ABSTRACT
BACKGROUND
Although guidelines recommend against prostate-specific antigen (PSA) screening in elderly men with limited life expectancy, screening is common.
OBJECTIVE
We sought to identify medical center characteristics associated with screening in this population.
DESIGN/PARTICIPANTS
We conducted a prospective study of 622,262 screen-eligible men aged 70+ seen at 104 VA medical centers in 2003.
MAIN MEASURES
Primary outcome was the percentage of men at each center who received PSA screening in 2003, based on VA data and Medicare claims. Men were stratified into life expectancy groups ranging from favorable (age 70–79 with Charlson score = 0) to limited (age 85+ with Charlson score ≥1 or age 70+ with Charlson score ≥4). Medical center characteristics were obtained from the 1999–2000 VA Survey of Primary Care Practices and publicly available VA data sources.
KEY RESULTS
Among 123,223 (20%) men with limited life expectancy, 45% received PSA screening in 2003. Across 104 VAs, the PSA screening rate among men with limited life expectancy ranged from 25-79% (median 43%). Higher screening was associated with the following center characteristics: no academic affiliation (50% vs. 43%, adjusted RR = 1.14, 95% CI 1.04–1.25), a ratio of midlevel providers to physicians ≥3:4 (55% vs. 45%, adjusted RR = 1.20, 95% CI 1.09–1.32) and location in the South (49% vs. 39% in the West, adjusted RR = 1.25, 95% CI 1.12–1.40). Use of incentives and high scores on performance measures were not independently associated with screening. Within centers, the percentages of men screened with limited and favorable life expectancies were highly correlated (r = 0.90).
CONCLUSIONS
Substantial practice variation exists for PSA screening in older men with limited life expectancy across VAs. The high center-specific correlation of screening among men with limited and favorable life expectancies indicates that PSA screening is poorly targeted according to life expectancy.
Key words: PSA screening, regional variation, elderly, life expectancy
INTRODUCTION
Guidelines from the U.S. Preventive Services Task Force (USPSTF), American Cancer Society, and American Urologic Association all recommend against prostate-specific antigen (PSA) screening in elderly men with limited life expectancy.1–3 Most screen-detected prostate cancers will not cause symptoms for many years. Thus, men with limited life expectancy have little chance of experiencing benefits from screening, which occur years in the future, yet are at high risk of experiencing immediate burdens, including unnecessary biopsies, as well as incontinence, impotence, proctitis, hot flashes, and other adverse effects from treatment of clinically inconsequential cancers.3–5
Prior studies have shown substantial PSA screening among American men aged 80 and older, although regional screening rates range from under 2% to 56%.6–11 Within the VA, 45% of men over 80 received PSA screening in 2003.7 High rates of screening in elderly men are also common in fee-for-service Medicare, especially in regions with higher expenditures, more intensive end-of-life care, more unique physicians seen, and greater use of specialists for ambulatory care.9 While studies have demonstrated geographic variation in PSA screening of elderly men across large hospital referral regions,9 we are unaware of studies examining variation at the level of the medical center, which is a key locus for implementing screening policies. Furthermore, we are unaware of studies of geographic variation focusing on elderly men with limited life expectancy.
To characterize variation in PSA screening of older men across VA medical centers according to life expectancy, we examined VA data and Medicare claims for men aged 70 and older seen at 104 VAs. We identified patient and medical center characteristics prior to 2003 and calculated the percent of men who received PSA screening at each center in 2003. We aimed to identify medical center characteristics associated with higher PSA screening among elderly men with limited life expectancy, in order to guide interventions to reduce screening in men for whom potential burdens outweigh potential benefits.
METHODS
Data Sources and Patients
We identified a cohort of screen-eligible men aged 70 and older on 10/1/02, following them through 9/30/03 (FY 2003) for receipt of PSA screening; hereafter all years refer to fiscal years. Men were assigned to the VA that ordered their first PSA test in 2003; if they were not screened, they were assigned to the center where they had the most outpatient visits in 2003. Data were collected from the VA National Data Systems, the central repository for VA data, including the National Patient Care Database (inpatient/outpatient VA claims), fee basis files (claims for non-VA services paid by the VA), and 2003 VA Decision Support System National Data Extracts Laboratory Results Data Set (lab results including PSA, available for 104 of 127 VAs).7 We used linked Medicare claims from the VA Information Resource Center to identify non-VA services paid by Medicare in our cohort.
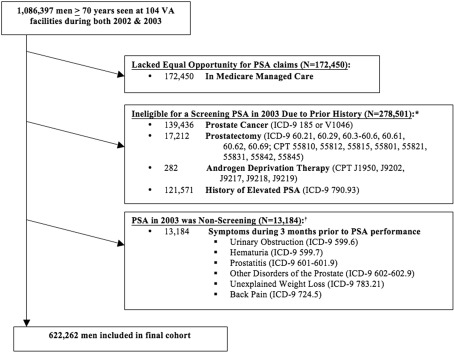
From these data sources, we identified 1,086,397 men aged 70 or older with at least one outpatient visit in both 2002 and 2003 at one of 104 VAs. After applying exclusions shown in Figure 1, 622,262 men remained in our screen-eligible cohort, seen at 104 VAs across the U.S.
