ABSTRACT
BACKGROUND
Primary care physicians with appropriate training may prescribe buprenorphine-naloxone (bup/nx) to treat opioid dependence in US office-based settings, where many patients prefer to be treated. Bup/nx is off patent but not available as a generic.
OBJECTIVE
We evaluated the cost-effectiveness of long-term office-based bup/nx treatment for clinically stable opioid-dependent patients compared to no treatment.
DESIGN, SUBJECTS, AND INTERVENTION
A decision analytic model simulated a hypothetical cohort of clinically stable opioid-dependent individuals who have already completed 6 months of office-based bup/nx treatment. Data were from a published cohort study that collected treatment retention, opioid use, and costs for this population, and published quality-of-life weights. Uncertainties in estimated monthly costs and quality-of-life weights were evaluated in probabilistic sensitivity analyses, and the economic value of additional research to reduce these uncertainties was also evaluated.
MAIN MEASURES
Bup/nx, provider, and patient costs in 2010 US dollars, quality-adjusted life years (QALYs), and incremental cost-effectiveness (CE) ratios ($/QALY); costs and QALYs are discounted at 3% annually.
KEY RESULTS
In the base case, office-based bup/nx for clinically stable patients has a CE ratio of $35,100/QALY compared to no treatment after 24 months, with 64% probability of being < $100,000/QALY in probabilistic sensitivity analysis. With a 50% bup/nx price reduction the CE ratio is $23,000/QALY with 69% probability of being < $100,000/QALY. Alternative quality-of-life weights result in CE ratios of $138,000/QALY and $90,600/QALY. The value of research to reduce quality-of-life uncertainties for 24-month results is $6,400 per person eligible for treatment at the current bup/nx price and $5,100 per person with a 50% bup/nx price reduction.
CONCLUSIONS
Office-based bup/nx for clinically stable patients may be a cost-effective alternative to no treatment at a threshold of $100,000/QALY depending on assumptions about quality-of-life weights. Additional research about quality-of-life benefits and broader health system and societal cost savings of bup/nx therapy is needed.
KEY WORDS: buprenorphine-naloxone, primary health care, cost-effectiveness, opioid substitution therapy
INTRODUCTION
In 2009 there were approximately 1.9 million individuals in the US with dependence on or abuse of opioid analgesics and 400,000 individuals with dependence on or abuse of heroin.1 Long-term opioid agonist treatment with methadone or buprenorphine-naloxone (bup/nx) is the most effective treatment for opioid dependence.2
Buprenorphine-naloxone is a partial opioid antagonist that is an effective maintenance treatment for opioid dependence when compared to no treatment and equivalent in efficacy to moderate doses of methadone.3–8 As a result of the Drug Addiction Treatment Act of 2000, bup/nx can be prescribed by appropriately trained office-based physicians and dispensed at a pharmacy or on-site.9 Approximately 580,000 patients received bup/nx in 2009, many from non-specialist office-based physicians.10,11
Lack of insurance reimbursement and limits on the duration of treatment are substantial barriers to the expansion of office-based bup/nx treatment.12,13 Although bup/nx is no longer patent protected in the US,14 there are no generic versions on the market, and the current cost of a 30-day prescription is at least seven times the medication cost of methadone.15 For many opioid-dependent patients, however, methadone maintenance is not a viable treatment option due to lack of access, the restrictiveness of daily methadone dosing, and social stigma.16 For these patients, office-based bup/nx is the only agonist maintenance treatment option, but the duration of their treatment may be limited by insurance coverage (unlike treatment for other chronic conditions) even though the need for maintenance treatment may be indefinite.12,17,18
Generic preference-weighted quality-of-life measures, which are used for cost-effectiveness analyses, vary with changes in substance abuse measures, including in patients with opioid dependence.19,20 We therefore evaluated the cost-effectiveness of long-term office-based bup/nx treatment for clinically stable patients compared to no treatment and the value of research to reduce uncertainties about the quality-of-life weights used in this analysis.
METHODS
Analytic Overview
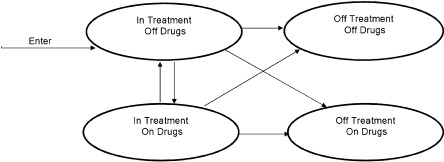
A decision analytic model with four health states was created to simulate the treatment outcomes, costs, and cost-effectiveness of office-based bup/nx treatment for clinically stable opioid-dependent patients who had been in treatment for 6 months (Fig. 1). Hypothetical clinically stable patients enter the model in the health state In Treatment Off Drugs. Each month the patient has a probability of remaining in that health state or transitioning to one of the other health states in the model: In Treatment On Drugs, Out of Treatment Off Drugs, and Out of Treatment On Drugs. Similarly, patients who transition to the health state In Treatment On Drugs can transition to any of the other health states. To reduce complexity, patients who transition to the health states Off Treatment Off Drugs or Off Treatment On Drugs remain in this health state for the duration remainder of the observation time and do not re-enter treatment.
