Abstract
Estimation of low density lipoprotein cholesterol (LDL-C) is crucial in management of coronary artery disease patients. Though a number of homogenous assays are available for estimation of LDL-C, use of calculated LDL-C by Friedewald’s formula (FF) is common in Indian laboratories for logistic reasons. Recently Anandaraja and colleagues have derived a new formula for calculating LDL-C. This formula needs to be evaluated before it is extensively applied in diagnosis. We measured LDL-C by homogenous method (D-LDL-C) in 515 fasting samples. Friedewald’s and Anandaraja’s formulas were used for calculation of LDL-C (F-LDL-C and A-LDL-C, respectively). The mean LDL-C levels were 123.3 ± 53.2, 112.4 ± 50.2 and 109.2 ± 49.8 mg/dl for D-LDL-C, F-LDL-C and A-LDL-C, respectively. There was a statistically significant difference between the results (P > 0.001) obtained by calculation formulas compared to the measured LDL-C. There was underestimation of LDL-C by 10.8 and 14 mg/dl by Friedewald’s and Anandaraja’s formulas respectively. The Pearson’s correlation between F-LDL-C and D-LDL-C was 0.931 and that between A-LDL-C and D-LDL-C was 0.930. Bland–Altman graphs showed a definite agreement between mean and differences of the calculation formulas and direct LDL-C with 95% of values lying with in ±2 SD limits. The mean percentage difference (calculated as {(Calculated LDL-C)-(D-LDL-C)}/D-LDL-C × 100) for F-LDL-C was maximum (−11.6%) at HDL-C ≥ 60 mg/dl and TG levels of 200–300 mg/dl (−10.4%) compared to D-LDL-C. A-LDL-C results gave highest mean percentage difference at total cholesterol concentrations <100 mg/dl (−37.3%) and HDL-C < 40 mg/dl (−17.1%), respectively. The results of our study showed that FF is better in agreement with D-LDL-C than Anandaraja’s formula for estimation of LDL-C by calculation though both lead to its underestimation.
Keywords: LDL-C, Friedewald’s formula, Anandaraja’s Formula
Introduction
The concentration of low density lipoprotein cholesterol (LDL-C) is one of the strongest markers of atherosclerosis and predictor for assessing the risk for coronary heart disease (CHD). A strong positive correlation between increased LDL-C and CHD has been well documented from various epidemiological and clinical studies [1–4]. According to the National Cholesterol Education Programme (NCEP) Adult Treatment Panel, LDL-C concentration is the primary basis for treatment and appropriate patient’s classification in risk categories [5]. The reference method for determining LDL-C is β-quantification [6]. It requires ultracentrifugation, uses large volumes of samples and is a time consuming and expensive technique. Therefore, this method is not suitable for routine laboratory testing [7]. In 1972, Friedewald et al. published a landmark report describing a formula to estimate LDL-C as an alternative to tedious ultra centrifugation. Because VLDL (very low density lipoprotein) carries most of the circulating triglycerides (TG), VLDL-C can be estimated reasonably well from the measured TG divided by 5 for mg/dl units. LDL-C is then calculated as total cholesterol (TC) minus high density lipoprotein cholesterol (HDL-C) minus estimated VLDL-C [8]. Although this estimation formula correlates highly with beta quantification, it has certain limitations: it is not valid for samples with chylomicrons, with TG > 400 mg/dl or in patients with dysbetalipoprotenemia. This formula assumes the ratio of total TG to VLDL-C to be constant in all samples. The formula will overestimate VLDL-C and underestimate LDL-C as a consequence if TG rich chylomicrons and chylomicron remnants are present in the serum sample (hence the requirement for a fasting sample) [9]. The use of this formula is not recommended for type 2 diabetes, nephrotic syndrome and chronic alcoholic patients because accompanying abnormalities in lipoprotein composition render the underlying assumptions invalid for assessment of cardiovascular risk in these patients and thus leading to erroneous results even when TG levels are between 200 and 400 mg/dl [10]. The NCEP working group on lipoprotein measurements has recommended that the LDL-C concentration be determined with a total analytical error not exceeding ±12% (≤4% imprecision and ≤4% inaccuracy) to guarantee correct patient classification into NCEP risk categories [11]. It is difficult to obtain this analytical quality with Friedewald’s formula (FF) because each component’s analytical error is added [7].