Figure 1.
Exclusions used to define the final cohort of elderly men eligible for PSA screening.
Data Collection and Measurement
Outcome Variable
We assessed receipt of PSA screening during 2003 across the VA system and Medicare. Screening within the VA was defined by an outpatient PSA in the 2003 VA Decision Support System National Data Extracts Laboratory Results Data Set. Completeness of extraction was confirmed by comparing our data with an independently extracted VA Decision Support System Laboratory Data Set.7 We also assessed PSA screening reimbursed by Medicare, since most VA users over age 65 are also enrolled in Medicare and may be screened in the community through Medicare. PSA screening reimbursed by Medicare was identified by Current Procedure Terminology (CPT) codes G0103 and 84153 from hospital outpatient records and physician/supplier files for each patient.12
Predictor Variables
Medical center characteristics hypothesized to influence PSA screening were obtained from the 1999–2000 VA Survey of Primary Care Practices, completed by primary care directors at 100 of 104 VAs,13,14 and publicly available VA data.15,16 Center characteristics obtained from the survey included 1) academic affiliation—formal arrangement with an academic training program for medical residents; 2) initial primary care clinic appointment length ≥45 minutes—greater than average time allotted for new patient appointments; 3) primary care geriatricians—having geriatricians providing primary care or direct primary care supervision; 4) ratio of midlevel providers [nurse practitioners (NP) and physician assistants (PA)] to physicians (MD) in primary care ≥3:4—a ratio of full-time equivalents for NP + PA/MD in the upper quartile of VAs; and 5) incentives for achieving primary care performance measures—awards (e.g., plaques, certificates) or monetary incentives (e.g., bonuses) for high primary care performance. Publicly available reports from the VA Office of Quality and Performance provided each VA’s 2003 performance scores on prostate cancer screening education (i.e., chart documentation of discussion of pros/cons of prostate cancer screening for men aged 50–69) and colorectal cancer screening (i.e., performance of timely colorectal cancer screening for patients in primary care).16 Medical center scores were dichotomized at the median and reflect targets for satisfactory performance. Additionally, the 2003 VA and DoD Healthcare Facilities Directory provided total reported healthcare expenditures and number of outpatient visits at each center in 2002, presented as tertiles.15 Medical center size was based on the number of men each center contributed to our cohort. Region within the U.S. was categorized into Midwest, Northeast, South, and West based on criteria from the 2000 U.S. Census.
Patient characteristics measured included age at the start of 2003 and comorbidity defined by the Deyo adaptation of the Charlson Comorbidity Index.17 Charlson scores were calculated from VA and Medicare inpatient/outpatient claims during 12 months prior to 2003. Men were categorized into three mutually exclusive groups based on life expectancy: 1) “favorable” life expectancy for those aged 70–79 with Charlson score = 0; 2) “limited” life expectancy for those aged 85 and older with Charlson score ≥1, or those aged 70 and older with Charlson score ≥4; and 3) everyone else. These categories identify one group with favorable life expectancy (> 10 years) for whom several guidelines recommend offering PSA screening, and another group (life expectancy ≤5 years) that all guidelines agree is unlikely to benefit from screening.18,19 Other patient characteristics known to influence cancer screening included race/ethnicity and marital status, ascertained from VA and Medicare data. We also used linkages to the 2000 U.S. Census to determine for each veteran’s ZIP code area the percentage of adults with a college education and the median income for adults aged 65 and older.
The Committee on Human Research at the University of California, San Francisco and the Committee for Research and Development at the San Francisco VA approved the study.
Statistical Analysis
The percentage of men who received PSA screening in 2003 was determined for each VA. We also determined the percentages of men with favorable and limited life expectancies who received PSA screening, to determine how well screening is targeted according to life expectancy. A Pearson correlation coefficient was calculated to describe the relationship between screening rates in men with limited and favorable life expectancies. For men with limited life expectancy, the associations between medical center/patient characteristics and receipt of PSA screening were determined by log-Poisson regression models with fixed effects for medical center/patient characteristics and random effects for medical centers. We used log-Poisson models to estimate unadjusted and adjusted risk ratios and 95% confidence intervals.20 The ratio of midlevel providers to physicians was missing at 10% of sites, so missing values were multiply imputed using five rounds of imputation.21 SAS procedures MI and MIANALYZE were used to generate and analyze multiply imputed datasets. Risk ratios for bivariate associations were reported using complete cases, and adjusted risk ratios were calculated using imputed data. Additionally, a sensitivity analysis was performed excluding men whose first PSA test in 2003 was through Medicare to focus solely on screening within the VA. All analyses were performed using SAS® version 9.2 and Stata version 10 statistical software packages.
RESULTS
Baseline Characteristics
Characteristics of the 622,262 elderly men in our cohort are presented in Table 1. 69% received care at a medical center with formal academic affiliation; 53% at a center with primary care performance incentives; 15% at a center in the West; and 11% at a center where the ratio of midlevel providers to physicians was ≥3:4.