Figure 1.
Treatment cohort simulation model.
Each health state is assigned a quality-of-life weight and monthly bup/nx medication cost, provider cost, and patient cost. Because no deaths were observed in the cohort data we used for the model, all patients are assumed to remain alive through the end of the analysis period. Cost-effectiveness ratios are calculated by comparing outcomes for simulated cohorts of opioid-dependent patients who are clinically stable after 6 months of bup/nx treatment and continue on office-based bup/nx (Treatment cohort) to outcomes for a simulated cohort of similar patients who do not continue office-based bup/nx (No Treatment cohort). Members of the simulated No Treatment cohort remain in Off Treatment On Drugs or Off Treatment Off Drugs health states for the duration of the analysis period. For each cohort, the monthly cost and quality-adjusted life years (QALYs) are summed for the period of the analysis, the incremental costs and QALYs of bup/nx treatment versus no treatment are compared, and a cost effectiveness ratio is calculated ($/QALY). Costs are in 2010 US dollars, and both costs and QALYs are discounted at 3% annually.21 The model was programmed in TreeAge Pro 2009 (TreeAge Software, Williamstown, MA).
In the base case, we use the current cost of bup/nx and a treatment duration of 24 months. In sensitivity analyses we reduce the cost of bup/nx, extend the treatment duration to 60 months, use an alternate source for quality-of-life weights, and vary other model parameters. We also compare results for injection drug user (IDU) and non-IDU populations because of differences in buprenorphine dose, frequency of urine toxicology testing, and quality-of-life weights. Probabilistic sensitivity analyses are used to evaluate model uncertainty about cost and quality-of-life inputs and the value of reducing this uncertainty by conducting further research, using a benchmark cost-effectiveness threshold of $100,000/QALY.22,23 The $100,000/QALY threshold is at approximately the midpoint of the one to three times GDP per capita threshold designated by the WHO-CHOICE Working Group.24
Treatment Retention and Drug Use
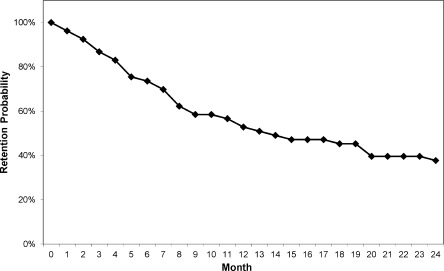
Model inputs for the monthly probabilities of staying in treatment with or without concomitant drug use were derived from a prospective observational cohort of 53 long-term opioid users treated with office-based bup/nx in a primary care setting.25 Patients who had previously completed 6 months of bup/nx treatment in a clinical trial and demonstrated clinical stability were allowed to enter the observational cohort. The clinical trial compared three office-based bup/nx treatment strategies (standard medical management with once- or thrice-weekly medication dispensing or enhanced medical management with thrice-weekly medication dispensing) and was unable to detect a difference in outcome among the treatments.26 We calculated the monthly probability of remaining in treatment starting from entry into the observational cohort, with patients considered in treatment until the month following their final visit with the provider. After 12 and 24 months of follow-up in the observational cohort, 51% and 38% of patients remained in treatment, respectively, and the median time in treatment was 13.4 months (Fig. 2). Retention at 12 months was similar to that reported in other observational cohorts of office-based bup/nx treatment.27,28
Figure 2.
Monthly probability of retention in treatment.
The probability of using drugs at any time while in treatment was 6%, based on analysis of urine toxicology test results. The probability of using drugs while out of treatment was 89%.29 In the absence of data on drug use patterns when bup/nx treatment is terminated, we assumed this same probability of drug use for simulated patients who drop out of bup/nx treatment in the Treatment cohort and for simulated patients in the No Treatment cohort, and vary this assumption in a sensitivity analysis (see below). For the 30% of the cohort, with a history of IDU,25 we assumed that those who use drugs either out of treatment in the Treatment cohort or in the No Treatment cohort returned to injecting or continued to inject drugs; similarly those with a history of IDU who continued to use drugs while in treatment were assumed to be injecting drugs. Data from the observational cohort did not indicate differences between IDUs and non-IDUs in transition probabilities or probabilities of drug use; different costs and quality-of-life weights were used for IDUs and non-IDUs (see below).
