Abstract
Vascular thrombotic disorders have emerged as a serious threat to our society. Platelet adhesion to fibrinogen, collagen and other platelet activators exposed over the atherosclerotic plaques can trigger platelet signaling events, activate platelets and lead to thrombotic events. Since anticoagulant and thrombolytic treatment strategies are usually associated with serious bleeding complications, preventing platelets adhesion may help to maintain platelets in an inactive state. In this study we tried to find out the effect of Silver nanoparticles, through their interaction with various platelet surface integrins on platelet adhesion on immobilized fibrinogen. Platelets, isolated from anti-coagulated human whole blood sample from healthy donors, were suspended in physiological buffer and each sample was divided into four tubes. In three of them 0.05, 0.5, and 5 μM concentrations of Silver nanoparticles were added, fourth tube served as control. Platelet adhesion on immobilized fibrinogen matrices and integrin mediated cell signaling events were studied in all the four samples. In the present study we show that nanosilver prevent platelet adhesion without conferring any lytic effect on them and effectively prevents integrin-mediated platelet responses in a concentration-dependent manner.
Keywords: Nanoparticles, Fibrinogen, Adhesion, Cytoskeleton
Introduction
Platelets, fascinating disc shaped subcellular fragments of megakaryocytes found circulating in the blood of all mammals, are chiefly known for their role in blood coagulation and hemostasis [1]. Once activated, platelets undergo a series of biochemical and morphological changes, which result in hemostasis and prevention of blood loss at the site of injury. Platelet plug formation requires spatially and temporally coordinated series of events [2], which result in arrest of circulating platelets on exposed collagen. Concomitantly, there is activation of platelets, recruitment of additional platelets to the site and formation of multicellular aggregates stabilized by fibrin [3].
Although hemostasis is the physiological response of functional platelets, any over-activity of platelets can lead to thrombotic situations. Thrombotic disorders have emerged as a major cause of morbidity and mortality in today’s medical scenario. Commonly used agents like cycloxygenase inhibitors aimed to prevent thrombus formation, or those which lyse an already formed thrombus like intravenous heparin [4], tissue plasminogen activator (TPA) and streptokinase [5], come with their own set of side effects [5–8]. P2Y12, integrin αIIbβ3 and phosphodiesterase blockers are the newer ones being evaluated, but their use are accompanied by side effects of serious bleeding, or even re-occlusion and re-infarction. As platelets play a central role in thrombosis, the preferred treatment goal would be to regulate and maintain these cells in an inactive state [9].
The molecular mechanisms of different signaling pathways in resting and activated platelets make a very important area of investigation.
Nanoparticles have been in medicinal use for thousands of years. Even ancient texts mention the use of nanoparticles as bhasma [10] for the treatment of various conditions. Nanoparticles are amiable to biological functionalization and can be synthesized and assembled in various shapes, sizes and architectures, explaining their potential biological and medical applications [11–15]. The size of these particles can be suitably manipulated to enable them to pass through biological membranes and affect cell physiology [16], which has been a challenge for the traditional medicines. Stable silver nanoparticles have been synthesized in the lab [17] and characterized under the scanning electron microscope for their uniformity of shape and size. The anti bacterial nature of silver nanoparticles by their ability to affect the cell signaling mechanisms have been proved by various studies [17]. In this study, we have tried to elucidate the anti thrombotic role of nano silver. There exists a report to suggest that nanotubes of carbon aggravate platelet aggregation [18], but the effect of silver nanoparticles on platelet reactivity is still unexplored. In this report we show that nano-sized silver particles effectively prevent platelet adhesion to immobilized matrices and the intra-cellular signaling events that follow in a concentration-dependent manner. Silver nanoparticles at the concentrations used in this study [19, 20], do not confer any cytotoxic effect.