Homogenous assays, developed in 1998 in an effort to overcome the limitations existing with both beta quantification and the Friedewald formula, represent the third generation of LDL-C measurements [12]. These homogenous direct methods use various physicochemical combinations of surfactants, polymeric complexes, and specific binding molecules to selectively measure cholesterol from LDL fraction [13]. There are five commercially available homogenous assays for LDL-C estimation and each of these has been certified by the Cholesterol Reference Method Laboratory Network of the Centres for Disease control and Prevention [14]. But these methods are not routinely used in most of the Indian laboratories as they are expensive which increase the cost of lipid profile estimation. Moreover many studies done to compare the direct methods with FF have shown to give the results comparable to the Friedewald calculation [14–16].
In spite of the technical disadvantages of FF, it is difficult to displace it from clinical practice unless a method with clear advantages in performance and overall cost effectiveness is developed. Recently a new formula for calculation of LDL-C has been proposed by Anandaraja et al. [17]. This formula uses only two analytes, TG and TC for calculation which may decrease the total error when compared to the FF in which analytical errors of three analytes get added in calculus. Since the formula does not require HDL-C result for calculation, it can prove to be more economical also.
Anandaraja’s formula has been approved for use in Brazilian and Greek population [18, 19]. There are no studies reporting use of this new formula in India. The formula needs to be validated before approval for routine use in clinical laboratories. The aim of this study is to compare the results obtained by direct homogenous assay for LDL-C to those obtained by Friedewald’s and Anandaraja’s formulas with the assumption that the results obtained by direct assay are the most accurate.
Materials and Methods
This was a comparative study for the estimation of LDL-C using two different formulas and direct estimation by a homogenous assay. The study was approved by the institutional ethical committee. Data was collected for the lipid profile samples received in the lab of a tertiary care hospital and included patients of at least 18 years of age. Patients were excluded if the lipid profile was incomplete. Lipid profiles with TG > 400 mg/dl were also excluded.
The serum samples were obtained by withdrawing 3 ml of venous blood after 10–12 h of overnight fasting and collected in plain vials. The serum was separated by centrifugation and analyzed on Hitachi 912 autoanalyser. TC and TG were measured enzymatically by CHOD-PAP [20] and Glycerol phosphate peroxidase-PAP [21] methods, respectively using reagent kits obtained from Narmis Diagnostics, Surrey, UK. TG and TC was calibrated using general chemistry calibrator provided by Narmis diagnostics. Level 1 and 2 control sera (Narmis Diagnostics) were used as quality control for these parameters.
Lipoprotein Analysis
The reagent kit for direct LDL-C assay (N-geneous LDL cholesterol reagent) was manufactured by Narmis Diagnostic Ltd, Surrey, UK. The homogenous assay is based on synthetic polymer method of Daiichi [14]. Detergent 1 causes release of cholesterol from HDL, VLDL and chylomicrons so that it can be removed. Reagent 2 contains another detergent which specifically acts on LDL to release cholesterol which can be estimated enzymatically by cholesterol oxidase-peroxidase.
HDL-C measurement was performed using a homogenous method without precipitation with the use of ultra N genous HDL cholesterol reagent provided by Narmis Diagnostic Ltd, Surrey, UK. Reagent 1 disrupts specifically HDL-C releasing cholesterol. In the second reaction concentration of HDL-C is measured enzymatically.
Narmis HDL/LDL cholesterol calibrator was used for the calibration of HDL-C and LDL-C.