Costs
Table 1 reports the monthly bup/nx, provider, and patient costs for office-based bup/nx treatment, based on data from the same prospective observational cohort.25 Costs were calculated separately for patients with a history of IDU because prior research, including a study with this cohort, has demonstrated that patients whose primary opioid of abuse is heroin are more likely to use opioids while receiving bup/nx than patients whose primary opioid of abuse is opioid analgesics.28,30 Average daily bup/nx cost while on treatment was calculated as the total of all daily doses for all non-IDU or IDU patients multiplied by the unit cost per mg per dose and divided by the total days in treatment. This unit cost was based on the published price of $1.90/mg for 2 mg buprenorphine/0.5 mg naloxone tablet and $0.85/mg for 8 mg buprenorphine/2 mg naloxone tablet, adjusted for discounts frequently available to large public and private insurers using the published local discount for all Medicaid drugs (14% discount plus $3.15 dispensing fee per 30-day prescription).15 All daily doses were calculated assuming the 8 mg buprenorphine/2 mg naloxone tablet could be split for a half-dose.
Table 1.
Monthly Cost Model Inputs for Office-Based Buprenorphine/Naloxone Treatment (2010 US dollars)
| Non-IDU mean (SD) | IDU mean (SD) | |
|---|---|---|
| Medication and provider costs | ||
| Buprenorphine-naloxone* | 354 (96) | 390 (86) |
| Physician time | 32 (26) | 32 (26) |
| Nursing time† | 17 (—) | 17 (—) |
| Adjustment for missed visits | 11 ( 6) | 11 (6) |
| Overhead | 28 (14) | 29 (14) |
| Urine toxicology test cost‡ | 31 (28) | 34 (34) |
| Liver function test cost§ | 5 (—) | 5 (—) |
| Patient costs | ||
| Travel time | 13 (10) | 13 (10) |
| Visit time | 5 (2) | 5 (2) |
| Transport cost | 23 (33) | 23 (33) |
Provider costs include physician time, nurse time, and laboratory tests. Average monthly costs for physician time were calculated as the total of all patient visits multiplied by the cost of these visits and divided by the total patient months in treatment. Patients had two nurse visits per month and an average of 0.8 physician visits per month, which is consistent with clinical guidelines that recommend bi-weekly or monthly monitoring of stable patients.18 The durations of physician and nurse visits have been reported in a previous cost study.31 Following methods used in that cost study, we applied local labor and fringe benefit rates for physicians and nurses to the visit durations to determine visit costs, adding an additional 22% of the cost to account for time lost due to missed visits and a 46% overhead rate.31,32 A similar approach was used to calculate average monthly utilization and costs for urine toxicology tests from the study data, and we also assumed liver enzyme tests (alanine aminotransferase, aspartate aminotransferase) occurred every 3 months while in treatment.25
Patient costs, including visit time, travel time, and monthly transportation costs, were derived from the previous cost study.31 Time costs were calculated by multiplying the duration of the visit and travel by the state minimum wage of $8.25 per hour,33 as more than half of the cohort reported not having full-time employment.25
Quality-of-Life Weights
Quality-of-life weights for each health state are defined on a scale from 0 (death) to 1 (perfect health). Quality-of-life weights are from a societal perspective from a study conducted in the United Kingdom.34 In this study, 22 members of a UK general population panel recruited to complete internet surveys assessing health states using standard gamble procedures35 evaluated descriptions of health states in and out of bup/nx treatment (Table 2).
Table 2.
Model Inputs for Quality-of-Life Weights
| Inputs | Mean (SD) | Source |
|---|---|---|
| Base case | ||
| In treatment, off drugs | 0.867 (0.152) | 34 |
| In treatment, on drugs, non-IDU | 0.683 (0.204) | 34 |
| In treatment, on drugs, IDU | 0.633 (0.208) | 34 |
| Out of treatment, off drugs | 0.867 (0.152) | 34 |
| Out of treatment, on drugs, non-IDU | 0.678 (0.207) | 34 |
| Out of treatment, on drugs, IDU | 0.588 (0.212) | 34 |
| Sensitivity analysis case | ||
| In treatment, off drugs | 0.856 (0.150) | Data analysis from39 |
| In treatment, on drugs, non-IDU and IDU | 0.876 (0.100) | Data analysis from39 |
| Out of treatment, off drugs | 0.856 (0.159) | Data analysis from39 |
| Out of treatment, on drugs, non-IDU and IDU | 0.806 (0.194) | Data analysis from39 |
SD, standard deviation; Non-IDU, non-intravenous drug user; IDU, intravenous drug user
Sensitivity Analyses
In one-way and two-way sensitivity analyses, we varied the following inputs. We reduced the cost of bup/nx by 20% to estimate the price reduction if there is one generic competitor, based on the price difference between branded and generic bup/nx,15 and by 50% to estimate the price reduction if there are multiple generic competitors.36,37 We extended the treatment duration to 60 months by projecting retention using a Weibull distribution fitted to data from the first 24 months (lambda = 0.089, gamma = 0.721), resulting in a median time in treatment of 24.3 months. We increased the probability of using drugs while in treatment to 50% and reduced the probability of using drugs while out of treatment and for those not in treatment to 50%. We examined results separately for individuals with and without a history of IDU.