Table 1.
Baseline Characteristics of Men 70 Years of Age or Older
| Characteristic | Total Cohort (N = 622,262) N (%) | Men with Limited Life Expectancy (N = 123,223) N (%) | Men with Favorable Life Expectancy (N = 136,839) N (%) |
|---|---|---|---|
| Attended a VA Medical Center with the Following Characteristics | |||
| Census Region of VA Medical Center | |||
| West | 96,125 (15.5) | 17,575 (14.3) | 22,665 (16.6) |
| Midwest | 186,472 (30.0) | 35,989 (29.2) | 41,857 (30.6) |
| Northeast | 84,885 (13.6) | 18,514 (15.0) | 18,188 (13.3) |
| South | 254,780 (40.9) | 51,145 (41.5) | 54,129 (39.6) |
| Academic Affiliation | 426,556 (68.6) | 84,993 (69.0) | 93,135 (68.1) |
| Incentives for Primary Care Performance* | 318,138 (52.9) | 63,379 (53.3) | 69,175 (52.3) |
| Primary Care Initial Appt Length ≥45 min* | 143,242 (23.8) | 28,897 (24.3) | 31,300 (23.7) |
| Primary Care Geriatricians* | 329,130 (55.2) | 65,954 (55.9) | 71,959 (54.8) |
| VA Size (number of men in cohort)† | |||
| Lowest Tertile (≤3,995 men) | 101,363 (16.3) | 19,317 (15.7) | 23,279 (17.0) |
| Middle Tertile (3,995–5,872 men) | 170,369 (27.4) | 33,794 (27.4) | 37,728 (27.6) |
| Highest Tertile ( ≥5,872 men) | 350,530 (56.3) | 70,112 (56.9) | 75,832 (55.4) |
| Performance on Prostate Cancer Education >90% | 306,868 (49.3) | 60,290 (48.9) | 67,557 (49.4) |
| Performance on Colorectal Cancer Screening >67% | 329,356 (52.9) | 65,396 (53.1) | 72,564 (53.0) |
| Number of Outpatient Visits in 2002 | |||
| Lowest Tertile ( ≤201,864 visits) | 125,863 (20.2) | 23,404 (19.0) | 29,184 (21.3) |
| Middle Tertile (201,864–365,000 visits) | 215,221 (34.6) | 42,852 (34.8) | 46,333 (33.9) |
| Highest Tertile (>365,000 visits) | 281,178 (45.2) | 56,967 (46.2) | 61,322 (44.8) |
| Total Health Care Expenditures in 2002 | |||
| Lowest Tertile ( ≤$88,687,612) | 122,957 (19.8) | 22,767 (18.5) | 28,274 (20.7) |
| Middle Tertile ($88,687,612–$177,724,499) | 195,444 (31.4) | 39,086 (31.7) | 42,221 (30.9) |
| Highest Tertile (>$177,724,499) | 303,861 (48.8) | 61,370 (49.8) | 66,344 (48.5) |
| Ratio of NP or PAs to MDs ≥3:4* | 57,657 (10.6) | 11,326 (10.5) | 13,039 (11.0) |
| Patient Characteristics | |||
| Age, years | |||
| 70–74 | 234,216 (37.6) | 33,010 (26.8) | 75,650 (55.3) |
| 75–79 | 219,951 (35.4) | 38,053 (30.9) | 61,189 (44.7) |
| 80–84 | 130,871 (21.0) | 24,603 (20.0) | 0 |
| ≥85 | 37,224 (6.0) | 27,557 (22.4) | 0 |
| Race/Ethnicity** | |||
| White | 556,384 (89.4) | 110,657 (89.8) | 121,352 (88.7) |
| Black | 44,550 (7.2) | 8,958 (7.3) | 10,146 (7.4) |
| White Hispanic | 11,290 (1.8) | 2,115 (1.7) | 2,619 (1.9) |
| Other | 10,038 (1.6) | 1,493 (1.2) | 2,722 (2.0) |
| Married* | 442,562 (71.7) | 85,120 (69.6) | 99,219 (73.2) |
| Charlson Score | |||
| 0 (good health) | 180,533 (29.0) | 0 | 136,839(100.0) |
| 1–3 (average health) | 338,925 (54.5) | 20419 (16.6) | 0 |
| ≥4 (poor health) | 102,804 (16.5) | 102804 (83.4) | 0 |
| Lived in ZCTA in which ≥25% of Adults Had a College Education* | 189,107 (31.2) | 38,328 (31.8) | 42,868 (32.3) |
| Median Annual Income of ZCTA* | |||
| Highest Tertile (≥$41,943) | 202,123 (33.4) | 39,581 (32.9) | 45,277 (34.1) |
| Middle Tertile ($32,980–$41,943) | 202,052 (33.3) | 39,850 (33.1) | 44,511 (33.5) |
| Lowest Tertile (≤$32,980) | 202,161 (33.3) | 41,020 (34.1) | 43,163 (32.5) |
