Abstract
OBJECTIVES
A 2007 systematic review compared angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) in patients with hypertension. Direct renin inhibitors (DRIs) have since been introduced, and significant new research has been published. We sought to update and expand the 2007 review.
DATA SOURCES
We searched MEDLINE and EMBASE (through December 2010) and selected other sources for relevant English-language trials.
STUDY ELIGIBILITY CRITERIA, PARTICIPANTS, AND INTERVENTIONS
We included studies that directly compared ACE inhibitors, ARBs, and/or DRIs in at least 20 total adults with essential hypertension; had at least 12 weeks of follow-up; and reported at least one outcome of interest. Ninety-seven (97) studies (36 new since 2007) directly comparing ACE inhibitors versus ARBs and three studies directly comparing DRIs to ACE inhibitor inhibitors or ARBs were included.
STUDY APPRAISAL AND SYNTHESIS METHODS
A standard protocol was used to extract data on study design, interventions, population characteristics, and outcomes; evaluate study quality; and summarize the evidence.
RESULTS
In spite of substantial new evidence, none of the conclusions from the 2007 review changed. The level of evidence remains high for equivalence between ACE inhibitors and ARBs for blood pressure lowering and use as single antihypertensive agents, as well as for superiority of ARBs for short-term adverse events (primarily cough). However, the new evidence was insufficient on long-term cardiovascular outcomes, quality of life, progression of renal disease, medication adherence or persistence, rates of angioedema, and differences in key patient subgroups.
LIMITATIONS
Included studies were limited by follow-up duration, protocol heterogeneity, and infrequent reporting on patient subgroups.
CONCLUSIONS AND IMPLICATIONS OF KEY FINDINGS
Evidence does not support a meaningful difference between ACE inhibitors and ARBs for any outcome except medication side effects. Few, if any, of the questions that were not answered in the 2007 report have been addressed by the 36 new studies. Future research in this area should consider areas of uncertainty and be prioritized accordingly.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1938-8) contains supplementary material, which is available to authorized users.
KEY WORDS: angiotensin converting enzyme inhibitors, angiotensin receptor blockers, direct renin inhibitors, hypertension, systematic review
CLINICAL CASE
A 54-year-old woman with a history of hypertension is seen by her doctor for persistently elevated blood pressure in spite of adherence to hydrochlorothiazide 25 mg daily. She is overweight and has a strong family history of coronary artery disease. To control her blood pressure, she and her doctor discuss adding an angiotensin-converting enzyme (ACE) inhibitor, an angiotensin II receptor blocker (ARB), or a direct renin inhibitor (DRI) to her regimen. She is primarily interested in avoiding the cardiovascular complications of hypertension, but does not want to take medication more than once a day, and she is concerned about side effects and the cost of her medication. What information is available to help guide her decision?
INTRODUCTION
Almost 75 million American adults have hypertension. Advances in antihypertensive therapy have dramatically reduced cardiovascular, cerebrovascular, and renal events.1–3 Among the effective pharmacotherapies are inhibitors of the renin-angiotensin-aldosterone (renin) system. In 2007 the Agency for Healthcare Research and Quality (AHRQ) sponsored a comparative effectiveness review of the two most common renin system inhibitors, ACE inhibitors and ARBs, to answer the following three key questions for adults with essential hypertension: Do ACE inhibitors and ARBs differ in the following: 1) blood pressure control, cardiovascular events, quality of life, and other outcomes; 2) safety, tolerability, persistence with therapy, or treatment adherence; and 3) effects within important subgroups of patients? We reported high-level evidence demonstrating that ACE inhibitors and ARBs had similar effects on blood pressure control, and that ACE inhibitors had higher rates of cough than ARBs; however, data regarding long-term cardiovascular outcomes, quality of life, progression of renal disease, medication adherence or persistence, rates of angioedema, and differences in key patient subgroups were limited.4,5
Since the 2007 review, several original research studies have directly compared ACE inhibitors and ARBs in patients with hypertension, and direct renin inhibitors (DRIs) have been introduced as a new class of medication targeting the renin system. In the present review, we sought to update the 2007 report on the comparative effectiveness of ACE inhibitors and ARBs, expand the review to include DRIs, and determine whether the conclusions of the initial review have changed in light of new evidence.
METHODS
The present manuscript is derived from a new comparative effectiveness review commissioned by AHRQ. In that review, the protocol used for the 2007 report, including the three key questions listed above, was adapted to include DRIs and applied to the direct comparison literature published since the 2007 report. Further details of our methods, results, and conclusions are available in the full AHRQ report.6
Data Sources and Searches
To identify relevant studies, we updated and expanded (to include DRIs) the original search, conducted through May 2006, using search terms for drug interventions, hypertension, and applicable study designs. We searched MEDLINE and EMBASE (the latter not included in the original search) through December 23, 2010; the Cochrane Central Register of Controlled Trials (Issue 2, 2006); a register of systematic reviews underway in the Cochrane Hypertension Review Group (December 1, 2010); and grey literature sources (e.g. regulatory data, clinical trial registries, and conference abstracts) identified by AHRQ’s Effective Health Care Program (Appendix Table A available online).