Result and Discussion
Effect of Silver Nanoparticles on Platelet Functions
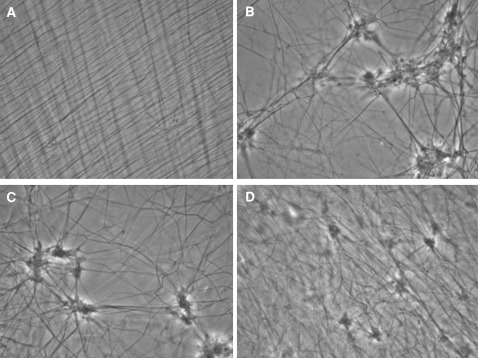
Platelets adhere on immobilized fibrinogen and undergo changes in size and shape characteristic of platelet activation (Fig. 1). Presence of nano silver decreased platelet adhesion, as shown by their fewer numbers per field (Fig. 2); slowed platelet spreading and degranulation, as seen under the Phase Contrast Microscope (Fig. 2); Platelet cytoskeletal changes like F-actin polymerization, as seen under the Fluorescent Microscope (Fig. 3), were also slowed by the presence of silver nano particles.
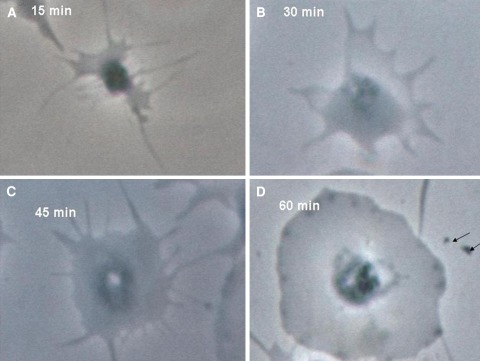
Fig. 1.
Phase contrast photograph of platelets immobilized on fibrinogen coated coverslips. In the control slides, it was found that platelet spreading, shape change and degranulation progress with time. Photos are under ×100 phase contrast from a single experiment representative of five different experiments
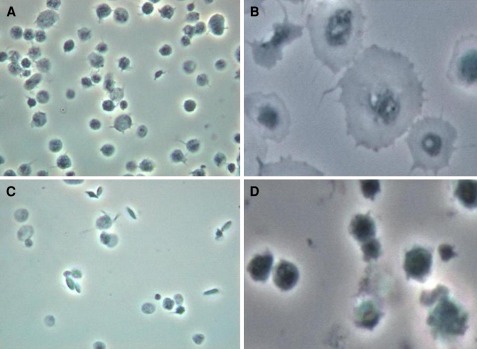
Fig. 2.
a Adhesion of platelets on immobilized fibrinogen, b their subsequent spread appearance after 15 min. c Platelets pretreated with 5 μM silver nanoparticles, showing less adhesion (as shown by their fewer numbers per field), and d hardly any spreading after 15 min. Photos are under ×100 phase contrast from a single experiment representative of five different experiments
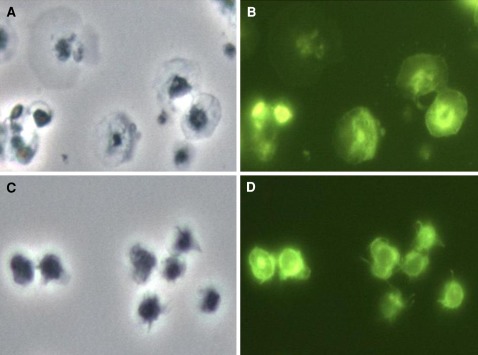
Fig. 3.
Photos of spread platelets at 15 min over immobilized fibrinogen. The left panels are phase contrast photographs, whereas the right ones are phalloidin-FITC tagged fluorescence photographs, at ×100. a and b are of the same field showing F-actin reorganization and spreading in control platelets while c and d show the same field of platelets pre-treated with 5 μM silver nanoparticles and minimal cytoskeletal changes
Protein Extraction from Adhered Platelets
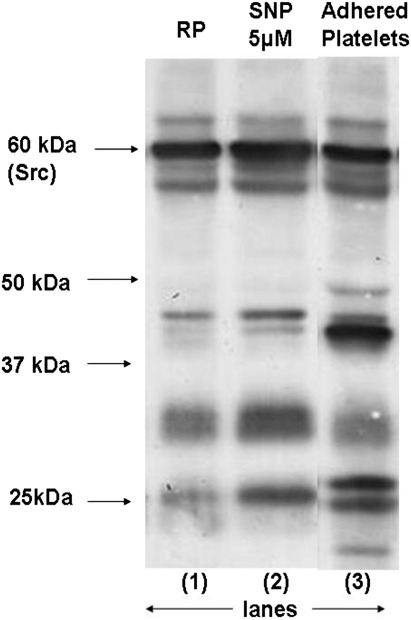
The phosphotyrosine analysis for platelet proteins adhered to immobilized fibrinogen (Fig. 4), showed decreased integrin mediated cell signaling and protein phosphorylation in the presence of silver nanoparticles.