*Data missing for these variables: Incentives for primary care performance (3%), Primary care initial appointment length (3%), Primary care geriatricians (4%), Ratio of NPs or PAs to MDs (10%), Married (1%), Lived in ZCTA where ≥25% of adults had a college education (3%), and Median annual income of ZCTA for adults 65+ (3%). ZCTA = Zip code tabulation area
†VA size based on the number of men each VA contributed to the cohort of 622,262 screen-eligible men
**22% of VA medical centers cared for populations that included ≥10% black patients; this center characteristic was not associated with PSA screening (p = 0.92)
PSA Screening Rates
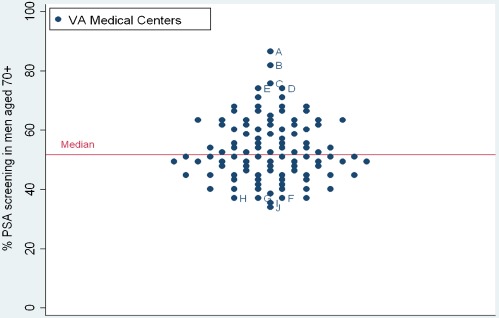
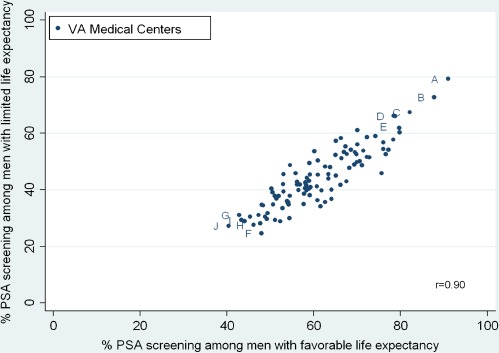
54% of our cohort of men aged 70 or older (338,407/622,262) received PSA screening in 2003. The percentage of men who received screening at each center ranged from 33-87%, illustrating wide variation in screening practices at VAs across the U.S. (Figure 2). The percentage of men with limited life expectancy who received PSA screening was somewhat lower at 45% and also varied substantially across centers, ranging from 25-79%. 31% (32/104) of centers screened over half of men with limited life expectancy. VAs with the highest overall screening (Centers A-E) had high screening in men with both limited and favorable life expectancies. Screening rates among men with limited and favorable life expectancies were highly correlated (r = 0.90) (Figure 3).
Figure 2.
Percentage of men aged 70 and older who received PSA screening at each of 104 VAs. VAs with the highest screening rates are identified A-E and those with the lowest screening rates are identified F-J.
Figure 3.
Correlation of PSA screening among older men with favorable and limited life expectancies at each of 104 VAs. Favorable life expectancy defined as men aged 70–79 with Charlson score = 0 (life expectancy >10 years). Limited life expectancy defined as men aged 85+ with Charlson score ≥1 or men aged 70+ with Charlson score ≥4 (life expectancy ≤5 years). Letters correspond to the highest and lowest screening VAs from Figure 2. The lower right quadrant is where screening is best targeted according to life expectancy (i.e., low screening in men with limited life expectancy and high screening in men with favorable life expectancy).
Medical Center and Patient Factors Associated with PSA Screening
The percentage of men with limited life expectancy who received PSA screening is presented by medical center/patient characteristics in Table 2. Center characteristics associated with higher screening in men with limited life expectancy included having no academic affiliation (50% vs. 43%, adjusted RR = 1.14, 95% CI 1.04-1.25), having a ratio of midlevel providers to physicians in primary care ≥3:4 (55% vs. 45%, adjusted RR = 1.20, 95% CI 1.09-1.32), and location in the South (49% vs. 39% in the West, adjusted RR = 1.25, 95% CI 1.12-1.40). Other center characteristics were not independently associated with PSA screening, including initial primary care appointment length, having primary care geriatricians, and performance scores on prostate cancer screening education and colorectal cancer screening (Table 2). Primary care performance incentives were associated with higher screening in bivariate analyses but not in adjusted analyses.
Table 2.