Study Selection
Title and abstract screening was performed by two independent reviewers. Articles that were included by either reviewer moved forward for full-text screening. Full-text screening was also performed by two independent reviewers; however, the reviewers worked together to reconcile most differences, with any remaining disagreement adjudicated by a third reviewer.
We included all clinical studies directly comparing ACE inhibitors, ARBs, and/or DRIs in at least 20 total adults with essential hypertension, provided they had at least 12 weeks of follow-up and reported at least one outcome of interest. Our inclusion criteria were identical to those in the 2007 report,4,5 with the addition of DRIs as a potential comparator. Fixed-dose combination medications were included if the non-ACE inhibitor/ARB/DRI medication was identical across treatment arms (e.g., studies with enalapril/hydrochlorothiazide compared to losartan/hydrochlorothiazide would be included if the hydrochlorothiazide dose was the same in both treatment arms). Because of the number of direct comparison studies, we did not include indirect comparisons. Studies not conducted solely in patients with hypertension had to report subgroup results for those with hypertension.
Data Extraction and Quality Assessment
Data were extracted using a standardized template (Appendix Table B available online). For each article, one investigator abstracted data, and a second over-read the abstraction for accuracy and completeness. Disagreements were resolved by consensus, or when necessary, by a third reviewer’s adjudication.
To assess the quality of clinical trials and cohort studies, we adapted criteria developed by the U.S. Preventive Services Task Force and the Centre for Reviews and Dissemination7,8 and categorized studies as “good,” “fair,” or “poor” in quality. We assessed the strength of the body of evidence for each key question using the approach recommended in AHRQ’s Methods Guide for Effectiveness and Comparative Effectiveness Reviews.9 This approach is conceptually similar to the GRADE framework10 used in the 2007 report. Table 1 summarizes the results of this grading for both the 2007 report and the current update.
Table 1.
Summary of Evidence on Comparative Long-term Benefits and Harms of ACE Inhibitors, ARBs, and DRIs in Patients with Essential Hypertension
| Key Question | Strength of Evidence, 2007 Report | Strength of Evidence, Updated Report | Conclusions |
|---|---|---|---|
| 1. Key Question 1. For adult patients with essential hypertension, how do ACE inhibitors, ARBs, and direct renin inhibitors differ in the following health outcomes: | High | High (ACE inhibitor vs. ARB); | ACE inhibitors and ARBs appear to have similar long-term effects on blood pressure among individuals with essential hypertension. This conclusion is based on evidence from 77 studies (70 RCTs, 5 nonrandomized controlled clinical trials, 1 retrospective cohort study, and 1 case–control study) in which 26,170 patients receiving an ACE inhibitor or an ARB were followed for periods from 12 weeks to 5 years (median 24 weeks). Blood pressure outcomes were confounded by additional treatments and varying dose escalation protocols |
| a. Blood pressure control? | |||
| Low (DRI vs. ACE inhibitor or ARB) | Evidence concerning the effect of DRIs on blood pressure is very limited and currently based on only three studies. These studies found the DRI to have a greater reduction in blood pressure compared to the ACE inhibitor ramipril (two studies) and no significant difference compared to the ARB losartan (one study) | ||
| b. Mortality and major cardiovascular events? | Moderate | Low (ACE inhibitor vs. ARB)* | Due to low numbers of deaths or major cardiovascular events reported, it was difficult to discern any differential effect of ACE inhibitors versus ARBs versus DRIs with any certainty for these critical outcomes. In 21 studies that reported mortality, MI, or clinical stroke as outcomes among 38,589 subjects, there were 38 deaths and 13 strokes reported. This may reflect low event rates among otherwise healthy patients and relatively few studies with extended followup |
| Insufficient (DRI vs. ACE inhibitor or ARB) | Only three of these 21 studies (including 1 death) evaluated DRIs versus ACE inhibitors or ARBs, and therefore the evidence to discern any differential effects between these drug classes on mortality and major cardiovascular events was insufficient | ||
| c. Quality of life? | Low | Low (ACE inhibitor vs. ARB); | No differences were found between ACE inhibitors and ARBs in measures of general quality of life; this is based on four studies, two of which did not provide quantitative data. |
| Insufficient (DRI vs. ACE inhibitor or ARB) | No study evaluated the comparative effectiveness of DRIs for quality-of-life outcomes | ||
| d. Rate of use of a single antihypertensive medication? | High | High (ACE inhibitor vs. ARB); | There was no statistically evident difference in the rate of treatment success based on use of a single antihypertensive for ARBs compared to ACE inhibitors. The trend toward less frequent addition of a second agent to an ARB was heavily influenced by retrospective cohort studies, where medication discontinuation rates were higher in ACE inhibitor-treated patients, and by RCTs with very loosely defined protocols for medication titration and switching |
| Insufficient (DRI vs. ACE inhibitor or ARB) | There were no relevant studies evaluating DRIs | ||
