invaluable advice and assistance throughout the project. Dr. Kwan-Jin Jung of the University of Pittsburgh Brain Imaging Research Center provided advanced directional image filtering of the MRI images that enhanced the contours of the basal ganglia. This research was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health, grant numbers R21-NS55197 and R01-NS070865 to RST. Vanessa Franco was supported by the National Institutes of Health, grant number T32-NS007433, and the Clinical and Translational Science Institute Multidisciplinary Predoctoral Fellowship program, awarded through the Clinical and Translational Science Institute and the Institute for Clinical Research Education at the University of Pittsburgh, grant 5TL1RR024155-04.
Abbreviations
- cis-flu
cis-flupenthixol
- DA
dopamine
- DT
dwell time
- MD
movement duration
- PD
Parkinson’s disease
- RT
reaction time
- SPHP
start position hold period
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–9. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, et al. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander AL, et al. PD Online Research. The Michael J. Fox Foundation for Parkinson’s Research; New York: 2009. Factors affecting drug distribution through infusion. [Google Scholar]
- Alexander G, et al. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander GE, et al. Basal ganglia thalamo-cortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Amalric M, Koob GF. Depletion of dopamine in the caudate nucleus but not in nucleus accumbens impairs reaction-time performance in rats. J Neurosci. 1987;7:2129–34. doi: 10.1523/JNEUROSCI.07-07-02129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, et al. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–99. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias P, Cudeiro J. Effects of rhythmic sensory stimulation (auditory, visual) on gait in Parkinson’s disease patients. Exp Brain Res. 2008;186:589–601. doi: 10.1007/s00221-007-1263-y. [DOI] [PubMed] [Google Scholar]
- Arias P, Cudeiro J. Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PLoS One. 2010;5:e9675. doi: 10.1371/journal.pone.0009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachlin M, et al. Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE Trans Inf Technol Biomed. 2010;14:436–46. doi: 10.1109/TITB.2009.2036165. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Benecke R, et al. Performance of simultaneous movements in patients with Parkinson’s disease. Brain. 1986;109:739–757. doi: 10.1093/brain/109.4.739. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, et al. Microinjections of flupenthixol into the caudate putamen of rats produce intrasession declines in food-rewarded operant responding. Pharmacol Biochem Behav. 1993;45:343–50. doi: 10.1016/0091-3057(93)90249-s. [DOI] [PubMed] [Google Scholar]
- Bergman H, et al. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–20. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, et al. Spatial and temporal properties of tic-related neuronal activity in the cortico-basal ganglia loop. J Neurosci. 2011;31:8713–21. doi: 10.1523/JNEUROSCI.0195-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Internal versus external cues and the control of attention in Parkinson’s disease. Brain. 1988;111:323–345. doi: 10.1093/brain/111.2.323. [DOI] [PubMed] [Google Scholar]
- Butterfield LC, et al. The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology. 2010;24:721–30. doi: 10.1037/a0019650. [DOI] [PubMed] [Google Scholar]
- Chan F, et al. Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia. 2005;43:784–96. doi: 10.1016/j.neuropsychologia.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Cooke JD, et al. Increased dependence on visual information for movement control in patients with Parkinson’s disease. Can J Neurol Sci. 1978;5:413–5. doi: 10.1017/s0317167100024197. [DOI] [PubMed] [Google Scholar]
- Cooper JA, et al. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Costall B, et al. Catalepsy and circling behaviour after intracerebral injections of neuroleptic, cholinergic and anticholinergic agents into the caudate-putamen, globus pallidus and substantia nigra of rat brain. Neuropharmacology. 1972;11:645–63. doi: 10.1016/0028-3908(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Cunningham LM, et al. A review of assistive technologies for people with Parkinson’s disease. Technol Health Care. 2009;17:269–79. doi: 10.3233/THC-2009-0547. [DOI] [PubMed] [Google Scholar]
- Cunnington R, et al. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–85. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Czernecki V, et al. Motivation, reward, and Parkinson’s disease: influence of dopatherapy. Neuropsychologia. 2002;40:2257–67. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Damier P, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–48. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Debeir T, et al. Effect of intra-striatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp Neurol. 2005;193:444–54. doi: 10.1016/j.expneurol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Turner RS. Testing Basal Ganglia motor functions through reversible inactivations in the posterior internal globus pallidus. J Neurophysiol. 2008;99:1057–76. doi: 10.1152/jn.01010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz MA, et al. Evaluation of a modified inverted walking stick as a treatment for parkinsonian freezing episodes. Mov Disord. 1990;5:243–7. doi: 10.1002/mds.870050311. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Eberling JL, et al. Tremor is associated with PET measures of nigrostriatal dopamine function in MPTP-lesioned monkeys. Exp Neurol. 2000;165:342–6. doi: 10.1006/exnr.2000.7470. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Verteilung von noradrenalin und dopamin (3-hydroxytyramin) im gehirn des menschen und ihr verhalten bei erkrankungen des extrapyramidalen systems. Klin Wschr. 1960;38:1236–1239. [Google Scholar]