Precision
The intraassay precision was calculated as the mean variance obtained for 20 replicate analyses at the same time in a day using Narmis Diagnostics level 2 general chemistry control for TG and TC and Narmis Diagnostics HDL and LDL-C control for HDL-C and LDL-C. The coefficient of variation (CV) for TG and TC at mean levels of 176 and 177 mg/dl respectively was 2.82 and 1.52% respectively. CV for HDL-C at mean level of 58 mg/dl was 0.5% and that for LDL-C at the mean level of 64 mg/dl was 0.9%. The interassay precision was analyzed by using a fresh aliquot of control material on each day for 20 consecutive days. The control material used were Narmis Diagnostics general chemistry control level 1 and 2 for TG and TC and Narmis Diagnostics HDL and LDL-C control for HDL-C and LDL-C. CV for TG at mean levels of 94.7 and 176 mg/dl was 2.4 and 3.2%, respectively. CV for TC at mean levels of 96.7 and 177 mg/dl was 2.7 and 1.5% respectively. CV for HDL-C was 2.1% at 58 mg/dl and that for LDL-C was 2.9% at mean value of 64 mg/dl.
Total error for LDL-C estimation was calculated by using the equation: TE = %bias + 1.96 (CVa) where % bias is the mean laboratory difference between the measured value for a control serum pool and the reference value for the pool. CVa is the overall analytical CV for the pool including within and among run variation and calculated as Standard deviation/lab mean × 100 [22].
The total error for the homogenous LDL-C assay was 5.6% which was within the allowable maximum error of 12% [11] as per NCEP guidelines.
LDL-C concentrations were also calculated by FF [8] and Anandaraja’s formula [17] as follows:
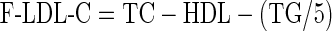 |
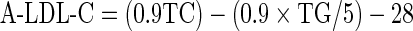 |
The mean percentage difference (%ΔLDL) defined as calculated LDL-C minus D-LDL-C compared to the direct measurement was calculated using the formula:
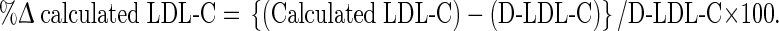 |
The performance of two formulas was compared at different levels of TC, TG and HDL-C.
Statistical Analysis
The statistical analysis was done using Microsoft Excel 2007 and SPSS version 16.0. Paired ‘t’ test and Pearson’s correlation coefficient were used for the analysis. Two tailed P value of <0.05 were considered statistically significant. To examine the degree of agreement between the values obtained by the two methods, Bland–Altman graphical plots were used.
Results
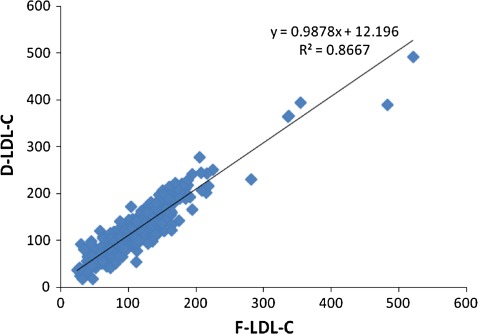
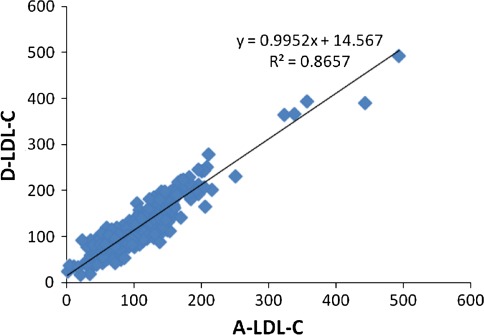
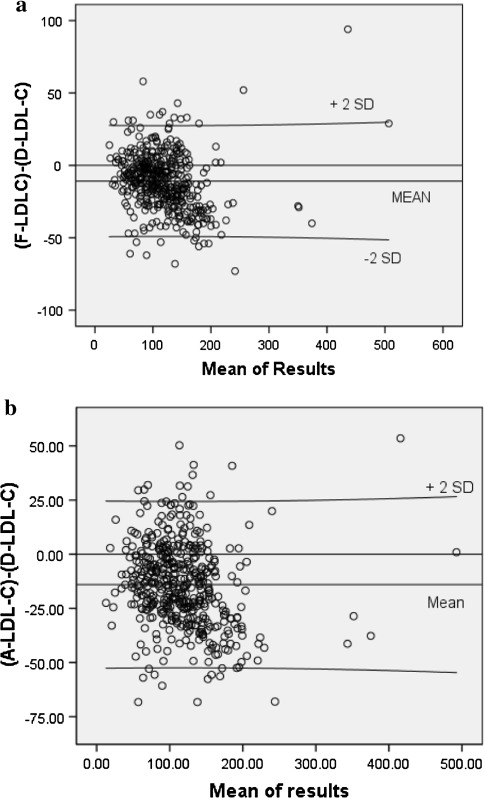