| e. Risk factor reduction and other intermediate outcomes? | Moderate (lipid levels, markers of carbohydrate metabolism/ diabetes control, progression of renal disease) | Moderate (lipid levels, markers of carbohydrate metabolism/ diabetes control, progression of renal disease) (ACE inhibitor vs. ARB); | There were no consistent differential effects of ACE inhibitors, ARBs, on several potentially important clinical outcomes, including lipid levels and markers of carbohydrate metabolism/diabetes control. There appears to be a small difference in change in renal function between ACE inhibitors and ARBs (favoring ACE inhibitors), but this difference is both small and most likely not clinically meaningful or significant. Relatively few studies assessed these outcomes over the long term |
| Insufficient (DRI vs. ACE inhibitor or ARB) | There were no studies that evaluated these outcomes in DRIs. | ||
| Low (progression to type 2 diabetes and LV mass / function) | Low (progression to type 2 diabetes and LV mass / function: (ACE inhibitor vs. ARB); Insufficient (DRI vs. ACE inhibitor or ARB) | There was no evidence for an impact of ACE inhibitors, ARBs, or DRIs on glucose or A1c, and no included studies evaluated rates of progression to type 2 diabetes mellitus. Although we included 13 studies of LV mass/function, these were dominated by poor-quality studies with small sample sizes, and only one study included evaluation of a DRI | |
| 2. Key Question 2. For adult patients with essential hypertension, how do ACE inhibitors, ARBs, and DRIs differ in safety, adverse events, tolerability, persistence with drug therapy, and treatment adherence? | High (cough, withdrawals due to adverse events) | Cough: High (ACE inhibitor vs. ARB); Insufficient (DRI vs. ACE inhibitor or ARB) | ACE inhibitors have been consistently shown to be associated with higher risk of cough than ARBs (odds ratio 4.74; 95% CI 3.56 to 6.31). For RCTs, this translates to a difference in rates of cough of 7.8%; however, for cohort studies with lower rates of cough, this translates to a difference of 1.2%. There were only two studies comparing DRIs to ACE inhibitors and these gave an estimated odds ratio of 0.333 (95% CI of 0.2241 to 0.4933) |
| Withdrawals due to adverse events: High (ACE inhibitor vs. ARB); Low (DRI vs. ACE inhibitor or ARB) | The withdrawal rate for ACE inhibitors was found to have an estimated odds ratio of 1.77 (95% CI 1.42 to 2.21) compared with ARBs. For RCTs, this translated to an absolute difference in withdrawals of 2.3% (3.1% versus 5.4%). The DRI trials did not find a statistically significant difference (odds ratio 0.886; 95% CI 0.458 to 1.714) when compared with the withdrawal rate associated with ACE inhibitors | ||
| There was no evidence of differences across treatments in rates of other commonly reported specific adverse events. | |||
| Low (angioedema) | Angioedema: Low (ACE inhibitor vs. ARB); Insufficient (DRI vs. ACE inhibitor or ARB) | Although several studies collected data on angioedema, the event rates were very low or zero for all studies; this limited our ability to accurately characterize the frequency of angioedema. In the four studies that did report episodes of angioedema, this adverse event was observed only in patients treated with an ACE inhibitor (five patients from three studies) or a DRI (one patient in one study) | |
| Moderate (persistence/adherence) | Persistence with drug therapy/ treatment adherence: Moderate (ACE inhibitor vs. ARB); Insufficient (DRI vs. ACE inhibitor or ARB) | ACE inhibitors and ARBs have similar rates of treatment adherence based on pill counts; this result may not be applicable outside the clinical trial setting. Rates of continuation with therapy appear to be somewhat better with ARBs than with ACE inhibitors; however, due to variability in definitions, limitations inherent in longitudinal cohort studies, and relatively small sample sizes for ARBs, the precise magnitude of this effect is difficult to quantify. The three included studies evaluating DRIs did not find evidence of differences in treatment adherence compared with ACE inhibitors or ARBs. Persistence was not evaluated in any of the studies including DRIs | |
| 3. Key Question 3. Are there subgroups of patients – based on demographic and other characteristics (i.e., age, race, ethnicity, sex, comorbidities, concurrent use of other medications) – a for whom ACE inhibitors, ARBs, or DRIs are more effective, are associated with fewer adverse events, or are better tolerated? | Very low | Insufficient (ACE inhibitor vs. ARB; DRI vs. ACE inhibitor or ARB) | Evidence does not support conclusions regarding the comparative effectiveness, adverse events, or tolerability of ACE inhibitors, ARBs, and DRIs for any particular patient subgroup |
*The reduction in the quality of evidence represents a difference in interpretation of the evidence that was suggested by reviewers of the full report
Abbreviations: ACE inhibitor(s) = angiotensin-converting enzyme inhibitor(s); ARB(s) = angiotensin II receptor blocker(s)/antagonist(s); CI = confidence interval; DRI (s) = direct renin inhibitor(s); GFR = glomerular filtration rate; LV = left ventricular; MI = myocardial infarction; RCTs = randomized controlled trials
Data Synthesis and Analysis
When evaluating groups of studies reporting the same or similar outcomes for potential data synthesis, we tended to be inclusive of individual studies unless their populations were clearly dissimilar. When calculating summary effect sizes, we stratified by study design, separating randomized controlled trials (RCTs) from observational studies. We used random-effects models to allow for statistical heterogeneity and calculated the Q statistic as a measure of this. Even in the presence of statistical heterogeneity, we performed meta-analysis if studies appeared to be clinically and methodologically similar. We examined the potential for publication bias, but found no statistical evidence for this. 6 We combined dichotomous events using odds ratios (ORs) and continuous measures using differences in means. We used Comprehensive Meta-Analysis Version 2 (Comprehensive Meta-Analysis Version 2, Biostat, Englewood, NJ) for all analyses.