Finally, we used alternative quality-of-life weights derived from the follow-up phase of a separate clinical trial evaluating the use of bup/nx for 14 days versus 12 weeks among opioid-dependent adolescents and young adults aged 15 to 21.38,39 In that study, at the 6-month follow-up appointment, 70 trial participants completed the EQ-5D quality-of-life questionnaire 40,41 and reported current use of illicit drugs and of prescribed bup/nx. The EQ-5D responses were converted to quality-of-life weights representing the US societal perspective,42 and the resulting quality-of-life weights are reported in Table 2. There were insufficient data to separately report quality-of-life weights for non-IDU and IDU participants.
For each one-way or two-way sensitivity analysis, we conducted probabilistic sensitivity analyses by running 10,000 Monte Carlo simulations that simultaneously varied all quality-of-life model inputs and all cost inputs except the price of bup/nx. For non-medication cost inputs we used lognormal distributions derived from the cohort data, and for quality of life inputs we used beta distributions derived from summary data (Table 2) using the method of moments.43 Results are reported as the probability that the cost-effectiveness ratio of bup/nx treatment versus no treatment is below $100,000 per QALY. We conducted expected value of partial perfect information (EVPPI) analyses to describe the value of obtaining better quality data to reduce uncertainties in quality-of-life and non-medication cost inputs. The EVPPIs represent the maximum value society should be willing to pay to eliminate all of the uncertainty about quality-of-life or non-medication cost model inputs.43
RESULTS
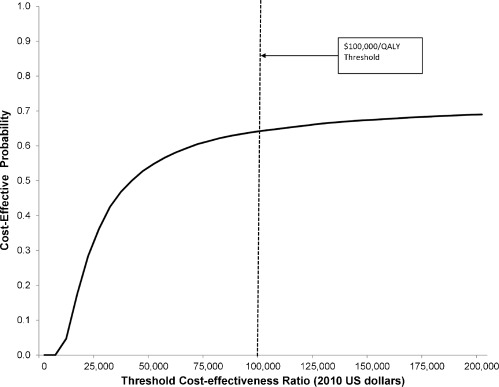
Table 3 presents the base case and sensitivity analysis results. In the base case, the total cost of treatment over 24 months is $7,700, and treatment results in 1.58 QALYs compared to 1.36 QALYs for no treatment, or an additional 0.22 QALYs. The cost-effectiveness ratio of office-based treatment in this clinically stable population is $35,100/QALY. In probabilistic sensitivity analysis, there is a 64% probability that this cost-effectiveness ratio is less than $100,000/QALY and a 55% probability that this cost-effectiveness ratio is less than $50,000/QALY (Fig. 3).
Table 3.
