Abstract
Stroke is the third major cause of death and foremost cause of disability worldwide. Cerebrovascular stroke remains largely a clinical diagnosis. The use of biomarkers in diagnosing stroke and assessing prognosis is an emerging and rapidly evolving field. The study aimed to investigate the predictive value of neurobiochemical marker of brain damage (neuron-specific enolase [NSE]) with respect to degree of disability at the time of admission and neurological worsening in acute ischemic stroke patients. We investigated 150 patients with cerebrovascular stroke who were admitted within 72 h of onset of stroke in the Department of Neurology at SAIMS. Venous blood samples were taken after admission and NSE was analyzed by solid enzyme linked immunosorbent assay using Analyzer and microplate reader from Biored: Code 680. In all patients, the neurological status was evaluated by a standardized neurological examination and the National Institutes of Health Stroke Scale on admission and on day 7. Serum NSE concentration was found to significantly correlate with both degree of disability and neurological worsening in acute ischemic stroke cases in the present study. The maximum serum NSE level within 72 h of admission was significantly higher in patients with greater degree of disability at the time of admission. Serum NSE levels were also found to be significantly elevated in patients with bad neurological outcome. Our study showed that serum NSE has high predictive value for determining severity and early neurobehavioral outcome after acute stroke.
Keywords: Ischemic stroke, Neuron specific enolase (NSE), National Institute of Health Stroke Scale (NIHSS), Degree of disability, Neurological worsening
Introduction
The diagnosis and management of stroke is limited by lack of rapid diagnostic assays for use in emergency settings. The CNS cellular response to stroke results in characteristic upregulation and release of particular neuronal markers into the CSF and blood-stream. Increased levels of neuronal isoenzymes and other molecules with CNS-specific expression signal damage to the brain parenchyma. Several neurobiochemical markers can evaluate neuronal injury and in recent years, neurobiochemical markers of brain damage have gained particular attention in the identification of stroke patients with an adverse neurological outcome. The serum neuron-specific enolase (NSE) level is one of these markers. NSE, a dimeric isoenzyme of the glycolytic enzyme enolase, is found in the cytoplasm of neurons and cells with neuroendocrine differentiation [1, 2]. The αγ and γγ (more common) isoforms are known as NSE because they initially were found in neurons and neuroendocrine cells. NSE is considered as specific neurobiochemical marker of brain damage after brain infarctions in humans [3, 4] or in animals [5–7]. It is assumed that NSE, being an enzyme from the cellular cytoplasm, is released during cell destruction.
Physiologically, NSE is present only in negligible amounts in the peripheral blood serum concentration is 8.7 ± 3.9 ng/ml (men 8.9 ± 3.9, women 8.3 ± 4.0) [8]. CSF-NSE concentration is 17.3 ± 4.6 ng/ml (men 17.4 ± 4.2, women 17.0 ± 5.2);
Subjects and Methods
The present study has been carried out on a total of 251 subjects aged between 35 and 85 years. Out of these 251 subjects, 101 adults with an apparently normal and healthy physique and presenting with no clinical signs or symptoms suggestive of cerebrovascular disease were used as the control group and 150 adults presenting with clinical signs and symptoms of cerebrovascular stroke were taken as the study group(cases).The subjects in the study group were selected from-
IPD wards and ICU from the Department of Neurology, SAIMS Medical College, Indore
IPD wards of Department of Medicine, SAIMS Medical College, Indore.
The Inclusion criteria for subjects in study group were -
Adult stroke (age > 21 years)
Within 72 h of admission
The Exclusion criteria for subjects in study group were-
CNS infection
Stroke more than 72 h
Peripartum stroke
Selected patients were subjected to the following protocol:
Detailed history
Detailed Neurological Examination using the National Institutes of Health Stroke Scale
Blood sampling for serum NSE
CT scan within 12 h of admission to exclude patients with stroke mimic.
The venous blood samples were drawn within 72 h from the onset of symptoms in 5 ml serum separator tubes, centrifuged at 3,000 rpm for 15 min and then aliquotted in 2 ml tubes. Samples were stored at −20°C until assays were run to evaluate. The serum NSE samples were analyzed using an enzyme immunoassay manufactured by DRG Instruments GmbH, Germany and is based on the sandwich technique including the solid-phase monoclonal antibody raised against γ, γ-NSE. The sensitivity of the NSE assay is 1 ng/ml and the overall intra assay coefficient of variation was 7.3% and overall inter assay Coefficient of variation was 9.6%. Hemolytic specimens were discarded because lysis of erythrocytes and platelets influenced the serum NSE level.