A total of 515 lipid profiles were assessed. Of these, 46 (8.9%) had TG levels more than 400 mg/dl and thus excluded from analysis. Out of the 469 samples for which analysis was done, 240 (51.2%) were received from the male patients and 229 (48.8%) were from females. The mean age of the patients was 48.8 ± 14.2 years. The mean TC was 187.4 ± 60.6 mg/dl. Paired ‘t’ test showed a statistically significant difference (P < 0.001) between the measured and the calculated LDL-C (Table 1). A good correlation was found between D-LDL-C and F-LDL-C (r = 0.931) (Fig. 1). Pearson’s correlation between D-LDL-C and A-LDL-C was 0.930 (Fig. 2). Bland–Altman plots indicated an obvious relationship between the differences and mean for both the calculation formulas and the measured LDL-C. The negative bias can be well appreciated in both the graphs (Fig. 3).
Table 1.
Paired samples statistics and correlations
| Mean ± SD (mg/dl) | Mean difference (mg/dl) | Correlation (r) | P value | %ΔLDL-C | |
|---|---|---|---|---|---|
| F-LDL-C vs. D-LDL-C | 112.4 ± 50.2 123.3 ± 53.2 |
−10.8 | 0.931 | <0.001 | −8.8 |
| A-LDL-C vs. D-LDL-C | 109.2 ± 49.8 123.3 ± 53.2 |
−14.0 | 0.930 | <0.001 | −11.4 |
Fig. 1.
Comparison of F-LDL-C vs. D-LDL-C. Scatter plot of F-LDL-C against directly measured LDL-C. There was a correlation of r2 = 0.867 and r = 0.931
Fig. 2.
Comparison of A-LDL-C vs. D-LDL-C. Scatter plot of A-LDL-C against directly measured LDL-C. There was a correlation of r2 = 0.866 and r = 0.930
Fig. 3.
a Bland–Altman plot for LDL-C estimated directly and by Friedewald’s calculation. b Bland–Altman plot for LDL-C estimated directly and by Anandaraja’s calculation
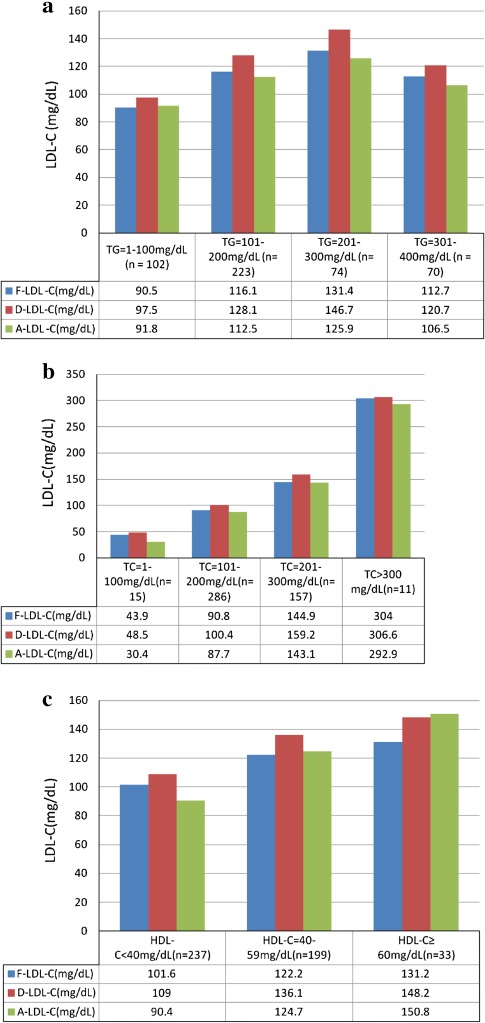
Comparison of LDL-C results at different levels of TGs showed statistically significant difference (P < 0.001) between measured values and those calculated by Friedewald’s and Anandaraja’s formulas. There was underestimation of LDL-C by calculation at all the levels of TGs which was maximum at TG levels of 200–300 mg/dl for both the formulas (Fig. 4a). Comparison of calculated and D-LDL-C at different levels of TC ranging from 45 to 635 mg/dl exhibited underestimation by calculation. The difference was statistically significant (P < 0.001) for A-LDL-C at all levels except at TC > 300 mg/dl. F-LDL-C had statistically significant difference from D-LDL-C at TC levels of 101–300 mg/dl (Fig. 4b). There was statistically significant (P < 0.001) underestimation of LDL-C by FF at all levels of HDL-C. A-LDL-C was statistically significantly lower than the D-LDL-C at all levels of HDL-C except at levels ≥60 mg/dl (Fig. 4c).