PSA Screening Rates in Men Aged 70+ with Limited Life Expectancy According to Medical Center and Patient Characteristics (N = 123,223)
| Characteristic | Screening PSA N (%) | Unadjusted Risk Ratio (95% CI) | Adjusted Risk Ratio* (95% CI) |
|---|---|---|---|
| VA Medical Center Characteristics | |||
| Census Region of VA Medical Center | |||
| West | 6922(39) | 1.0 | 1.0 |
| Midwest | 15411(43) | 1.07(0.93,1.22) | 1.15 (1.02,1.30) |
| Northeast | 8667(47) | 1.10(0.95,1.28) | 1.14(0.99,1.31) |
| South | 24828(49) | 1.15(1.02,1.30) | 1.25 (1.12,1.40) |
| Academic Affiliation | |||
| No | 19167(50) | 1.15(1.05–1.27) | 1.14(1.04–1.25) |
| Yes | 36661(43) | 1.0 | 1.0 |
| Incentives for Primary Care Performance | |||
| No | 23920(43) | 1.0 | 1.0 |
| Yes | 30266(48) | 1.15(1.05,1.27) | 1.06(0.98,1.15) |
| Primary Care Initial Appt Length ≥45 min | |||
| No | 40871(45) | 1.0 | 1.0 |
| Yes | 13315(46) | 0.95(0.85,1.07) | 0.98(0.89,1.09) |
| Primary Care Geriatricians | |||
| No | 23990(46) | 1.0 | 1.0 |
| Yes | 29831(45) | 1.06(0.96,1.16) | 0.94(0.86,1.03) |
| VA Size (number of men in cohort) | |||
| Lowest Tertile (≤3,995 men) | 8945(46) | 1.0 | 1.0 |
| Middle Tertile (3,995–5,872 men) | 32375(46) | 0.93(0.83,1.04) | 0.98(0.88,1.10) |
| Highest Tertile (≥5,872 men) | 14508(43) | 0.97(0.87,1.09) | 1.00(0.88,1.15) |
| Performance on Prostate Cancer Education | |||
| >90% | 27194(45) | 1.0 | 1.0 |
| ≤90% | 28634(46) | 0.97(0.88,1.07) | 1.00(0.91,1.08) |
| Performance on Colorectal Cancer Screening | |||
| >67% | 29497(45) | 1.0 | 1.0 |
| ≤67% | 26331(46) | 1.02(0.93,1.12) | 0.95(0.87,1.05) |
| Number of Outpatient Visits in 2002 | |||
| Lowest Tertile (≤201,864 visits) | 10864 (46) | 1.0 | 1.0 |
| Middle Tertile (201,864–365,000 visits) | 18260 (43) | 0.97(0.84–1.10) | 1.00(0.87–1.16) |
| Highest Tertile (>365,000 visits) | 26704 (47) | 1.01(0.88–1.14) | 1.20(0.97–1.47) |
| Total Health Care Expenditures in 2002 | |||
| Lowest Tertile (≤$88,687,612) | 10762 (47) | 1.0 | 1.0 |
| Middle Tertile ($88,687,612–$177,724,499) | 17217 (44) | 0.95(0.82–1.07) | 0.92(0.79–1.06) |
| Highest Tertile (>$177,724,499) | 27849 (45) | 0.96(0.84–1.09) | 0.82(0.66–1.02) |
| Ratio of NP or PAs to MDs | |||
| <3:4 | 43514 (45) | 1.0 | 1.0 |
| ≥3:4 | 6176 (55) | 1.18(1.02–1.35) | 1.20(1.09–1.32) |
| Patient Characteristics | |||
| Age, years | |||
| 70–74 | 18224(55) | 1.0 | 1.0 |
| 75–79 | 18387(48) | 0.87(0.85,0.89) | 0.87(0.85,0.89) |
| 80–84 | 9745(40) | 0.72(0.70,0.73) | 0.71(0.70,0.73) |
| ≥85 | 9472(34) | 0.62(0.61,0.64) | 0.62(0.61,0.64) |
| Race/Ethnicity | |||
| White | 51028(46) | 1.0 | 1.0 |
| Black | 3288(37) | 0.82(0.79,0.85) | 0.79(0.76,0.82) |
| White Hispanic | 987(47) | 0.86(0.80,0.92) | 0.88(0.82,0.94) |
| Other | 525(35) | 0.81(0.74,0.88) | 0.83(0.76,0.90) |
| Married | |||
| No | 14720(40) | 1.0 | 1.0 |
| Yes | 40742(48) | 1.18(1.16,1.21) | 1.13(1.11,1.16) |
| Lived in ZCTA in which ≥25% of Adults Had a College Education | |||
| No | 36680(45) | 1.0 | 1.0 |
| Yes | 17878(47) | 1.04(1.02,1.05) | 1.06(1.03,1.08) |
| Median Income of ZCTA | |||
| Highest Tertile (≥41,943) | 18788(46) | 1.0 | 1.0 |
| Middle Tertile (32,980,41,943) | 17990(45) | 0.98(0.96,1.00) | 1.00(0.98,1.03) |
| Lowest Tertile (≤32,980) | 17788(45) | 0.94(0.92,0.96) | 0.99(0.96,1.02) |
*Adjusted for all characteristics in Table 2
Among men with limited life expectancy, several patient characteristics were associated with PSA screening. White men were 20% more likely to be screened than black men. Men who were younger, married, and had higher socioeconomic status were also more likely to be screened (Table 2).
To rule out that variation in PSA screening across centers was driven by community screening, we performed a sensitivity analysis including only PSA tests within the VA; all associations remained the same. Overall, 40% of men in our cohort received PSA screening within the VA and 14% within Medicare. Using VA data alone, percentages of men screened at each center ranged from 18-85%, indicating that variation was not driven by Medicare testing.