RESULTS
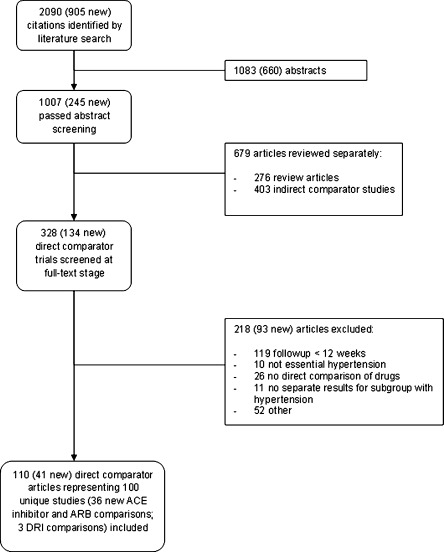
A total of 2090 citations were identified by the literature search (905 new since the 2007 report), of which 100 distinct studies described in 110 articles were included in the updated review (36 new ACE inhibitor versus ARB comparisons; three DRI studies; Fig. 1). The new studies contributed 176,308 additional patients. Table 2 summarizes the total number of studies, number of new studies, study design, quality ratings, and number of participants for each outcome. The specific agents compared are summarized in Appendix Table C (available online).
Figure 1.
Study flow diagram.
Table 2.
Summary of Reviewed Studies
| Outcome | Study Design | Studies, n (New Studies) | Study Quality, n (New Studies) | Participants, n (New Patients)† | ||
|---|---|---|---|---|---|---|
| Good | Fair | Poor | ||||
| Blood pressure control | RCT | 74 (27)* | 15 (10) | 41 (10) | 18 (7) | 22,953 (10,658) |
| Non-RCT | 4 (3) | 0 | 0 | 4 (3) | 222 (160) | |
| Retrospective cohort | 1 (0) | 0 | 1 | 0 | 1087 | |
| Case–control | 1 (0) | 0 | 0 | 1 | 88 | |
| Death and major cardiovascular events | RCT | 17 (8)* | 8 (5) | 6 (1) | 4 (2) | 10,281 (6944) |
| Non-RCT | 1 (1) | 1 (1) | 71 (71) | |||
| Retrospective cohort | 3 (3) | 2 (2) | 1 (1) | 24,129 (24,129) | ||
| Quality of life | RCT | 4 | 0 | 4 | 0 | 1142 |
| Rate of use of a single antihypertensive | RCT | 26 (7)* | 9 (6) | 14 (1) | 3 | 9450 (4599) |
| Retrospective cohort | 2 (0) | 0 | 2 | 0 | 7071 | |
| Case–control | 1 (0) | 0 | 0 | 1 | 88 | |
| Lipid levels | RCT | 18 (7) | 4 (2) | 9 (2) | 5 (3) | 6250 (4637) |
| Non-RCT | 1 (1) | 1 (1) | 36 (36) | |||
| Case–control | 1 (0) | 0 | 0 | 1 | 88 | |
| Progression to type 2 diabetes | – | – | – | – | – | – |
| Markers of carbohydrate metabolism or diabetes control | RCT | 18 (7) | 3(1) | 10 (3) | 5 (3) | 6026 (4412) |
| Non-RCT | 3 (2) | 0 | 1 (1) | 2 (1) | 169 (107) | |
| Retrospective cohort | 1 (1) | 0 | 0 | 1 (1) | 100 | |
| Case–control | 1 (0) | 0 | 0 | 1 | 88 | |
| Measures of left ventricular mass or function | RCT | 11 (5)* | 4 (4) | 3 | 4 (1) | 1253 (687) |
| Non-RCT | 1 (0) | 0 | 0 | 1 | 62 | |
| Case–control | 1 (0) | 0 | 0 | 1 | 88 | |
| Measures of kidney disease (creatinine, glomerular filtration rate, proteinuria) | RCT | 25 (9)* | 6 (4) | 15 (4) | 4 (1) | 8733 (6159) |
| Non-RCT | 2 (1) | 0 | 1 (1) | 1 | 133 (71) | |
| Retrospective cohort | 1 (1) | 1 (1) | 100 (100) | |||
| Cross-sectional cohort | 1 (0) | 0 | 0 | 1 | 49 | |
| Case–control | 1(0) | 0 | 0 | 1 | 88 | |
| Serious adverse events (overall rates) | RCT | 14 (7)* | 6 (5) | 7 (1) | 1 (1) | 10 219 (6390) |
| Adverse events (overall rates) | RCT | 48 (18)* | 13 (9) | 28 (7) | 7 (2) | 19 667 (9185) |
| Cough | RCT | 39 (13)* | 9 (5) | 26 (6) | 4 (2) | 18 445 (8375) |
| Prospective cohort | 2 (0) | 0 | 0 | 2 | 51 859 | |
| Cross-sectional cohort | 1 (0) | 0 | 0 | 1 | 49 | |
| Angioedema | RCT | 4 (1)* | 1 (1) | 3 | 0 | 2051 (842) |