Fig. 4.
Comparison of calculated and direct LDL-C results at different levels of TG, TC and HDL-C. a Comparison of calculated and direct LDL-C results at different levels of TG. b Comparison of calculated and direct LDL-C values at different levels of TC. c Comparison of calculated and direct LDL-C values at different levels of HDL-C
Discussion
Strategies for the treatment of lipid abnormalities are primarily based on the concentrations of LDL-C. Therefore, LDL-C must be accurately determined to establish CHD risk profile in order to initiate dietary adjustments, drug therapy and to monitor their effects. Beta quantification, which is the reference method [6] for LDL-C estimation is time consuming and expensive and is not suitable for routine laboratory testing [7]. Homogenous methods developed during last few years are expensive and have failed to show clear advantages in terms of performance when compared to Friedewald’s calculation [14–16]. But FF has its well known limitations [9, 10], the most important being difficulty in obtaining recommended analytical quality of <12% total error [7]. In the past few decades attempts have been made to derive more accurate formulas for LDL-C calculation [23–27]. Anandaraja et al. [17] described a new formula for calculation of LDL-C in Indian population of 1000 patients by applying multiple linear regression analysis and validated its accuracy in 1008 patients. They confirmed a reduction in false overestimation of LDL-C compared with FF. The present study was designed to evaluate the performance of this formula in another set of Indian patients. Anandaraja et al. measured direct LDL-C by precipitation method. In our study detergent based homogenous method of Daiichi was used. The correlation between F-LDL-C and D-LDL-C in their study was 0.88. We have found a correlation of 0.931 between these two. Other studies have reported a correlation 0.86 [28] and 0.88 [16] and 0.786 [12], respectively. In a study done in Japan, a positive correlation was found between F-LDL-C and D-LDL-C with r2 = 0.975 [29]. Anandaraja et al. [17] reported the Pearson’s correlation of 0.97 between LDL-C measured by their formula and D-LDL-C which was better as compared to that for F-LDL-C. This correlation was 0.930 in our study which is similar to that obtained for F-LDL-C (Table 1). Vujovic et al. have reported a correlation of 0.89 between A-LDL-C and D-LDL-C in the study done in Serbian population [30]. Kamal et al. [12] have also reported a good correlation between these with r = 0.810.