- Ellenbroek B, et al. Muscular rigidity and delineation of a dopamine-specific neostriatal subregion: tonic EMG activity in rats. Brain Res. 1985;345:132–40. doi: 10.1016/0006-8993(85)90843-1. [DOI] [PubMed] [Google Scholar]
- Emborg ME. Nonhuman primate models of Parkinson’s disease. ILAR J. 2007;48:339–55. doi: 10.1093/ilar.48.4.339. [DOI] [PubMed] [Google Scholar]
- Espay AJ, et al. Impairments of speed and amplitude of movement in Parkinson’s disease: a pilot study. Mov Disord. 2009;24:1001–8. doi: 10.1002/mds.22480. [DOI] [PubMed] [Google Scholar]
- Evarts EV, et al. Reaction time in Parkinson’s disease. Brain. 1981;104:167–86. doi: 10.1093/brain/104.1.167. [DOI] [PubMed] [Google Scholar]
- Fahn S. The freezing phenomenon in parkinsonism. Adv Neurol. 1995;67:53–63. [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory sstem. Projections from physiologically mapped body-part representations. J Neurophysiol. 1991;66:1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- Flowers KA. Visual “closed-loop” and “open-loop” characteristics of voluntary movement in patients with Parkinsonism and intention tremor. Brain. 1976;99:269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Forsayeth J, et al. Gene therapy for Parkinson’s disease: where are we now and where are we going? Expert Rev Neurother. 2010;10:1839–45. doi: 10.1586/ern.10.161. [DOI] [PubMed] [Google Scholar]
- Francois C, et al. Dopaminergic innervation of the subthalamic nucleus in the normal state, in MPTP-treated monkeys, and in Parkinson’s disease patients. J Comp Neurol. 2000;425:121–9. doi: 10.1002/1096-9861(20000911)425:1<121::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Francois C, et al. A stereotaxic atlas of the basal ganglia in macaques. Brain Res Bull. 1996;41:151–8. doi: 10.1016/0361-9230(96)00161-x. [DOI] [PubMed] [Google Scholar]
- Francois C, et al. Calbindin D-28k as a marker for the associative cortical territory of the striatum in macaque. Brain Res. 1994;633:331–6. doi: 10.1016/0006-8993(94)91557-1. [DOI] [PubMed] [Google Scholar]
- Freeman A, et al. Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann Neurol. 2001;50:321–9. doi: 10.1002/ana.1119. [DOI] [PubMed] [Google Scholar]
- Fudge JL, et al. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Comp Neurol. 2004;476:330–47. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CL, et al. A longitudinal study of motor performance and striatal [18F]fluorodopa uptake in Parkinson’s disease. Brain Imaging Behav. 2011;5:203–11. doi: 10.1007/s11682-011-9124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinier J, et al. Interactions between lateralized choices of hand and target. Exp Brain Res. 2006;170:149–59. doi: 10.1007/s00221-005-0193-9. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJH. An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol. 1977;171:369–386. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Harman PJ, Carpenter MB. Volumetric comparisons of the basal ganglia of various primates including man. J Comp Neurol. 1950;93:125–37. doi: 10.1002/cne.900930107. [DOI] [PubMed] [Google Scholar]
- Hauber W, et al. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A(2A) receptors in the caudate-putamen of rats. Eur J Neurosci. 2001;14:1287–93. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, et al. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–84. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O, Kish S. Biochemical pathophysiology of Parkinson’s disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- Horstink MW, et al. Bimanual simultaneous motor performance and impaired ability to shift attention in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1990;53:685–90. doi: 10.1136/jnnp.53.8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttel J, et al. Receptor-binding profiles of neuroleptics. Psychopharmacology Suppl. 1985;2:9–18. doi: 10.1007/978-3-642-70140-5_2. [DOI] [PubMed] [Google Scholar]
- Inase M, et al. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- Jan C, et al. Dopaminergic innervation of the pallidum in the normal state, in MPTP-treated monkeys and in parkinsonian patients. Eur J Neurosci. 2000;12:4525–35. [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63(2):363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Jordan N, et al. A component analysis of the generation and release of isometric force in Parkinson’s disease. J Neurol Neurosurg Psychiatr. 1992;55:572–576. doi: 10.1136/jnnp.55.7.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, et al. MK 801 reverses haloperidol-induced catalepsy from both striatal and extrastriatal sites in the rat brain. Eur J Pharmacol. 1997;332:153–60. doi: 10.1016/s0014-2999(97)01078-9. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–59. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Kimber TE, et al. Voluntary movement after pallidotomy in severe Parkinson’s disease. Brain. 1999;122:895–906. doi: 10.1093/brain/122.5.895. [DOI] [PubMed] [Google Scholar]