| Withdrawals due to adverse events | RCT | 39 (17)* | 12 (10) | 24 (6) | 3 (1) | 12 736 (5222) |
| Non-RCT | 1 (0) | 0 | 0 | 1 | 62 | |
| Case–control | 1 (0) | 0 | 0 | 1 | 88 | |
| Adherence or persistence | RCT | 26 (18)* | 8 (8) | 15 (8) | 3 (2) | 8880 (5514) |
| Retrospective cohort | 13 (4) | 1 (1) | 11 (3) | 1 | 341 438 (141 332) | |
*Includes studies with a direct renin inhibitor
†Represents number included in ACE inhibitor and ARB treatment groups or, in the case of RCTs, number randomly assigned
Abbreviations: RCT = randomized controlled trial; non-RCT = a controlled trial that was not randomized
Comparisons with Direct Renin Inhibitors
Two good-quality RCTs compared the DRI aliskiren at a maximum dose of 300 mg to the ACE inhibitor ramipril at a maximum dose of 10 mg.11–13 In both studies, aliskiren produced a greater reduction in blood pressure at 12 weeks, with between-group blood pressure (SBP/DBP) differences of −2.7/-1.611,12 and −2.3/-1.5 mmHg.13 These studies also reported safety, adverse events, persistence, and renal function, but did not find any differences in these outcomes. One good-quality RCT compared aliskiren up to 300 mg to the ARB losartan at a maximum dose of 100 mg and did not find any significant between-group difference for any outcome.14 Results from these studies are noted under the relevant specific outcomes below.
Blood Pressure Control
The updated literature search identified an additional 30 studies (three evaluating a DRI) that reported a blood pressure outcome, for a total of 80 studies.11–97 Study durations ranged from 12 weeks to 5 years, with a median of 24 weeks. Only nine studies reported enrollment of at least 10% black patients.11,12,24,47,51,61,63,74,86,90 The funding source was reported in 47 studies (59%),11–15,17–24,26,27,30,32,42–44,47,49,51–55,57–62,66,67,69,70,72–76,78–80,82–84,86–88,92,97 with the majority of these (32 studies) funded by the manufacturer of one of the study medications.
For the overall comparison of blood pressure lowering between ACE inhibitors and ARBs, 58 studies (74%) reported no difference,15,17–31,33,35,37,40,41,44–49,51,52,56–63,66,67,69,70,73,75–93,95,97 two studies (3%) favored ACE inhibitors,64,65,74 11 studies (14%) favored ARBs,16,34,36,43,50,53–55,68,72,94 and 6 studies (8%) did not report a statistical comparison between the two agents.32,38,39,42,71,96 Because of substantial heterogeneity in study protocols, quantitative meta-analysis was not performed for blood pressure lowering.
Successful Blood Pressure Control with a Single Antihypertensive Agent
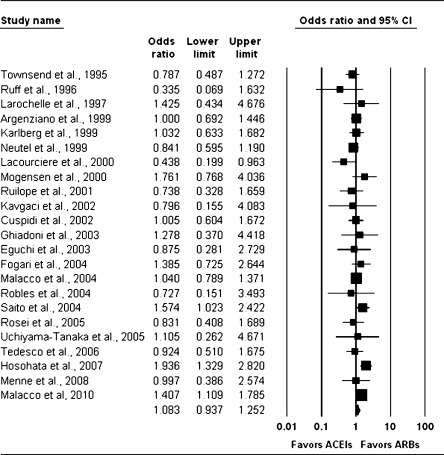
We identified seven new studies in the update (three evaluating a DRI) that reported the outcome of successful blood pressure control with a single antihypertensive agent, for a total of 29 studies.11–14,17,27,31,34,40,42,44–47,49,51–53,58,60,63,71,73–76,89–92,98 Sample sizes ranged from 30 to 13,303 patients. The rates of successful blood pressure control with a single agent ranged from 6% to 93.3% (median 55%).