We have found measured LDL-C to be higher than that obtained by calculation using both the formulas. The only exception was higher A-LDL-C results compared to the measured LDL-C when HDL-C levels were ≥60 mg/dl though the difference was statistically insignificant. Kamal et al. [12] have reported an underestimation of 17 and 22 mg/dl by Friedewald’s and Anandaraja’s formulas respectively. This difference in our study was 10.8 and 14 mg/dl respectively. Kamazeki et al. [29] have reported an underestimation of 5.9 mg/dl by FF compared to the directly measured LDL-C. Vujovic et al. [30] have also reported higher values for D-LDL-C. They have found a percentage difference of −6.9 for F-LDL-C and −3.9% for A-LDL-C. In our study %ΔLDL-C for Anandaraja’s formula was higher at −11.4% compared to that for FF at −8.8% (Table 1). In the study by Agrawal et al. [31], comparison of F-LDL-C results with measured LDL-C during three different periods with three different homogenous assays was done. A substantial lack of agreement between direct and calculated LDL-C with higher D-LDL-C values by all the methods in spite of having good correlation coefficients was reported by the authors. Some studies have reported opposite trends with higher results with calculated LDL-C by FF as compared to measured LDL-C [16, 18]. In the study by Gasko et al. [18], results by Anandaraja’s formula were closer to direct measurement with a mean difference of −1 mg/dl.
The difference between measured and calculated LDL-C results can be significant in terms of patients’ risk classification for coronary artery disease. According to NCEP ATP III, LDL-C levels of 160, 130 and 100 mg/dl are the treatment goals for low risk, moderate risk and high risk patients for CHD, respectively [5]. We have found a statistically significant difference in risk classification of patients when direct LDL-C was used instead of the calculated one (Table 2). Similar results have been reported by other authors also [12, 29, 31]. Direct measurement leads to approximately 10% more patients being candidate for lipid lowering drug therapy as compared to the use of calculated LDL-C. Use of Anandaraja’s formula does not produce any significant effect on patient risk classification when compared to FF (Table 2).
Table 2.
Comparison of patient risk classification on basis of LDL-C levels using direct measurement and calculation formulas
| LDL-C (mg/dl) | Number of patients by direct estimation | Number of patients by FF | Number of patients by Anandaraja’s formula |
|---|---|---|---|
| >100 | 305 (65.0%) | 271 (57.8%) | 260 (55.4%) |
| >130 | 189 (40.3%) | 147 (31.4%) | 141 (30.1%) |
| >160 | 93 (19.8%) | 49 (10.4%) | 46 (9.8%) |
Comparison of LDL-C results obtained by both the formulas at different levels of the three analytes indicates that low TC levels of <100 mg/dl produce maximum difference in A-LDL-C results. Low cholesterol levels produce a %ΔA-LDL-C of −37.3% which is a very significant difference for reporting. HDL-C levels <40 mg/dl is also a major source of difference with −17.1% error in results. Paz et al. [32] have demonstrated that A-LDL-C were underestimated or overestimated compared to LDL-C electrophoresis and depended on HDL-C concentrations. Vujovic et al. have also supported their observation and commented that HDL-C should not be omitted from the formula. Our results are similar to their findings. Error in F-LDL-C results was maximum at HDL-C ≥ 60 mg/dl (−11.6%) and TG concentration of 201–300 mg/dl (−10.4%). No other study has reported the effect of high HDL-C levels on the results obtained by FF to the best of our knowledge. As TG levels increase, increase in mean difference between the results of direct and F-LDL-C has been reported in previous studies [12, 33]. Our results support this finding except at TG > 300 mg/dl when mean error was less than that obtained for TG levels of 200–300 mg/dl (Fig. 4a).
Conclusion
Calculated LDL-C results obtained by Friedewald’s and Anandaraja’s formulas show very good correlation with the measured LDL-C. But the negative bias in results is responsible for producing statistically significantly different results compared to the directly measured LDL-C. Using direct LDL-C for reporting should be the obvious choice in this situation. But that will not only increase the cost of the test but also put more patients on drug therapy, exposing patients to more potential adverse effects and at a much greater cost with little evidence of benefit [31]. The reason being, most of the clinical trials documenting the benefits of lipid lowering drugs have used FF for reporting of LDL-C except for the Heart protection study [28, 34]. Compared to FF, Anandaraja’s formula tends to give higher percentage error and does not perform well especially in patients with low HDL-C and TC. We conclude that in spite of its limitations, FF is better than Anandaraja’s formula for estimation of LDL-C by calculation.