- Kish SJ, et al. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kishore A, et al. Unilateral versus bilateral tasks in early asymmetric Parkinson’s disease: differential effects on bradykinesia. Mov Disord. 2007;22:328–33. doi: 10.1002/mds.21238. [DOI] [PubMed] [Google Scholar]
- Krauze MT, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–9. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Lees J, Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106:257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, et al. Cognitive impairment and dementia in patients with Parkinson disease. Curr Top Med Chem. 2009;9:903–12. [PMC free article] [PubMed] [Google Scholar]
- Lyons KE, Pahwa R. Deep brain stimulation and tremor. Neurotherapeutics. 2008;5:331–8. doi: 10.1016/j.nurt.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, et al. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–78. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Malach R, Graybiel AM. Mosaic architecture of the somatic sensory-recipient sector of the cat’s striatum. J Neurosci. 1986;6:3436–58. doi: 10.1523/JNEUROSCI.06-12-03436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese R, et al. The role of sensory cues in the rehabilitation of parkinsonian patients: a comparison of two physical therapy protocols. Mov Disord. 2000;15:879–83. doi: 10.1002/1531-8257(200009)15:5<879::aid-mds1018>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Martin RF, Bowden DM. A stereotaxic template atlas of the macaque brain for digital imaging and quantitative neuroanatomy. Neuroimage. 1996;4:119–50. doi: 10.1006/nimg.1996.0036. [DOI] [PubMed] [Google Scholar]
- McCairn KW, et al. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132:2125–38. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- McDowell SA, Harris J. Irrelevant peripheral visual stimuli impair manual reaction times in Parkinson’s disease. Vision Res. 1997;37:3549–58. doi: 10.1016/S0042-6989(97)00188-0. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38:329–37. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CH. Akinesia in Parkinsonism. Relation between spontaneous movement (other than tremor) and voluntary movements made on command. J Neurol Neurosurg Psychiatry. 1982;45:582–5. doi: 10.1136/jnnp.45.7.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, et al. Stereotactic neurosurgical planning, recording, and visualization for deep brain stimulation in non-human primates. J Neurosci Methods. 2007;162:32–41. doi: 10.1016/j.jneumeth.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, et al. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119(Pt 2):551–68. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- Morrish PK, et al. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119:585–91. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci. 2006;26:5360–9. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandhagopal R, et al. Longitudinal progression of sporadic Parkinson’s disease: a multi-tracer positron emission tomography study. Brain. 2009;132:2970–9. doi: 10.1093/brain/awp209. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, et al. A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil Rehabil. 1998;20:142–50. doi: 10.3109/09638289809166074. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, et al. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci. 2009;29:1422–30. doi: 10.1111/j.1460-9568.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- Oguru M, et al. Apathy and depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2010;23:35–41. doi: 10.1177/0891988709351834. [DOI] [PubMed] [Google Scholar]
- Oiwa Y, et al. Progressive and extensive dopaminergic degeneration induced by convection-enhanced delivery of 6-hydroxydopamine into the rat striatum: a novel rodent model of Parkinson disease. J Neurosurg. 2003;98:136–44. doi: 10.3171/jns.2003.98.1.0136. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, et al. Micrographia in Parkinson’s disease: the effect of providing external cues. J Neurol Neurosurg Psychiatry. 1997;63:429–33. doi: 10.1136/jnnp.63.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–51. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Parent A, et al. Calcium-binding proteins in primate basal ganglia. Neurosci Res. 1996;25:309–34. doi: 10.1016/0168-0102(96)01065-6. [DOI] [PubMed] [Google Scholar]
- Parkinson J. An essay on the shaking palsy. Wittingham & Rowland; London: 1817. [Google Scholar]
- Pavese N, et al. Progression of monoaminergic dysfunction in Parkinson’s disease: a longitudinal 18F-dopa PET study. Neuroimage. 2011;56:1463–8. doi: 10.1016/j.neuroimage.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, et al. Impairment of context-adapted movement selection in a primate model of presymptomatic Parkinson’s disease. Brain. 2003;126:1392–408. doi: 10.1093/brain/awg139. [DOI] [PubMed] [Google Scholar]