The severity of stroke was scored on admission and after 7 days by the neurologist, using the National Institute of Health Stroke Scale (NIHSS). The NIHSS score consists of 15 items and a total score of 42 points. Score of 0 = no stroke, 1–4 = minor stroke, 5–20 = moderate stroke, 21–42 = severe stroke. Early neurological worsening was diagnosed as an increase in National Institute of Health Stroke Score (NIHSS) by two or more points (or stroke-related death) between admission and day 5 [9] and who remained stable or improved in the same period were classified as no worsening. The protocol was approved by the local Ethics Committee; Informed consent was given by patients themselves or by relatives as legally required.
Results
Out of total of 251 subjects, 150 cases of cerebrovascular stroke were included in the study group and 101 subjects as control.
Tables 1, 2, 3, 4, 5 and 6 clearly depicts that the maximum number of patients (58.66%) in the study group (cases) were in 46–65 years age group and minimum (16.66%) were in 30–45 years age group. In the control group also most of the patients (82.17%) belonged to 46–65 years age group and least (5.9%) were above 66 years age group. In the study group percentage of males (63.33%) was more as compared to females (36.66%).Incidence of hypertension (78.66%), atrial fibrillation (61.33%), Diabetes Mellitus (44%) was more in the study group as compared to other risk factors.
Table 1.
Demographic profile of study group (cases) and controls
| Demographical variables | Subjects (n = 251) | ||
|---|---|---|---|
| Cases (n = 150) | Control (n = 101) | ||
| Age category (years) | 30–45 | 25 | 45 |
| 46–65 | 88 | 50 | |
| 66–85 | 37 | 6 | |
| Sex | Female | 55 | 34 |
| Male | 95 | 67 | |
| Hypertensive’s | No | 32 | 65 |
| Yes | 118 | 36 | |
| Smokers | No | 108 | 76 |
| Yes | 42 | 25 | |
| Atrial fibrillation | No | 58 | 88 |
| Yes | 92 | 13 | |
| Diabetes mellitus | No | 84 | 75 |
| Yes | 66 | 26 | |
| Alcohol | No | 96 | 79 |
| Yes | 54 | 22 | |
Table 2.
Serum NSE in controls and study group
| Variable ↓ | Subject | N | Mean | SD | t | df | P value |
|---|---|---|---|---|---|---|---|
| NSE score ng/ml | Control | 101 | 7.48 | 1.52 | 23.54 | 165.88 | <0.001 |
| Cases | 150 | 22.68 | 7.69 |
Table 3.
Serum NSE levels in controls and study group
| NSE score category | Total | ||||
|---|---|---|---|---|---|
| 15.1–25 ng/ml | 25.1–35 ng/ml | 35.1–45 ng/ml | |||
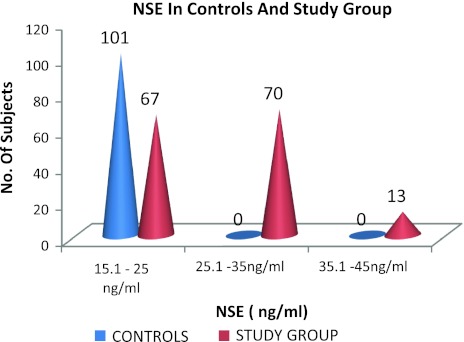
| Subject | Cases | 67 | 70 | 13 | 150 |
| Control | 101 | 0 | 0 | 101 | |
| Total | 168 | 70 | 13 | 251 | |
Table 4.
Serum NSE, disability score (0 day) in study and control group
| N | Mean | SD | t | df | Significance | |
|---|---|---|---|---|---|---|
| NIHSS score0 day | 150 (study group) | 16.8467 | 6.84609 | −14.960 | 149 | <0.001 |
| NSE score ng/ml | 22.6779 | 7.68773 | ||||
| NIHSS score 0 day | 101 (control group) | 0.257 | 0.44 | −44.96 | 100 | <0.001 |
| NSE score ng/ml | 7.48 | 1.51 |
Table 5.
Serum NSE levels and degree of disability (0 day) in study group
| NSE (ng/ml) | NIHSS (0 day) category | Spearman correlation | Approx. sig | ||
|---|---|---|---|---|---|
| Mild 0–4 | Moderate 5–20 | Severe 21–42 | |||
| 15.1–25 | 28 | 36 | 3 | 0.776 | <0.001 |
| 25.1–35 | 0 | 17 | 53 | ||
| 35.1–45 | 0 | 0 | 13 | ||
Table 6.