Contributor Information
Shalini Gupta, Phone: +91-9872667625, Email: shalinidr2000@yahoo.com.
Minni Verma, Email: minni.verma909@gmail.com.
Kamaljit Singh, Email: kamaljit29@yahoo.ca.
References
- 1.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group: prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. [DOI] [PubMed]
- 3.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis. J Am Med Assoc. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 4.Keevil JG, Cullen MW, Gangnon R, McBride PE, Stein JH. Implications of cardiac risk and low density lipoprotein cholesterol distributions in the United States for the diagnosis and treatment of dyslipidemia: data from National Health and Nutrition Examination Survey 1999 to 2002. Circulation. 2007;115:1363–1370. doi: 10.1161/CIRCULATIONAHA.106.645473. [DOI] [PubMed] [Google Scholar]
- 5.National Cholesterol Education Program (NCEP) Expert panel. Third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation and treatment of high cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed]
- 6.Bachorik PS. Measurement of low density lipoprotein cholesterol. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of lipoprotein testing. Washington: AACC Press; 1997. pp. 145–160. [Google Scholar]
- 7.Turkalp I, Cil Z, Ozkazanç D. Analytical performance of a direct assay for LDL-cholesterol: a comparative assessment versus Friedewald’s formula—original investigation. Anadolu Kardiyol Derg. 2005;5:13–17. [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy Rl, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.McNamara JR, Cohn JS, Wilson PW, Schaefer EJ. Calculated values for low density lipoprotein cholesterol in the assessment of lipid abnormalities and coronary disease risk. Clin Chem. 1990;36:36–42. [PubMed] [Google Scholar]
- 10.Rubies-Prat J, Revere RJ, Senti M, et al. Calculated low-density lipoprotein cholesterol should not be used for management of lipoprotein abnormalities in patients with diabetes mellitus. Diabetes Care. 1993;16:1081–1086. doi: 10.2337/diacare.16.8.1081. [DOI] [PubMed] [Google Scholar]
- 11.Bachorik PS, Ross JW. National Education Program recommendations for measurements of low density lipoprotein cholesterol: executive summary. National Cholesterol Education Program Working Group on Lipoprotein Measurements. Clin Chem. 1995;41:1414–1420. [PubMed] [Google Scholar]
- 12.Kamal AHM, Hossain M, Chowdhury S, Mahmud NU. A comparison of calculated with direct measurement of low density lipoprotein cholesterol level. JCMCTA. 2009;20:19–23. [Google Scholar]
- 13.Miller WG, Waymack PP, Anderson FP, Ethridge SF, Jayne EC. Performance of four homogenous direct methods for LDL-cholesterol. Clin Chem. 2002;48:489–498. [PubMed] [Google Scholar]
- 14.Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogenous assays versus calculation. Clin Chem. 2002;48:236–254. [PubMed] [Google Scholar]
- 15.Sakaue T, Hirano T, Yoshino G, Sakai K, Takeuchi H, Adachi M. Reactions of direct LDL cholesterol assays with pure LDL fraction and LDL: comparison of three homogenous methods. Clin Chim Acta. 2000;295:97–106. doi: 10.1016/S0009-8981(00)00200-X. [DOI] [PubMed] [Google Scholar]
- 16.Sahu S, Chawla R, Uppal B. Comparison of two methods of estimation of low density lipoprotein cholesterol. The direct versus Friedewald estimation. Ind J Clin Biochem. 2005;20:54–61. doi: 10.1007/BF02867401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anandaraja S, Narang R, Godeswar R, Laksmy R, Talwar KK. Low density lipoprotein cholesterol estimation by a new formula in Indian population. Int J Cardiol. 2005;102:117–120. doi: 10.1016/j.ijcard.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Gasko R. Low density lipoprotein cholesterol estimation by the Anandaraja’s formula-confirmation. Lipids Health Dis. 2006;5:18. doi: 10.1186/1476-511X-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazi IF, Elisaf M. LDL-cholesterol calculation formulas in patients with or without the metabolic syndrome. Int J Cardiol. 2007;119:414–415. doi: 10.1016/j.ijcard.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 20.Meiattini F, Prencipe L, Bardeii F, Giannini G, Tarli P. The 4-hydroxybenzoate/4-aminophenazone chromogenic system used in the enzymatic determination of serum cholesterol. Clin Chem. 1978;24:2161–2165. [PubMed] [Google Scholar]