Discussion
Despite long-standing guidelines recommending against PSA screening in elderly men with life expectancy less than 10 years,1–3 we found high screening in this population with substantial variation across VAs. Nearly one-third of medical centers screened more than half of men with limited life expectancy. VAs in the South, without academic affiliation, and with a high ratio of midlevel providers to physicians in primary care screened the most men with limited life expectancy, even after adjusting for patient characteristics. Additionally, at each VA the percentages of men screened with limited and favorable life expectancies were highly correlated, indicating that screening is being poorly targeted.
There is growing literature examining regional variation in cancer screening, although it has rarely assessed variation according to life expectancy. The literature examining PSA screening in the U.S. has described screening of older men based on age cut-offs without consideration of comorbidity, showing wide variations by region.6,9–11 Using national surveys or Medicare claims, studies have found that Southern regions screen at higher rates, and describe twofold to 20-fold regional variations in the percentage of older men screened.6,9,22,23 Similarly, we found that VAs in the South screen at higher rates than in the West, including for men with limited life expectancy. Even across the nationally integrated VA system, we found threefold differences in screening of men with limited life expectancy. One VA in the Midwest screened nearly 80% of this population. Possible explanations for differences include regional physician attitudes about resource allocation24—with the South and Midwest generally having high healthcare expenditures,25 beliefs about the effectiveness of PSA screening,26,27 or regional patient preferences for screening.28 Studies outside the VA suggest the latter is less likely.10,24,28
In addition to region, other center characteristics associated with high PSA screening of men with limited life expectancy include lack of academic affiliation and a high ratio of midlevel providers (NPs and PAs) to physicians in primary care. The negative association of academic affiliation with PSA screening has been found previously29–31 and explained by a greater emphasis of evidence-based medicine at academic centers, although there is substantial variation in PSA knowledge and practices even within a single center.26,29,32 Furthermore, prior studies suggest a weaker malpractice climate in academic settings may lead to less defensive medicine.27,29,33 The evidence is less consistent regarding the role of midlevel providers in screening.34,35 Studies show increased colorectal cancer screening with higher use of NPs and PAs,35 and decreased PSA screening of men aged 75 and older.36 Although the latter study appears to conflict with our results, the difference might be explained by the fact that the study utilized provider-level data, while we examined data at the level of the medical center. A high ratio of midlevel providers to physicians at a medical center may be more indicative of tight hospital resources or high patient load, rather than screening practices of individual providers.
Incentives for primary care performance and scores on prostate cancer screening education did not significantly affect PSA screening practices. These programs have been criticized by some as encouraging a one-size-fits-all approach to care.37,38 We found that medical centers are uniformly not targeting screening according to life expectancy, regardless of performance measures or whether a center has a high or low overall screening rate. Not only do high screening VAs screen many men with limited life expectancy who should not be screened, but centers screening few men with limited life expectancy also screen few men with favorable life expectancy, for whom some guidelines recommend screening. One explanation for poor targeting at low screening centers may be an overall attitude that PSA is a poor test that causes more harm than good, which is a position now supported by 2011 USPSTF guidelines.24,27,39,40
Although we found only a few center characteristics associated with PSA screening in men with limited life expectancy, this does not suggest that individual VA culture and policies do not influence screening.41 While we did not measure medical center screening policies in this study, we did speak with the Quality Assurance Coordinator for Primary Care at the highest screening VA (Site A) and learned that PSA is included in the annual wellness labs for all men. This decision has contributed to a very high rate of PSA screening (79%) among elderly men with limited life expectancy at this VA. Meanwhile, one of the lowest screening sites (Site I) is only 300 miles from Site A. In this case, individual VA culture and policies trump regional culture when explaining variation in screening practices.
Additionally, while many medical center characteristics are difficult to change, individual center policies, such as including PSA in routine labs, are highly amenable to change so they follow guidelines to involve patients in decision-making and reduce harm to the sickest patients. Aids to assist physicians assess life expectancy in elders may be useful to reduce screening in patients with limited life expectancy.18,42 Other tools, such as educational videos or pamphlets, may help physicians communicate with elders to guide decisions that will be best for patients’ health.43,44 Furthermore, although performance measures have seen varied success, it may be useful to consider a new kind of measure that rewards physicians for not ordering a screening PSA when it is not appropriate, such as in the oldest and sickest men.
This study has several limitations. First, the study population was veterans, so results may not be generalizable outside the VA. Nevertheless, the VA is one of the largest healthcare systems for elderly men in the U.S., so understanding variations across the VA is important in its own right. Second, the rate of PSA screening at each VA reflects screening both in the community and at the VA, since these are not independent events; veterans who are screened using Medicare do not need a repeat test at a VA. To rule out that our findings were driven by community screening, we performed sensitivity analyses including only PSA tests within the VA; all associations remained the same. Third, data are from 2003 and may not reflect current screening practices. Nevertheless, a recent study indicates that annual PSA screening among men aged 75–79 remains high at 57% in 2005 and 2008.45 Finally, because some PSA tests in the community may have been paid by sources other than Medicare, some screening may not be captured in our data. Thus, PSA screening among elderly veterans may in fact be higher than presented in this study.