Fig. 4.
Phosphotyrosine profile for platelets adhered to immobilized fibrinogen. Lane 1 shows resting platelets, lanes 2 and 3 show adhered platelets: 2 in the presence and 3 in the absence of 5 μM silver nanoparticles
Clot retraction Studies
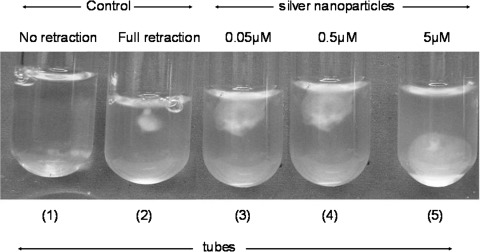
Subsequently we studied the effect of nanoparticles on fibrin clot retraction, which results from the interaction between platelet integrin αIIbβ3 and fibrin [21]. Pretreatment of platelets with increasing concentration of silver nanoparticles led to progressive inhibition in the extent of retraction (up to 40% inhibition in presence of 5 μM nanosilver) (Fig. 5).
Fig. 5.
Fibrin clot retraction for platelets. Tube 1 Clot formation was induced by thrombin in all the tubes. In the first tube there was no WP (negative control), in the second there was no Silver nanoparticles (positive control), tubes 3, 4 and 5 show progressive inhibition of clot retraction the presence of increasing concentration of silver nanoparticles
Clot Retraction Microscopy
The clot retraction experiment reproduced on glass cover slips (Fig. 6), showed the micro structure of a fibrin mesh, and how contraction of platelet cytoskeleton cause retraction of this mesh. Silver nanoparticles impeded the platelet–fibrin interaction and inhibited fibrin clot retraction in a concentration dependant manner.
Fig. 6.
Fibrin clot seen as on a glass slide under ×100 phase contrast microscope. a Without platelets. b–d, show clot retraction in the presence of platelets. c and d show clot retraction inhibition in the presence of 0.05 and 5 μM of silver nanoparticles, respectively
Materials and Methods
The study has been conducted in the Department of Biochemistry, Subharti Medical College, Meerut, Uttar Pradesh, in collaboration with the Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi.
Platelet Isolation
Platelets were isolated by differential centrifugation from fresh human blood. Whole blood sample from healthy volunteers was collected in citrate–phosphate–dextrose–adenine and centrifuged at 180 g for 20 min. PRP (platelet-rich plasma) was incubated with 1 mM acetylsalicylic acid for 15 min at 37°C. After the addition of EDTA (ethylenediaminetetraacetic acid) (5 mM), platelets were sedimented by centrifugation at 800 g for 15 min. Cells were washed in buffer A (20 mM Hepes, 138 mM NaCl, 2.9 mM KCl, 1 mM MgCl2, 0.36 mM NaH2PO4, 1 mM EGTA (ethylene glycol tetraacetic acid), supplemented with 5 mM glucose, and 0.6 ADPase units of apyrase/ml, pH 6.2). Platelets were finally resuspended in buffer B (pH 7.4), which was the same as buffer A but without EGTA and apyrase. The final cell count was adjusted to 0.5–0.8 × 109/ml. All steps were carried out under clean conditions, and precautions were taken to maintain the cells in an inactivated state.