Cost Effectiveness of Office-Based Buprenorphine/Naloxone Treatment Compared to No Treatment
| Treatment cost (2010 US dollars) | Quality-adjusted life years (QALYs) | Incremental QALYs (compared to no treatment) | CE ratio ($/QALY)* | Percent of probabilistic sensitivity analysis CE ratios < $100,000/QALY | |
|---|---|---|---|---|---|
| Base case | |||||
| No Treatment | 0 | 1.36 | --- | --- | --- |
| Treatment | |||||
| Current bup/nx cost | 7,700 | 1.58 | 0.22 | 35,100 | 64 |
| 20% bup/nx cost reduction | 6,700 | 1.58 | 0.22 | 30,300 | 66 |
| 50% bup/nx cost reduction | 5,100 | 1.58 | 0.22 | 23,000 | 69 |
| 60-month treatment duration | |||||
| No treatment | 0 | 3.18 | --- | --- | --- |
| Treatment | |||||
| Current bup/nx cost | 12,200 | 3.53 | 0.35 | 35,200 | 58 |
| 20% bup/nx cost reduction | 10,500 | 3.53 | 0.35 | 30,300 | 60 |
| 50% bup/nx cost reduction | 8,000 | 3.53 | 0.35 | 23,100 | 62 |
| Sensitivity analysis quality-of-life weights | |||||
| No treatment | 0 | 1.64 | --- | --- | --- |
| Treatment | |||||
| Current bup/nx cost | 7,700 | 1.69 | 0.05 | 138,000 | 41 |
| 20% bup/nx cost reduction | 6,700 | 1.69 | 0.05 | 119,000 | 41 |
| 50% bup/nx cost reduction | 5,100 | 1.69 | 0.05 | 90,600 | 43 |
| Probability in treatment on drugs = 0.50 (0.06 in base case) | |||||
| No treatment | 0 | 1.36 | --- | --- | --- |
| Treatment | |||||
| Current bup/nx cost | 7,700 | 1.48 | 0.12 | 63,800 | 53 |
| 20% bup/nx cost reduction | 6,700 | 1.48 | 0.12 | 55,000 | 54 |
| 50% bup/nx cost reduction | 5,100 | 1.48 | 0.12 | 41,900 | 56 |
| Probability off treatment or no treatment on drugs = 0.50 (0.89 in base case) | |||||
| No treatment | 0 | 1.53 | --- | --- | --- |
| Treatment | |||||
| Current bup/nx cost | 7,700 | 1.65 | 0.12 | 65,700 | 55 |
| 20% bup/nx cost reduction | 6,700 | 1.65 | 0.12 | 56,600 | 57 |
| 50% bup/nx cost reduction | 5,100 | 1.65 | 0.12 | 43,100 | 60 |
| Non-IDU only | |||||
| No treatment | 0 | 1.41 | --- | --- | --- |
| Treatment | |||||
| Current bup/nx cost | 7,600 | 1.60 | 0.19 | 39,300 | 57 |
| 20% bup/nx cost reduction | 6,500 | 1.60 | 0.19 | 34,000 | 57 |
| 50% bup/nx cost reduction | 5,000 | 1.60 | 0.19 | 25,900 | 59 |
| IDU only | |||||
| No treatment | 0 | 1.25 | --- | --- | --- |
| Treatment | |||||
| Current bup/nx cost | 8,200 | 1.53 | 0.28 | 28,500 | 66 |
| 20% bup/nx cost reduction | 7,000 | 1.53 | 0.28 | 24,500 | 66 |
| 50% bup/nx cost reduction | 5,300 | 1.53 | 0.28 | 18,600 | 69 |
QALY, quality-adjusted life year; CE, cost-effectiveness; bup/nx, buprenorphine-naloxone; Non-IDU, non-intravenous drug user; IDU, intravenous drug user
*Cost-effectiveness ratios may not match previous columns due to rounding
Figure 3.
Cost-effectiveness acceptability curve for office-based buprenorphine-naloxone treatment versus no treatment (base case).
Reducing the cost of bup/nx by 20% and 50% results in cost-effectiveness ratios of $30,300/QALY and $23,000/QALY, respectively. At current bup/nx costs, the cost-effectiveness ratio is $35,200/QALY when the treatment duration is extended to 60 months, $32,400/QALY when patient costs are excluded, $39,300 when the analysis is limited to non-IDUs, and $28,500/QALY when the analysis is limited to IDUs. The benefits of lower bup/nx costs in reducing (improving) cost-effectiveness ratios are similar to the base case, and the probability that the cost-effectiveness ratio is less than $100,000/QALY is between 57% and 69%. When we varied the probability of using drugs while in treatment from 6% to 50%, the cost-effectiveness ratio was less than $100,000 with 53% certainty. When we varied the probability of using drugs while out of treatment and for those not in treatment from 89% to 50%, the cost-effectiveness ratio was less than $100,000 with 55% certainty.
Using the sensitivity analysis quality-of-life weights that do not distinguish between non-IDUs and IDUs, bup/nx treatment results in 1.69 QALYs compared to 1.64 QALYs, or an additional 0.05 QALYs (compared to an additional 0.27 QALYs in the base case). The smaller QALY gain leads to higher (less attractive) cost-effectiveness ratios than in the base case: $138,000/QALY at current bup/nx costs, $119,000/QALY with a 20% bup/nx cost reduction, and $90,600/QALY with a 50% bup/nx reduction. The probability that these cost-effectiveness ratios are less than $100,000/QALY is between 41% and 43%; the probability that they are less than $50,000/QALY is between 34% and 38%.