- 21.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- 22.Rifai N, Bachorik PS, Albers JJ. Lipids, lipoproteins and apolipoproteins. In: Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry. 3. New Delhi: WB Saunders Company; 1998. pp. 809–861. [Google Scholar]
- 23.Nauck M, Graziani MS, Bruton D, et al. Analytical and clinical performance of a detergent based homogenous LDL-cholesterol assay: a multicenter evaluation. Clin Chem. 2000;46:506–514. [PubMed] [Google Scholar]
- 24.Nakanishi N, Matsuo Y, Yoneka H, Nakamura K, Suzuki K, Tatara K. Validity of the conventional indirect methods including Friedewald method for determining serum low density lipoprotein cholesterol level: comparison with the direct homogenous enzymatic analysis. J Occup Health. 2002;42:130–137. doi: 10.1539/joh.42.130. [DOI] [Google Scholar]
- 25.Bairaktari ET, Tzallas C, Kalientzidou M, et al. Evaluation of alternative calculation methods for determining low density lipoprotein cholesterol (LDL-C) in haemodialysis patients. Clin Biochem. 2004;37:937–940. doi: 10.1016/j.clinbiochem.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Wagner AM, Zapico E, Bonet R, Perez A, Oedonez-Llanos J. The effect of VLDL particles on the accuracy of a direct LDL-cholesterol method in type 2 diabetic patients. Clin Biochem. 2003;36:177–183. doi: 10.1016/S0009-9120(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Zhang X, Pan B, et al. A modified formula for calculating low density lipoprotein cholesterol values. Lipids Health Dis. 2010;9:52. doi: 10.1186/1476-511X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faas FH, Earleywine A, Smith WG, Simmons DL. How should low-density lipoprotein cholesterol concentration be determined? J Fam Pract. 2002;51:973–975. [PubMed] [Google Scholar]
- 29.Kamezaki F, Sonoda S, Nakata S, Otsuji Y. A direct measurement for LDL-cholesterol increases hypercholesterolemia prevalence: comparison with Friedewald calculation. J UOEH. 2010;32:211–220. doi: 10.7888/juoeh.32.211. [DOI] [PubMed] [Google Scholar]
- 30.Vujovic A, Stevulijevic JK, Spasic S, et al. Evaluation of different formulas for LDL-C calculation. Lipids Health Dis. 2010;9:27. doi: 10.1186/1476-511X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrawal M, Spencer HJ, Faas FH. Method of LDL cholesterol measurement influences classification of LDL cholesterol treatment goals: clinical research study. J Investig Med. 2010;58:945–949. doi: 10.231/JIM.0b013e3181fb7ca7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paz E, Hermida J, Bouzas L, Brenlla J, Tutor JC. LDL cholesterol estimation using the Anandaraja’s and Friedewald’s formulas in schizophrenic patients treated with antipsychotic drugs. Clin Biochem. 2008;41:1002–1007. doi: 10.1016/j.clinbiochem.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Jun KR, Park HI, Chun S, Park H, Min WK. Effects of total cholesterol and triglyceride on the percentage difference between the low-density lipoprotein cholesterol concentrations measured directly and calculated using the Friedewald formula. Clin Chem Lab Med. 2008;46:371–375. doi: 10.1515/CCLM.2008.064. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM, Cleeman JI, Merz NB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2005;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]