Synthesis of Silver Nanoparticles
A solution of 0.01 M silver ions was prepared by dissolving 0.017 g AgNO3 in 100 ml of deionized water. During the process additives like ammonia (30%) are added drop wise to form a stable soluble complex of silver ions. This was used as the precursor for the silver nanoparticles. A blend of reducing agents like d-glucose and hydrazine was used during the synthesis of the nanoparticles such that an optimum rate was achieved. To ensure complete reduction of the silver ions, about 110 ml of such blend of reducing agents (at concentration of 0.01 M) was incorporated into 100 ml of silver nitrate stock solution (0.01 M) with continuous stirring which yielded stable nano silver particles of concentration 0.005 M in aqueous media. The nanoparticles were examined for stability by carrying out centrifugation of a month old samples at 15,000 g for 15 min at room temperature. No noticeable precipitation was obtained. The colour and pH of the solution were also checked at regular intervals, which hardly showed any change. Before every experiment the nanoparticles were sonicated and filtered through 0.20 μm filter (Milipore) to ensure the absence of micro particles.
Consistent with recent reports [22, 23], silver nanoparticles (13–15, 30–35, and 40–45 nm size ranges, respectively) inhibited aggregation by similar extents.
The protocol followed for synthesis and characterization of Silver nanoparticles are described in detail in http://pubs.acs.org.
Preparation of Various Immobilized Matrices
Glass slides (Blue Star, India) were taken cleaned with tissue soaked in acetone and allowed to dry at room temperature. Cover slips (Number 1, Blue star, India) were similarly cleaned, labeled and placed on the slide. Fibrinogen (100 μg/ml) or poly-l-lysine (100 μg/ml) as appropriate was pipetted over the cover slip, so as to cover almost its entire surface. The slides with the cover slips were then kept inside a moist chamber to prevent drying at room temperature for 1 h for coating the glass surface with the appropriate matrix. Next, each cover slip was washed with normal saline (0.9% NaCl) from a pipette, 300 μl each time thrice, to wash away the non-adhered fibrinogen etc.
Platelet Adhesion on Immobilized Matrices
The control platelet suspension in buffer B or platelets following appropriate treatments (e.g., with varying concentrations of silver nanoparticles for a period of 10 min) at counts of 1 × 105 cells/ml were pipetted on to the coated cover slips and allowed to adhere. After appropriate time, or otherwise after 30 min, the unadhered platelets were washed off with normal saline from a pipette, 300 μl each time thrice. A droplet of mounting medium (DABCO) was placed on the slide and the cover slip was placed on this drop with the coated surface down. They were then viewed under the microscope, with ×100 oil immersion attachment.
Protein Extraction from Adhered Platelets
As per the experimental protocol, a parallel set of slides were prepared using 10 × 10 cm glass plates, preferably mini gel electrophoresis plates, kept in the moist chamber. The plates were cleaned with acetone and dried before use. The plates were coated with fibrinogen and platelet suspension were allowed to sample lysis buffer was put on the plate at the appropriate points of time. Adhered cells were scrapped with a clean new shaving blade broken into half. The sample was saved in labeled microfuge tubes, heated in Laemmli lysis buffer and preserved in −20°C deep freezer till further analysis.
Fluorescent Antibody Tagging of Platelets
Platelets suspended in buffer B were fixed with equal volume of 4% paraformaldehyde at room temperature for 30 min. Triton-×100 (0.1%) and phalloidin tagged with FITC were added and incubated in the dark for 30 min at room temperature. (This was done in a 1,500 μl microfuge plastic tube, wrapped with aluminum foil, or stored inside the drawer). After this, platelets were released on the appropriate coated cover slips and processed as mentioned before. These slides were visualized under the fluorescence microscope.
Phase Contrast and Fluorescence Microscopy
The phase contrast and the fluorescence studies were done using the OLYMPUS CX 41 RF, model ULH 50 HG or the Leica DM-LB2 microscopes. The room lights were kept off during the procedure, to avoid photo quenching of the fluorescent dye. A drop of Leica immersion oil was placed over the cover slip, the slide was viewed and the cell populations most representative of the whole were photographed first under fluorescence followed quickly by the phase contrast, taking care not to move the field being viewed. Thus, it was possible to acquire a phase contrast image of the field same as the fluorescence image.