We performed a meta-analysis of data from the 26 studies directly comparing ACE inhibitors and ARBs. Data from RCTs gave an estimated OR of 1.08 (95% CI 0.94 to 1.25), suggesting that the odds of successful blood pressure control are not statistically significantly different with an ARB alone versus an ACE inhibitor alone (Fig. 2). The relationship between the summary estimated OR, number of randomized patients, and number of comparative studies is shown in Appendix Figure 1 (available online) and demonstrates minimal change in the estimated effect in spite of multiple new studies over the last several years.
Figure 2.
Random-effects meta-analysis of RCTs evaluating successful blood pressure control on monotherapy. Angiotensin-converting enzyme (ACE) inhibitors versus angiotensin II receptor blockers (ARBs); RCT = randomized controlled trial.
Death and Major Cardiovascular Events
An additional 12 studies with 31,144 patients that reported how many patients died or had myocardial infarction or stroke were published since the 2007 review, for a total of 21 studies (three evaluating DRIs).11–14,17–21,23,43,46,47,49,52,58,75,80,84,87,94,95,99–102 Most of these studies excluded high risk patients and reported only one or two clinical events. The study by Barnett et al.23 provided the largest sample and had the longest duration of follow-up. This study evaluated telmisartan versus enalapril in 250 patients with hypertension, type 2 diabetes and early nephropathy over a 5-year treatment period. Cardiovascular events occurred at a similar rate in both treatment groups: there were six strokes in each group; nine nonfatal MIs in the telmisartan group and six in the enalapril group; and nine patients with heart failure in the telmisartan group and six in the enalapril group. This study also reported 12 deaths, six in the telmisartan group and six in the enalapril group.
Large direct comparison trials in high-risk patients, such as the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), did not meet our inclusion criteria. Despite a 10-fold increase in the number of patients since the initial review, only 38 deaths were reported, 22 of which were new since the original report.
Lipid Levels and Glucose Control
Twenty studies (eight new, none involving a DRI) met our inclusion criteria and evaluated serum lipid changes,19,29,33,35,40,43,46,49,62,65,73,80,81,85,89,91,92,95,96 although none addressed the use of lipid-lowering agents during the study period. Of 12 studies reporting direct statistical comparisons,29,33,35,43,62,65,73,81,85,87,92,95 only three found different effects between the medications compared, with results favoring ACE inhibitors in one study and ARBs in the other two.
Twenty-three studies (10 new) measured serum glucose or hemoglobin A1c.19,22,29,33,35,40,43,46,49,60,62,65,77,80,81,85,87,91,92,95,96,99,102 None addressed hypoglycemic therapy during the study. No change occurred in hemoglobin A1c either between or within groups in the 16 studies reporting this outcome.
Left Ventricular Mass or Function
Thirteen studies (five new, including one evaluating a DRI) presented original results on left ventricular mass or function.14,22,25,27,28,41,69,78,79,84,88,89,92 Apart from one study in which there was a greater reduction of left ventricular hypertrophy with an ARB,89 there were no differences between ACE inhibitor and ARB groups. Only one study evaluated a DRI; it found similar effects between aliskiren and an ARB.14
Serum Creatinine Level, Glomerular Filtration Rate, and Proteinuria
Thirty studies (11 new, including two evaluating a DRI) presented original results on either serum creatinine/GFR,13,19,22,28,33,43,59,72,82,87,91,92 proteinuria,11,29,58,60,80,103 or both.23,30,46,49,56,62,65,73,81,97,99,102 The ten new studies that described changes in creatinine or glomerular filtration rate did not consistently demonstrate differential effects with use of ACE inhibitors versus ARBs, nor did the two studies comparing a DRI with the ACE inhibitor ramipril;11,13 these findings were similar to those from the 2007 report. The analysis of all studies gave an estimated standardized mean difference of 0.11 (95% CI −0.05 to 0.27), suggesting that mean posttreatment creatinine levels were non-significantly higher for the ARB studies.
As in the 2007 report, the six new studies that quantitatively described changes in urine albumin or protein excretion also consistently demonstrated no differential effects with use of ACE inhibitors versus ARBs.30,58,62,81,97,102 None evaluated DRIs, all were conducted in non-U.S. sites, and most had between 6 and 12 months follow-up.30,58,62,81
Quality of Life, Adverse Events, Persistence and Adherence
Quality of Life
The updated search identified no new studies that reported quality-of-life outcomes.
Serious and Overall Adverse Events
Fourteen studies (seven new, including two evaluating a DRI) met our inclusion criteria and reported overall rates of serious adverse events.11,14,15,43,45,50,52,53,58,59,72,73,87,94 The nature of serious adverse event reporting was inconsistent, and overall rates were low (0 to 6%); thus, data on these events are insufficient to quantitatively assess differential effects.
One serious adverse event, angioedema, has been reported to occur in ACE inhibitor-treated patients with much greater frequency than in ARB-treated patients.101 However, this outcome was reported in only four studies (one new).11,45,57,63 One of the reported cases occurred in a patient treated with a DRI; the other five cases were in patients treated with an ACE inhibitor.