In conclusion, PSA screening in veterans aged 70 and older with limited life expectancy varies greatly across VA medical centers with a threefold difference in screening between the highest and lowest screening VAs. These findings indicate the need for interventions to reduce inappropriate screening in elderly men, including efforts to change individual center practices that promote high screening regardless of life expectancy; PSA should not be included in routine labs. Additionally, guidelines and performance measures must do more to encourage individualized screening decisions that consider life expectancy. New communication tools are needed to help elderly men weigh benefits and harms of PSA screening, since current decision-aids focus on younger men.43,44 Initially, interventions should be directed at centers with high screening of men with limited life expectancy in order to minimize harms of screening. However, all VAs would benefit from these interventions because despite wide variation in PSA screening rates, medical centers are uniformly not targeting screening according to life expectancy.
Acknowledgments
Funders
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R01 CA134425) to [LW, RH, AP, and SF]; the Medical Student Training in Aging Research Program at the American Federation for Aging Research to [CS]; the National Institute on Aging (grant number K-01AG025444) to [KM]; the New Mexico VA Health Care System to [RH]; the White River Junction VA to [BS]; and the VA HSR&D Research Career Development Award (Project #05-195) to [EY].
Prior presentations
This work was presented at the American Geriatrics Society Annual Scientific Meeting, May 2010, Orlando, FL.
Conflicts of Interest
The funding sources had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication. The authors report no conflicts of interest related to the work described in this manuscript.
References
- 1.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society Guidelines for the early detection of cancer, 2003. CA Cancer J Clin. 2003;53:27–43. doi: 10.3322/canjclin.53.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182(5):2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 3.Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2002;137:915–6. [DOI] [PubMed]
- 4.Crawford ED, Grubb R, 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29(4):355–361. doi: 10.1200/JCO.2010.30.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamatiou K, Alevizos A, Mariolis A, et al. Do clinically insignificant tumors of the prostate exist? Urol Int. 2008;81(4):379–382. doi: 10.1159/000167832. [DOI] [PubMed] [Google Scholar]
- 6.Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004;38(6):732–744. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 8.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289(11):1414–1420. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 9.Bynum J, Song Y, Fisher E. Variation in prostate-specific antigen screening in men aged 80 and older in fee-for-service Medicare. J Am Geriatr Soc. 2010;58(4):674–680. doi: 10.1111/j.1532-5415.2010.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu-Yao G, Stukel TA, Yao SL. Prostate-specific antigen screening in elderly men. J Natl Cancer Inst. 2003;95(23):1792–1797. doi: 10.1093/jnci/djg104. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Zhao G, Pollack LA, Smith JL, Joseph DA. Use of the prostate-specific antigen test among men aged 75 years or older in the United States: 2006 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2010;7(4):A84. [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman JL, Klabunde CN, Schussler N, Warren JL, Virnig BA, Cooper GS. Measuring breast, colorectal, and prostate cancer screening with Medicare claims data. Med Care. 2002;40(suppl IV):36–42. doi: 10.1097/00005650-200208001-00005. [DOI] [PubMed] [Google Scholar]
- 13.Yano EM, Simon B, Canelo I, Mittman B, Rubenstein LV. 1999 VHA Survey of Primary Care Practices, Technical Monograph #00-MC12. Sepulveda, CA: VA HSR&D Center of Excellence for the Study of Healthcare Provider Behavior; 2000.
- 14.Yano EM, Soban LM, Parkerton PH, Etzioni DA. Primary care practice organization influences colorectal cancer screening performance. Health Serv Res. 2007;42(3, pt 1):1130–1149. doi: 10.1111/j.1475-6773.2006.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federal Practitioner. 2003 Directory: VA and DoD Health Care Facilities. Chatham, NJ: Quadrant HealthCom Inc; 2003.
- 16.VA Office of Quality and Performance. FY 2003. VHA performance measurement system technical manual 2003. Available at: http://vaww.archive.oqp.med.va.gov/oqp_services/performance_measurement/tech_man.asp. Accessed February 28, 2011.