Clot-Retraction Study
For fibrin clot retraction assay the experimental method described by Osdoit and Rosa [21] was essentially followed. Washed platelets (0.6 × 109 cells/ml) were incubated without or with different concentrations of Silver nanoparticles at 37°C for 2 min. Ca2+ was added 90 s prior to completion of this incubation. Fibrinogen (2 mg/ml) was added to the platelets thereafter. Fibrin clot retraction was initiated by addition of thrombin (1 IU/ml). Pictures of the clot thus formed were taken at different time intervals.
Microscopy of Clot Retracted Samples
This was an adaptation of fibrin clot retraction assay, the experimental method described by Osdoit and Rosa [21]. 500 μl microfuge tubes were used for this experiment. 200 μl of platelet suspension in buffer B at counts of 6 × 108/ml was taken and reagents were added to achieve the following respective final concentrations. Ca++ (2 mM), fibrinogen (2 mg/ml). Fibrin clot retraction was initiated by addition of thrombin (1U/ml), and the time was counted henceforth. Immediately after addition of thrombin the sample was mixed thoroughly and 20 μl each was pipetted out over several labeled glass slides, covered with a coverslip and maintained at 37°C in a moist chamber. The slides were seen at the appropriate time points under the microscope at ×100 with phase contrast attachment.
Immuno Blotting
Platelet proteins were separated on 10% or 10–18% gradient SDS–PAGE as needed and were electrophoretically transferred to PVDF membranes (Bio-Rad Laboratories, CA, USA) by using a wet system (TARSONS, India). The membranes were blocked with 5% bovine serum albumin in 10 mM Tris–HCl, 150 mM NaCl, pH 8.0, (TBS) containing 0.05% Tween-20 for 2 h at room temperature blots were incubated for 2 h with monoclonal antibody against phosphotyrosine (clone 4G10), followed by horseradish peroxidase-labeled antimouse IgG for 1 h. Antibody binding was detected using enhanced chemiluminescence and quantified in an Agfa Duoscan T1200 flatbed scanner using Gene Tools software (Syngene).
Conclusion
The biggest challenge presented to researchers studying platelet biology is to relate the significance of platelet signaling and function in vitro to the in vivo situation of hemostasis and thrombosis. We demonstrated here that silver nanoparticles effectively inhibited integrin-mediated platelet functional responses like adhesion to immobilized fibrinogen, F-actin reorganization and platelet cytoskeletal changes namely fibrin clot retraction in a dose–dependent manner, irrespective of the nature of agonists used.
Thus, significant inhibition of platelet functions with a relatively low dose of nanosilver, raise the hope for its use as an antiplatelet therapeutic agent. However, like any nascent drug, critical analysis of the toxicity profile of the silver nano particles is warranted. The translation of this basic research may 1 day establish silver nanoparticles as a new strategy to maintain platelets in a low activation state and prevent vascular thrombosis.
Acknowledgments
This research was supported in part by grants received by Prof. D. Dash (Professor and Head, Department of Biochemistry, Institute of Medical Sciences, BHU, Varanasi) from the Department of Biotechnology (DBT), Government of India, and the DST Unit on Nanoscience and Technology (DST-UNANST), Banaras Hindu University.
References
- 1.White JG. Anatomy and structural organization of the platelet. In: Colman et al. Hemostasis and thrombosis. Philadelphia: J. B. Lippincott Company; 1994. p. 397–413.