The EVPPI analyses confirmed the value of improving certainty in the quality-of-life weight estimates. Using the $100,000/QALY threshold, the EVPPI for eliminating uncertainty about base case quality-of-life weights is $6,400 per person eligible for 24-month office-based bup/nx treatment. At 20% and 50% reductions in bup/nx costs, the EVPPIs per person are $5,800 and $5,100, respectively. In contrast, at each specified bup/nx cost level, the value from reducing uncertainty about the non-medication cost items was less than $5 per person eligible for office-based bup/nx.
DISCUSSION
With the recent implementation of the Mental Health Parity and Addiction Equity Act of 2008,44 more individuals will be able to obtain private and public health insurance coverage for substance abuse treatment on the same basis as medical treatment. Using cost-effectiveness methods routinely applied to medical treatments, our study found that extended office-based bup/nx treatment for clinically stable patients compared to no treatment has a cost-effectiveness ratio of $35,100/QALY and remains consistent as treatment duration is extended. The cost-effectiveness ratio is lower (more attractive) assuming a lower cost for bup/nx due to generic competition, but the impact is limited unless there are assumed to be multiple generic competitors. Currently, interventions with a cost-effectiveness ratio below $100,000/QALY are considered to be good value in the US, although the threshold choice varies among decision makers. Many studies continue to use a threshold of $50,000/QALY45 from the mid-1990s, while others have argued for an even higher threshold of $100,000/QALY to $300,000/QALY despite the potential to encourage further increases in health care expenditures.22,23 Taking into account uncertainty in our model estimates for non-medication cost and quality-of-life, extended bup/nx maintenance treatment has a 64% chance of being cost-effective at a $100,000/QALY threshold.
Three previous studies evaluated the cost-effectiveness of bup/nx compared to no treatment in populations with different costs, treatment durations, or sources of benefit, and found similar cost-effectiveness results. The UK study conducted for the National Institute for Clinical Excellence found bup/nx maintenance therapy to have a cost-effectiveness ratio of £26,429/QALY compared to no treatment from the national health system perspective in 2004, or $60,400/QALY in 2010 US dollars.34 The probability that the cost-effectiveness ratio was less than £65,000/QALY (approximately $100,000/QALY) in this study was approximately 60%. The recent study conducted among US adolescents found that 12-week outpatient bup/nx compared to a 2-week bup/nx taper had a cost-effectiveness ratio of $25,000/QALY ($28,800/QALY in 2010 dollars) using a 12-month period of analysis when considering outpatient substance abuse treatment costs.38 There was an 86% probability that the cost-effectiveness ratio was less than $100,000. An earlier study conducted in the US in 1998 found that if bup/nx increases access to opioid agonist treatment by 10%, it has a cost-effectiveness ratio less than $45,000/QALY ($70,700/QALY in 2010 US dollars), but this study only considered benefits attributable to reduced HIV transmission.46
Our results are sensitive to quality-of-life weights, as indicated by the less attractive cost-effectiveness ratios when we used a different source for these weights. Further analysis using the EVPPI framework emphasizes the importance of conducting additional research to collect accurate data on quality of life on and off bup/nx treatment in a relevant population. Spending $6,400 per eligible patient on quality of life research is economically justifiable according to this analysis, suggesting a rationale for a total budget of over $735 million on this type of research assuming just 5% of the 2.3 million opioid-dependent individuals in the US are eligible for office-based bup/nx treatment. In fact, the actual cost of observational studies that collect quality-of-life data are much lower.
There are several limitations to the analysis. Data on treatment retention, drug use, and costs were from a single-site prospective cohort. Results may not be generalizeable to other office-based setting or populations that were not previously involved in a clinical trial, although other observational studies have demonstrated similar levels of stability for selected patients receiving bup/nx in primary care.27,28,47,48 Costs and outcomes may not reflect current less-intensive patterns of care for clinically stable patients on bup/nx. Relapse to drug use after leaving office-based bup/nx treatment has not been documented, and drug use while in treatment was only 6% in this population; results were consistent when we varied these proportions in sensitivity analyses.
We were unable to consider the potential cost impact of office-based bup/tx treatment on the use of other health services; for example, in the UK successful treatment of opioid dependence is associated with reductions in the use of mental health services and increases in the use of primary care services.34 We also did not include potential benefits from improved workforce participation and decreased criminal activity, although some of these benefits are presumed to be included in quality-of-life measures, nor from behavioral changes that decrease the risk of transmission of HIV and hepatitis C.21
The sources that we used for quality-of-life weights had small sample sizes and were not drawn from the same population that we modeled. The sensitivity analysis data have additional limitations in that they are derived from a young adult US population based on self-reported prescription bup/nx treatment that occurred outside of a trial protocol and were not independently documented. This may have contributed to the non-intuitive finding of a higher quality-of-life weight for those in treatment who continued to use drugs than for those in treatment who did not use drugs. Nevertheless, these are the only quality-of-life weights we are aware of collected from bup/nx treatment patients in the US using a standard research technique (EQ-5D).