- Pifl C, et al. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropoyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience. 1991;44:591–605. doi: 10.1016/0306-4522(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Pirker W. Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord. 2003;18(Suppl 7):S43–51. doi: 10.1002/mds.10579. [DOI] [PubMed] [Google Scholar]
- Poliakoff E, et al. Orienting of attention and Parkinson’s disease: tactile inhibition of return and response inhibition. Brain. 2003;126:2081–92. doi: 10.1093/brain/awg210. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat FM. Failed suppression of direct visuomotor activation in Parkinson’s disease. J Cogn Neurosci. 2001;13:31–43. doi: 10.1162/089892901564153. [DOI] [PubMed] [Google Scholar]
- Praamstra P, et al. Reliance on external cues for movement initiation in Parkinson’s disease. Evidence from movement-related potentials. Brain. 1998;121:167–77. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Redgrave P, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold M, et al. Occupancy of dopamine D(1), D (2) and serotonin (2A) receptors in schizophrenic patients treated with flupentixol in comparison with risperidone and haloperidol. Psychopharmacology (Berl) 2007;190:241–9. doi: 10.1007/s00213-006-0611-0. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T. Extrastriatal dopaminergic circuits of the Basal Ganglia. Front Neuroanat. 2010;4:139. doi: 10.3389/fnana.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–62. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- Salamone JD, et al. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, et al. The role of brain dopamine in response initiation: effects of haloperidol and regionally specific dopamine depletions on the local rate of instrumental responding. Brain Res. 1993;628:218–26. doi: 10.1016/0006-8993(93)90958-p. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intra-striatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Scatton B, et al. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–8. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Scatton B, et al. Dopamine deficiency in the cerebral cortex in Parkinson disease. Neurology. 1982;32:1039–40. doi: 10.1212/wnl.32.9.1039. [DOI] [PubMed] [Google Scholar]
- Seibyl JP, et al. Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol. 1995;38:589–98. doi: 10.1002/ana.410380407. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of cortico-striatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selikhova M, et al. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132:2947–57. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- Shen W, et al. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, et al. Oculomotor abnormalities in Parkinson’s disease. Arch Neurol. 1979;36:360–4. doi: 10.1001/archneur.1979.00500420070009. [DOI] [PubMed] [Google Scholar]
- Takada M, et al. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci. 2001;14:1633–50. doi: 10.1046/j.0953-816x.2001.01789.x. [DOI] [PubMed] [Google Scholar]
- Temperli P, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Turner R, DeLong M. Corticostriatal activity in primary motor cortex of the macaque. J Neurosci. 2000;20:7096–108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar P, et al. Neuronal activity in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 1999;82:934–45. doi: 10.1152/jn.1999.82.2.934. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, et al. Temporary inactivation in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 2000;83:2780–90. doi: 10.1152/jn.2000.83.5.2780. [DOI] [PubMed] [Google Scholar]
- Vogelbaum MA. Convection enhanced delivery for the treatment of malignant gliomas: symposium review. J Neurooncol. 2005;73:57–69. doi: 10.1007/s11060-004-2243-8. [DOI] [PubMed] [Google Scholar]
- Wakeman DR, et al. Cell transplantation and gene therapy in Parkinson’s disease. Mt Sinai J Med. 2011;78:126–58. doi: 10.1002/msj.20233. [DOI] [PubMed] [Google Scholar]
- Walter BL, Vitek JL. Surgical treatment for Parkinson’s disease. Lancet Neurol. 2004;3:719–28. doi: 10.1016/S1474-4422(04)00934-2. [DOI] [PubMed] [Google Scholar]
- White OB, et al. Ocular motor deficits in Parkinson’s disease. I. The horizontal vestibulo-ocular reflex and its regulation. Brain. 1983;106:555–570. doi: 10.1093/brain/106.3.555. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wichmann T, et al. Milestones in research on the pathophysiology of Parkinson’s disease. Mov Disord. 2011;26:1032–41. doi: 10.1002/mds.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex. 2009;19:1844–56. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]
- Worbe Y, et al. Discontinuous Long-Train Stimulation in the Anterior Striatum in Monkeys Induces Abnormal Behavioral States. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr063. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Van Hoesen GW. Cortico-striate projections in the Rhesus monkey: The organization of certain cortico-caudate connections. Brain Research. 1978;139:43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, et al. Distinct sites of dopaminergic and glutamatergic regulation of haloperidol-induced catalepsy within the rat caudate-putamen. Brain Res. 1994;639:139–48. doi: 10.1016/0006-8993(94)91774-4. [DOI] [PubMed] [Google Scholar]
- Zetusky WJ, Jankovic J. Laterality and symptom association in Parkinson’s disease. Arch Neurol. 1985;42:1132–3. doi: 10.1001/archneur.1985.04060110010001. [DOI] [PubMed] [Google Scholar]