Specific Adverse Events
Forty-eight studies (18 new, including two evaluating a DRI) reported rates of one or more specific adverse events.11,13–16,19,24,26–30,34,36,37,43,45,47–55,57–59,61–63,65,68,72,74,75,78,81,87,89,90,94,95,97,103–105 Given the large number of commonly reported specific adverse events, we focused on three specific events with the largest difference in absolute rates across studies: dizziness, headache, and cough.
Dizziness
Rates of dizziness in 31 studies reporting this event ranged from 1% to 20% in ARB-treated groups (mean 3.7%), 0% to 18% in ACE inhibitor-treated groups (mean 4.4%), and 3% to 8% in DRI-treated groups (mean 6.0%).
Headache
Rates of headache in the 30 included studies ranged from 1% to 22% in ARB-treated groups (mean 5.8%), 0% to 34% in ACE inhibitor-treated groups (mean 7.0%), and 9% to 11% in DRI-treated groups (mean 10.0%). This compares to mean rates of 6.3% in ARB-treated groups and 7.9% in ACE inhibitor-treated groups reported in the 2007 review.
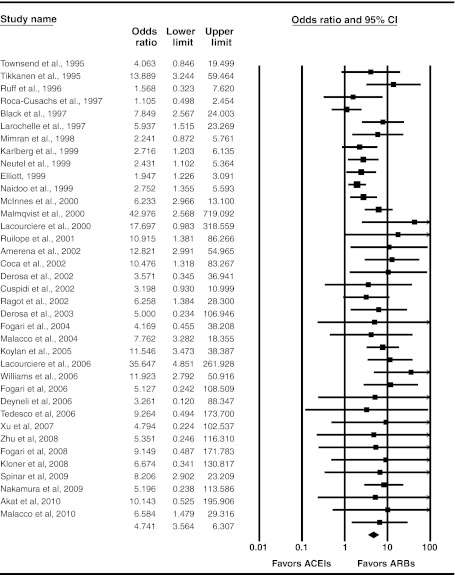
Cough
Forty-two studies (13 new, including two evaluating a DRI) reported cough as an adverse event; of these, 40 studies compared rates of cough in subjects treated with ACE inhibitors or ARBs,15,16,19,24,26–30,34,36,37,45,47–53,55,57,59,61–63,65,68,72,74,75,87,89,90,94,95,97,103–105 and two in subjects treated with an ACE inhibitor or a DRI.11,13 Rates of cough ranged from 0 to 13% for ARB-treated groups (mean 2.2%) and 0 to 23% in ACE inhibitor-treated groups (mean 8.7%), and were 4% in DRI-treated patients. Based on our meta-analysis of RCTs, ACE inhibitors have consistently been shown to be associated with higher risk of cough than ARBs (OR 4.74; 95% CI 3.56 to 6.31) (Fig. 3); the new evidence did not significantly improve precision of the 2007 estimate (Appendix Figure 2 available online).
Figure 3.
Random-effects meta-analysis of RCTs evaluating cough as an adverse event. Angiotensin-converting enzyme (ACE) inhibitors versus angiotensin II receptor blockers (ARBs); RCT = randomized controlled trial.
Withdrawals Due to Adverse Events
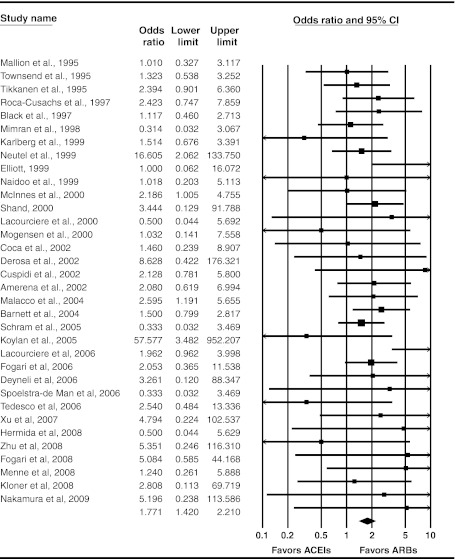
Forty-one studies (17 new, including 3 evaluating a DRI) reported withdrawals due to adverse events.11,13,14,16,19,22–24,26–28,30,36,37,43,45,47–50,52–54,57–63,65,72,78,80,82,88–90,92,95,97 Thirty-six trials reported withdrawals due to adverse events for both ACE inhibitors and ARBs; in 28 of these trials (78%) there were more withdrawals in the ACE inhibitor-treated groups.
Meta-analysis of RCTs reporting this outcome yielded an estimated OR of 1.77 (95% CI 1.42 to 2.21) (Fig. 4); however, absolute rates of withdrawal due to an adverse event were low: 5.4% for ACE inhibitors and ∼3% for ARBs. In spite of increasing evidence, this estimated OR has been remarkably stable over the last decade (Appendix Figure 3 available online).
Figure 4.
Random-effects meta-analysis of RCTs evaluating withdrawals due to adverse events. Angiotensin-converting enzyme (ACE) inhibitors versus angiotensin II receptor blockers (ARBs); RCT = randomized controlled trial.