Importantly, this cost effectiveness analysis does not consider the costs and benefits of the initial 6-months of treatment. Patients who were eligible for and initiated long-term treatment in the observational cohort represented 32% of those who were randomized in the initial 6-month trial,26 and this yield is likely to vary in different populations. If the costs are higher or benefits are lower for those who drop out of treatment, the cost-effectiveness ratio from the perspective of initiating treatment will likely be higher (less attractive). Finally, the results are not generalizable to a population for whom methadone maintenance would be an appropriate and available alternative.
Our findings demonstrate the potential economic value of office-based bup/nx for clinically stable opioid-dependent patients. We found that office-based bup/nx for these patients can be a cost-effective alternative to no treatment at an accepted threshold of $100,000/QALY depending on assumptions about quality-of-life weights. Additional research about quality of life on and off bup/nx therapy can improve the certainty that the cost-effectiveness result meets this threshold. Further research about broader health system and societal cost savings associated with bup/nx treatment would also likely lead to lower cost-effectiveness ratio estimates.
Acknowledgements
This research was supported in part by the Robert Wood Johnson Foundation’s Substance Abuse Policy Research Program grants nos. 63625 and 55396. The Robert Wood Johnson Foundation had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. This study was also supported by the National Institute on Drug Abuse K01 DA01719 (Dr. Schackman), R01 DA017221 (Dr. Polsky), K01 DA022398 (Dr. Moore), and R01 DA0009803 (Dr. Fiellin).
Portions of earlier versions of this paper were presented at the 2009 Addiction Health Services Research Conference, the 2009 Society for Medical Decision Making Annual Meeting, and the 2009 Substance Abuse and Mental Health Services Administration/National Institute on Drug Abuse Buprenorphine Summit.
We would like to thank Dr. George Woody and the National Institute on Drug Abuse Clinical Trials Network (CTN) for the use of summary results from CTN-0010, “Buprenorphine/Naloxone (Bup/Nx) Facilitated Rehabilitation for Heroin Addicted Adolescents/Young Adults” (NCT 00078130). We acknowledge the members of the Using Modeling to Inform Public Health (UMPH!) seminar at Yale University School of Medicine and the students in Industrial and Systems Engineering 191 at the University of Wisconsin-Madison for their valuable comments.
Conflicts of interest
Dr. Polsky has been a consultant to GlaxoSmithKline, Precision Economics (project funded by Pfizer), and SDIHealth (project funded by Amgen). Dr. Fiellin has received honoraria for serving on an external advisory board monitoring diversion and abuse of buprenorphine from Pinney Associates and Paragon Rx.
References
- 1.Results from the 2009 National Survey on Drug Use and Health: Volume 1. Summary of National Findings. http://oas.samhsa.gov/NSDUH/2k9NSDUH/2k9Results.htm. Accessed November 10, 2011.
- 2.National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction Effective medical treatment of opiate addiction. JAMA. 1998;280(22):1936–1943. doi: 10.1001/jama.280.22.1936. [DOI] [PubMed] [Google Scholar]
- 3.Fiellin DA, Friedland GH, Gourevitch MN. Opioid dependence: rationale for and efficacy of existing and new treatments. Clin Infect Dis. 2006;43(Suppl 4):S173–177. doi: 10.1086/508180. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40(1):17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 5.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 6.Ling W, Charuvastra C, Collins JF, et al. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93(4):475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 7.Mattick RP, Ali R, White JM, O’Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003;98(4):441–452. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 8.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2003(2):CD002207. [DOI] [PubMed]
- 9.CSAT Buprenorphine Information Center. Drug Addiction Treatment Act of 2000. http://buprenorphine.samhsa.gov/data.html. Accessed November 10, 2011.
- 10.Arfken CL, Johanson CE, Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J Subst Abuse Treat. 2010;39(2):96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Clark HW. The State of Buprenorphine Treatment. Paper presented at: Buprenorphine in the Treatment of Opioid Addiction: Reassessment 2010; May 10, 2010; Washington, DC.
- 12.Evaluation of the Buprenorphine Waiver Program: Buprenorphine Reimbursement and Availability Tracking Study. http://www.avisagroup.com/images/Final_Report_of_Tracking_Study.pdf. Accessed November 10, 2011.