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 19.Kistler CE, Kirby KA, Lee D, Casadei MA, Walter LC. Long-term outcomes following positive fecal occult blood test results in older adults: benefits and burdens. Arch Intern Med. 2011;171(15):1344–1351. doi: 10.1001/archinternmed.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 21.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 22.Shaw PA, Etzioni R, Zeliadt SB, et al. An ecologic study of prostate-specific antigen screening and prostate cancer mortality in nine geographic areas of the United States. Am J Epidemiol. 2004;160(11):1059–1069. doi: 10.1093/aje/kwh336. [DOI] [PubMed] [Google Scholar]
- 23.Henderson JA, Espey DK, Jim MA, German RR, Shaw KM, Hoffman RM. Prostate cancer incidence among American Indian and Alaska Native men, US, 1999–2004. Cancer. 2008;113((5)(suppl)):1203–1212. doi: 10.1002/cncr.23739. [DOI] [PubMed] [Google Scholar]
- 24.Sirovich B, Gallagher PM, Wennberg DE, Fisher ES. Discretionary decision making by primary care physicians and the cost of U.S. Health care. Health Aff (Millwood) 2008;27(3):813–823. doi: 10.1377/hlthaff.27.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper MM. The Dartmouth Atlas of Health Care: what is it telling us? Health Syst Rev. 1996;29(3):44–45. [PubMed] [Google Scholar]
- 26.Collins MM, Barry MJ. Controversies in prostate cancer screening. Analogies to the early lung cancer screening debate. JAMA. 1996;276(24):1976–1979. doi: 10.1001/jama.1996.03540240054031. [DOI] [PubMed] [Google Scholar]
- 27.Purvis Cooper C, Merritt TL, Ross LE, John LV, Jorgensen CM. To screen or not to screen, when clinical guidelines disagree: primary care physicians’ use of the PSA test. Prev Med. 2004;38:181–191. doi: 10.1016/j.ypmed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Anthony DL, Herndon MB, Gallagher PM, et al. How much do patients’ preferences contribute to resource use? Health Aff (Millwood) 2009;28(3):864–873. doi: 10.1377/hlthaff.28.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss JD, Schectman JM. Prostate cancer screening practices and beliefs. J Gen Intern Med. 2001;16(12):831–837. doi: 10.1046/j.1525-1497.2001.10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin OJ, Valente S, Hasse LA, Kues JR. Determinants of prostate-specific antigen test use in prostate cancer screening by primary care physicians. Arch Fam Med. 1997;6(5):453–458. doi: 10.1001/archfami.6.5.453. [DOI] [PubMed] [Google Scholar]
- 31.Hicks RJ, Hamm RM, Bemben DA. Prostate cancer screening. What family physicians believe is best. Arch Fam Med. 1995;4(4):317–322. doi: 10.1001/archfami.4.4.317. [DOI] [PubMed] [Google Scholar]
- 32.Tasian GE, Cooperberg MR, Cowan JE, et al. Prostate specific antigen screening for prostate cancer: Knowledge of, attitudes towards, and utilization among primary care physicians. Urol Oncol. Epub 2010 [DOI] [PubMed]
- 33.Hartz A, Lucas J, Cramm T, et al. Physician surveys to assess customary care in medical malpractice cases. J Gen Intern Med. 2002;17(7):546–555. doi: 10.1046/j.1525-1497.2002.10740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menees SB, Patel DA, Dalton V. Colorectal cancer screening practices among obstetrician/gynecologists and nurse practitioners. J Womens Health (Larchmt) 2009;18(8):1233–1238. doi: 10.1089/jwh.2008.1117. [DOI] [PubMed] [Google Scholar]
- 35.Hudson SV, Ohman-Strickland P, Cunningham R, Ferrante JM, Hahn K, Crabtree BF. The effects of teamwork and system support on colorectal cancer screening in primary care practices. Cancer Detect Prev. 2007;31(5):417–423. doi: 10.1016/j.cdp.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerfoot BP, Holmberg EF, Lawler EV, Krupat E, Conlin PR. Practitioner-level determinants of inappropriate prostate-specific antigen screening. Arch Intern Med. 2007;167(13):1367–1372. doi: 10.1001/archinte.167.13.1367. [DOI] [PubMed] [Google Scholar]
- 37.Walter LC, Davidowitz NP, Heineken PA, Covinsky KE. Pitfalls of converting practice guidelines into quality measures: lessons learned from a VA performance measure. JAMA. 2004;291(20):2466–2470. doi: 10.1001/jama.291.20.2466. [DOI] [PubMed] [Google Scholar]
- 38.Casalino LP. The unintended consequences of measuring quality on the quality of medical care. N Engl J Med. 1999;341(15):1147–1150. doi: 10.1056/NEJM199910073411511. [DOI] [PubMed] [Google Scholar]
- 39.Fisher M. Is prostate-specific antigen (PSA) screening indicated for any subgroup of men? J Fam Pract. 2002;51(2):113. [PubMed] [Google Scholar]
- 40.U.S. Preventive Services Task Force. Screening for Prostate Cancer: Draft Recommendation Statement. Available at: www.uspreventiveservicestaskforce.org/draftrec3.htm. Accessed October 16, 2011.
- 41.Hudson SV, Ohman-Strickland P, Ferrante JM, Lu-Yao G, Orzano AJ, Crabtree BF. Prostate-specific antigen testing among the elderly in community-based family medicine practices. J Am Board Fam Med. 2009;22(3):257–265. doi: 10.3122/jabfm.2009.03.080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med. 1996;124(6):577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 43.Volk RJ, Hawley ST, Kneuper S, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33(5):428–434. doi: 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 44.Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing internet and video to facilitate patient education for men considering the prostate specific antigen test. J Gen Intern Med. 2003;18(10):781–787. doi: 10.1046/j.1525-1497.2003.20911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellizzi KM, Breslau ES, Burness A, Waldron W. Still screening after all these years. Prevalence and correlates of cancer screening in older racially diverse adults. Arch Intern Med. Forthcoming 2011. [DOI] [PubMed]