Comparison of serum NSE, with neurological worsening
| NSE (ng/ml) | Neurological worsening | |
|---|---|---|
| Yes | No | |
| 15.1–25 | 3 | 64 |
| 25.1–35 | 47 | 23 |
| 35.1–45 | 13 | 0 |
Among subjects with cerebrovascular stroke 70 cases (46.666%) had serum NSE > 25 ng/ml and 13 cases (8.666%) had serum NSE level > 35 ng/ml. In controls all 101 subjects had serum NSE level ≤ 25 ng/ml. Calculated Chi- Square value of 83.497 was found to be significant at 1% level of significance.
A positive correlation(r = .919; P < 0.001) was observed between serum NSE levels and severity of stroke at the time of admission in study group. From the present study increased NSE level seen is related to cerebrovascular stroke.
A highly statistically significant correlation (P < 0.001) was observed between serum NSE and degree of disability categorized into mild, moderate and severe NIH Stroke Scale. The calculated chisquare value of 94.905 was found to be significant at 1% level of significance.
A highly significant statistical correlation was observed between serum NSE (r = 0.706; P < 0.001) and neurological worsening as seen by increase in NIH stroke score by ≥ 2 after 7 days of admission. The calculated chisquare value of 74.842 was found to be significant at 1% level of significance.
Discussion
Stroke causes a vast amount of death and disability throughout the world. Its impact on individual patients, their families and society as a whole is immense. Rapid management of acute stroke in the emergency setting, especially if the CT scan is normal or MRI is contraindicated or not available, is essential to ensure that patients receive thrombolysis within the therapeutic window.
Out of these 251 subjects, 101 adults with an apparently normal and healthy physique and presenting with no clinical signs or symptoms suggestive of cerebrovascular disease were taken as the control group and 150 adults presenting with clinical signs and symptoms of cerebrovascular stroke were taken as the study group (cases).
The mean serum NSE, in patients with cerebrovascular stroke was found to be significantly higher (P < 0.001) than in controls.
Fassbender et al. [10], Casmiro et al. [8], Anand et al. [11] in their studies have observed serum NSE levels to be less sensitive, rarely exceeding the reference range of normals and observed that greater extent of injury is required to produce a consistently measurable change. Stevens et al. [12], Hill et al. [13], Schaarschmidt et al. [14] have demonstrated an increase in serum NSE level after acute focal ischemia in humans.
During stroke, the blood–brain barrier (BBB) is compromised by endothelial cell death, and cytosolic contents released from injured brain tissues have the potential to cross the BBB. Physiologically, NSE, which is a highly soluble cytoplasmic protein, is present only in negligible amounts in the peripheral blood and consequently, is readily released by tissue damage in blood where it has a half life of around 48 h. Altered blood brain barrier and astroglial disintegration substantially makes the NSE leak into cerebral and systemic circulation.
Elevated levels of serum NSE, have been found to significantly correlate (P < 0.001) with disability score and degree of disability.
Hill et al. [13], Wunderlich et al. [15], Ferrarese et al. [16], Smith et al. [17] in their studies showed a positive correlation between the serum NSE level and the neurological outcome. Fassbender et al. [10], Cunningham et al. [4], Missler et al. [18] failed to demonstrate the significant correlation between NSE and neurological outcome.
From the present study NSE levels have been found to be related to brain parenchymal damage and secondary mechanisms of neuronal damage due to edema and increase of intracranial pressure in cerebrovascular stroke.
A positive correlation was observed between serum NSE levels and neurological worsening in study group.
Cunningham et al. [4], Schaarschmidt et al. [14] have also reported that increased levels of Serum NSE correlated with worse clinical outcome. According to Wunderlich et al. [15] observed that serum levels of NSE would be expected to rise as long as damage due to the infarction continued and NSE was washing out of the brain tissue (Fig. 1).
Fig. 1.
Comparison of NSE levels in subjects (controls and study group)
As neurological recovery depends on initial stroke severity, patients with higher serum NSE levels at the time of admission were more prone to bad neurological outcome in the present study.
Conclusion
Raised serum levels of brain biomarker NSE, in cerebrovascular stroke patients as compared to controls provides insight into the pathophysiologic mechanisms of brain injury.
Higher serum levels of NSE, were associated with greater degree of disability and neurological worsening as observed after 7 days in stroke patients suggesting the role of these biomarkers in predicting the neurobehavioral outcome after stroke.