- 13.Schackman BR, Merrill JO, McCarty D, Levi J, Lubinski C. Overcoming policy and financing barriers to integrated buprenorphine and HIV primary care. Clin Infect Dis. 2006;43(Suppl 4):S247–253. doi: 10.1086/508190. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed November 10, 2011.
- 15.Murray L, editor. Red book 2010 pharmacy’s fundamental reference. Montvale, NJ: Thomson PDR; 2010. [Google Scholar]
- 16.Fiellin DA. The first three years of buprenorphine in the United States: experience to date and future directions. J Addict Med. 2007;1(2):62. doi: 10.1097/ADM.0b013e3180473c11. [DOI] [PubMed] [Google Scholar]
- 17.Clark RE, Samnaliev M, Baxter JD, Leung GY. The Evidence Doesn’t Justify Steps By State Medicaid Programs To Restrict Opioid Addiction Treatment With Buprenorphine. Health Aff (Millwood) 2011;30(8):1425–1433. doi: 10.1377/hlthaff.2010.0532. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction.Treatment Improvement Protocol (TIP) Series 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed]
- 19.Pyne JM, Tripathi S, French M, McCollister K, Rapp RC, Booth BM. Longitudinal association of preference-weighted health-related quality of life measures and substance use disorder outcomes. Addiction. 2011;106(3):507–515. doi: 10.1111/j.1360-0443.2010.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosyk B, Sun H, Guh DP, et al. The quality of eight health status measures were compared for chronic opioid dependence. J Clin Epidemiol. 2010;63(10):1132–1144. doi: 10.1016/j.jclinepi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 22.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46(4):349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 23.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 24.Tan-Torres Edejer T, Baltussen R, Adam T, et al., eds. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva: World Health Organization; 2003.
- 25.Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- 26.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 27.Alford DP, Labelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat. 2009;37(4):426–430. doi: 10.1016/j.jsat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Gossop M, Marsden J, Stewart D, Kidd T. The National Treatment Outcome Research Study (NTORS): 4-5 year follow-up results. Addiction. 2003;98(3):291–303. doi: 10.1046/j.1360-0443.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 30.Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones ES, Moore BA, Sindelar JL, O’Connor PG, Schottenfeld RS, Fiellin DA. Cost analysis of clinic and office-based treatment of opioid dependence: results with methadone and buprenorphine in clinically stable patients. Drug Alcohol Depend. 2009;99(1–3):132–140. doi: 10.1016/j.drugalcdep.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Department of Labor Bureau of Labor Statistics. National Compensation Survey. http://data.bls.gov. Accessed November 10, 2011.
- 33.State of Connecticut Department of Labor, History of minimum wage laws. http://www.ctdol.state.ct.us/wgwkstnd/wage-hour/history.htm. Accessed November 10, 2011.
- 34.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 35.Stein K, Dyer M, Crabb T, et al. A pilot Internet "value of health" panel: recruitment, participation and compliance. Health Qual Life Outcomes. 2006;4:90. doi: 10.1186/1477-7525-4-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank RG, Salkever DS. Generic entry and the pricing of pharmaceuticals. J Econ Manage Strategy. 1997;6(1):75–90. doi: 10.1162/105864097567039. [DOI] [Google Scholar]
- 37.Santell JP. Projecting future drug expenditures–1996. Am J Health Syst Pharm. 1996;53(2):139–150. doi: 10.1093/ajhp/53.2.139. [DOI] [PubMed] [Google Scholar]
- 38.Polsky D, Glick HA, Yang J, Subramaniam GA, Poole SA, Woody GE. Cost-effectiveness of extended buprenorphine-naloxone treatment for opioid-dependent youth: data from a randomized trial. Addiction. 2010;105(9):1616–1624. doi: 10.1111/j.1360-0443.2010.03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Group The EuroQol. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 41.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 42.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Briggs A, Schulpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford, United Kingdom: Oxford University Press; 2006. [Google Scholar]
- 44.The Mental Health Parity and Addiction Equity Act. http://cciio.cms.gov/programs/protections/mhpaea/mhpaea_factsheet.html. Accessed November 10, 2011.
- 45.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 46.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96(9):1267–1278. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- 47.Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20(11):1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parran TV, Adelman CA, Merkin B, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106(1):56–60. doi: 10.1016/j.drugalcdep.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. Department of Health & Human Services. Centers for Medicare & Medicaid Services. http://www.cms.hhs.gov/. Accessed November 10, 2011.