- 2.Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989;69:58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- 3.Porter JC, Hogg N. Integrins take partners: cross-talk between integrin and other membrane receptors. Trends Cell Biol. 1998;8:390–396. doi: 10.1016/S0962-8924(98)01344-0. [DOI] [PubMed] [Google Scholar]
- 4.Watson RD, Chin BS, Lip GY. Antithrombotic therapy in acute coronary syndromes. Br Med J. 2002;325:1348–1351. doi: 10.1136/bmj.325.7376.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arcasoy SM, Kreit JW. Thrombolytic therapy of pulmonary embolism. Chest. 1999;115:1695–1707. doi: 10.1378/chest.115.6.1695. [DOI] [PubMed] [Google Scholar]
- 6.Patel SC, Mody A. Cerebral hemorrhagic complications of thrombolytic therapy. Prog Cardiovasc Dis. 1999;42:217–233. doi: 10.1016/S0033-0620(99)70004-6. [DOI] [PubMed] [Google Scholar]
- 7.Haines ST, Busse HI. Thrombosis and the pharmacology of antithromboic agents. Ann Pharmacother. 1995;29:892–904. doi: 10.1177/106002809502900912. [DOI] [PubMed] [Google Scholar]
- 8.Khalid A. Is thrombolytic therapy effective for pulmonary embolism? Am Fam Physician. 2002;65:1097–1102. [PubMed] [Google Scholar]
- 9.Severin S, Gratacap MP, Lenain N, Alvarez L, Hollande E, Penninger JM, Gachet C, Plantavid M, Payrastre B. Deficiency of Src homology 2 domain containing Inositol-5-phosphatase-I affects platelet responses and thrombus growth. J Clin Invest. 2007;117:944–952. doi: 10.1172/JCI29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopher L Brown, Gillian Bushell, Michael W Whitehouse, DS Agrawal, SG Tupe, KM Paknikar, Edward RT Tiekink. Nanogoldpharmaceutics (i) the use of colloidal gold to treat experimentally-induced arthritis in rat models; (ii) characterization of the gold in swarna bhasma, a microparticulate used in traditional Indian medicine. Gold Bull. 2007;40:245–251.
- 11.Kubik T, Bogunia-Kubik K, Sugisaka M. Nanotechnology on duty in medical applications. Curr Pharm Biotechnol. 2005;6:17–33. doi: 10.2174/1389201053167248. [DOI] [PubMed] [Google Scholar]
- 12.Bhirde AA, Patel V, Gavard J, Zhang U, Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS, Rusling JF. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao H, Nehl CL, Hafner JH. Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine. 2006;1:201–208. doi: 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Jain P, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007;2:681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 15.Silva GA. Introduction to nanotechnology and its applications to medicine. Surg Neurol. 2004;61:216–220. doi: 10.1016/j.surneu.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Brooking J, Davis SS, Illum L. Transport of nanoparticles across the rat nasal mucosa. J Drug Target. 2001;9:267–279. doi: 10.3109/10611860108997935. [DOI] [PubMed] [Google Scholar]
- 17.Shrivastava S, Bera T, Roy A, Singh G, Ramchandrarao P, Dash D. Characterization of enhanced antibacterial effect of novel silver nanoparticles. Nanotechnology. 2007;18:225103–225111. doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]
- 18.Radomski A, Jurasz P, Escolano DA, Drews M, Morandi M, Malinski Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu J, Ji J, Fan D, Shen J. Construction of antibacterial multilayer films containing nanosilver via layer-by-layer assembly of heparin and chitosan_silver ion complex. J Biomed Mater Res A. 2006;79:665–674. doi: 10.1002/jbm.a.30819. [DOI] [PubMed] [Google Scholar]
- 20.Ji JH, Jung JH, Kim SS, Yoon JU, Park JD, Choi BS, Chung YS, Kwon IH, Jeong J, Han BS, Shin JH, Sung JH, Song KS, Yu IJ. Twenty-eight-day inhalation toxicity study of silver nanoparticles in sprague dawley rats. Inhal Toxicol. 2007;19(10):857–871. doi: 10.1080/08958370701432108. [DOI] [PubMed] [Google Scholar]
- 21.Osdoit S, Rosa JP. Fibrin clot retraction by human platelets correlates with αIIbβ3 integrin-dependent protein tyrosine dephosphorylation. J Biol Chem. 2001;276(9):6703–6710. doi: 10.1074/jbc.M008945200. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size- dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 23.Chithrani BD, Chan WCW. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7(6):1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]