A detailed study of brain biomarker is essential for planning effective preventive measures. Biomarkers in stroke provide insight into the pathophysiologic mechanisms of brain injury. Furthermore, a simple blood test for acute stroke could be of benefit in hospitals where CT is not yet available.
Acknowledgment
Reviewers are acknowledged in the January issue for the previous year.
References
- 1.Marangos PJ. Neuron specific enolase: a clinically useful marker of neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–295. doi: 10.1146/annurev.ne.10.030187.001413. [DOI] [PubMed] [Google Scholar]
- 2.Barone FC, Clark RK, Price WJ, White RF, Feuerstein GZ, Storer BL, Ohlstein EH. Neuron-specific enolase increases in cerebral and systemic circulation following focal ischemia. Brain Res. 1993;623:77–82. doi: 10.1016/0006-8993(93)90012-C. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth RJ, Wassif WS, Sherwood RA, Gerges A, Poyser KH, Garthwaite J, Peters TJ, Bath PMW. Serum neuron-specific enolase, carnosinase, and their ratio in acute stroke. Stroke. 1996;27:2064–2068. doi: 10.1161/01.STR.27.11.2064. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham RT, Watt M, Winder J, McKinstry S, Lawson JT, Johnston CF, Hawkins SA, Buchanan KD. Serum neurone-specific enolase as an indicator of stroke volume. Eur J Clin Invest. 1996;26:298–303. doi: 10.1046/j.1365-2362.1996.129282.x. [DOI] [PubMed] [Google Scholar]
- 5.Hårdemark HG, Ericsson N, Kotwica Z, Rundströ G, Mendel-Hartvig I, Olsson Y, Påhlman S, Persson L. S-100 protein and neuron-specific enolase in CSF after experimental traumatic or focal ischemic brain damage. J Neurosurg. 1989;71:727–731. doi: 10.3171/jns.1989.71.5.0727. [DOI] [PubMed] [Google Scholar]
- 6.Hatfield RH, McKernan RM. CSF neuron-specific enolase as a quantitative marker of neuronal damage in a rat model. Brain Res. 1992;577:249–252. doi: 10.1016/0006-8993(92)90280-M. [DOI] [PubMed] [Google Scholar]
- 7.Horn M, Seger F, Schlote W. Neuron-specific enolase in gerbil brain and serum after transient cerebral ischemia. Stroke. 1995;26:290–297. doi: 10.1161/01.STR.26.2.290. [DOI] [PubMed] [Google Scholar]
- 8.Casmiro M, Maitan S, Pasquale F. Cerebrospinal fluid and serum neuron-specific enolase concentrations in a normal population. Eur J Neurol. 2005;12(5):369–374. doi: 10.1111/j.1468-1331.2004.01021.x. [DOI] [PubMed] [Google Scholar]
- 9.Kwan J. Hand P Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM. 2006;99:625–633. doi: 10.1093/qjmed/hcl082. [DOI] [PubMed] [Google Scholar]
- 10.Fassbender K, Schmidt R, Schreiner A, et al. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J Neurol Sci. 1997;148(1):101–105. doi: 10.1016/S0022-510X(96)05351-8. [DOI] [PubMed] [Google Scholar]
- 11.Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis. 2005;20(4):213–219. doi: 10.1159/000087701. [DOI] [PubMed] [Google Scholar]
- 12.Stevens H, Jakobs C, Jager AE, Cunningham RT, Korf J. Neurone-specific enolase and N-acetyl-aspartate as potential peripheral markers of ischaemic stroke. Eur J Clin Invest. 1999;29:6–11. doi: 10.1046/j.1365-2362.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 13.Hill MD, Jackowski G, Bayer N, Lawrence M, Jaeschke R. Biochemical markers in acute ischemic stroke. CMAJ. 2000;162:1139–1140. [PMC free article] [PubMed] [Google Scholar]
- 14.Schaarschmidt H, Prange HW, Reiber H. Neuron-specific enolase concentrations in blood as a prognostic parameter in cerebrovascular disease. Stroke. 1994;25:558–565. doi: 10.1161/01.STR.25.3.558. [DOI] [PubMed] [Google Scholar]
- 15.Wunderlich MT, Ebert AD, Kratz T, Goertler M, Jost S, Herrmann M. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke. 1999;30:1190–1195. doi: 10.1161/01.STR.30.6.1190. [DOI] [PubMed] [Google Scholar]
- 16.Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M, Begni B. Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab. 1999;19:1004–1009. doi: 10.1097/00004647-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Smith CJ, Emsley HCA, Gavin CM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.STR.28.10.1956. [DOI] [PubMed] [Google Scholar]