Two studies (both RCTs) compared a DRI with an ACE inhibitor for this outcome. Combining these studies yielded an estimated OR of 0.89 (95% CI 0.46 to 1.71) for DRI-treated patients relative to ACE inhibitor-treated patients.
Adherence and Persistence
Thirty-nine studies (22 new, including three evaluating a DRI) reported at least some quantitative information on persistence or adherence.11,13,14,16,26,30,36,37,42–44,47,48,50,55,57,58,62,73,76,81,88,89,93–95,97,98,106–118 Adherence to study protocol, in terms of pill counts, was universally high: at least 97% in five of the nine studies assessed and above 90% in all nine, without significant between group differences. Persistence with ARBs was modestly better than with ACE inhibitors, primarily due to adverse effects.
Effects in Subgroups of Patients
There was limited reporting of the comparative effectiveness of ACE inhibitors, ARBs, and DRIs in particular subgroups of patients. Among the 80 studies reporting blood pressure outcomes, four (none new) reported data for women,35,45,54,55 nine studies (three new) reported results in patients ≥65 years old,13,17,34,38,45,53,54,75,90 five studies (one new) reported data in a subgroup of black patients,13,17–21,61,74,90 and 10 studies (two new) reported results for patients with diabetes and hypertension.23,29,32,33,46,47,49,60,73,80 None of the results for these subgroups differed appreciably from the combined results for all patients.
DISCUSSION
Since the 2007 report on the comparative effectiveness of ACE inhibitors and ARBs in the treatment of hypertension, substantially more original research directly comparing these agents has been published (59% more studies). However, data from these new studies have not significantly changed the conclusions or the strength of evidence from the original report. As demonstrated in Appendix Figures 1, 2, and 3 (available online), the number of new studies is increasing, but with little added precision for our estimates of effects on blood pressure lowering, withdrawals due to adverse events, and cough, all of which are known with a high level of certainty. Conversely, the new evidence reported here did not significantly add to our understanding of long-term outcomes, quality of life, renal outcomes, medication adherence, or differences in key patient subgroups. New evidence on the comparative effectiveness of DRIs versus either ACE inhibitors or ARBs was limited to three studies with 2049 patients and did not allow definitive conclusions for any of our included outcomes. More long-term studies directly comparing DRIs with ACE inhibitors and ARBs are needed before this new medication class can be recommended.
The lack of clinically useful new information, in spite of many more new publications, is likely explained by several factors. There could be a mismatch between the areas of greatest research value and the actual research being funded. Formal analysis of the value of new research Information may be useful for guiding decisions about the use of future research funds.119 Even if value-of-information methods were applied to this area, the incentives for comparative effectiveness research investment differ between public funders and private, for-profit corporations. Therefore, the highest value research may not be the most likely to be funded.
The primary outcomes of many of the new studies were biochemical, and the clinical information abstracted from their reports was sparse. Even among our clinically measured outcomes, many are only a proxy for the clinically meaningful outcomes. The importance of directly comparing these medications’ effects on clinical outcomes is particularly important considering the mixed results of other placebo-controlled and direct comparison studies of ACE inhibitors and ARBs for these outcomes.120–122
Our review has some limitations. While our results are restricted to patients with essential hypertension, the agents studied here have been compared in large studies and in systematic reviews for related conditions such as congestive heart failure, ischemic heart disease, and chronic kidney disease.120,123,124 These systematic reviews, like ours, have limited inclusion to studies conducted in patients with the target condition; however, these reviews have examined an overlapping set of efficacy and safety outcomes. As a result, important direct comparison trials are often excluded from reviews because they do not meet the target condition inclusion criteria. Such was the case with ONTARGET, which was excluded from this review because no results were reported exclusively for patients with hypertension. It is likely that combining studies reporting identical outcomes, but in different target populations, may yield important new information, particularly for rarer events.125
The ability to evaluate heterogeneity in treatment response is limited by infrequent reporting of subgroup results. This limitation could be overcome if individual patient-level data were available for meta-analyses. This analysis would be logistically challenging given the proprietary nature of the data and a lack of uniform data quality standards; however, there are successful examples demonstrating such broad collaboration.126
There continue to be significant efforts to differentiate the comparative benefits and harms of the vast array of available pharmacotherapies for reducing vascular risk. Many of the differences between medication classes are likely small but nevertheless important when applied to the large population of patients. Detecting these differences may depend on the ability to maximize the information available across completed research studies and prioritization of future research around remaining areas of uncertainty. Coordinating these efforts across the broad spectrum of stakeholders would require a significant commitment from private and public sponsors of research, but one that may better serve a public interest in research efficiency.
RESOLUTION OF CLINICAL CASE
Based on the available evidence you first recommend against a DRI because there is not enough evidence to support its use. You advise the patient that an ACE inhibitor or an ARB would likely lower her blood pressure to a similar degree. You cannot be sure which one will provide greater risk reduction for a heart attack, stroke, kidney failure, or development of diabetes; however, when these have been directly compared in research studies, there was no significant difference. You advise her that approximately 9% of people who start taking an ACE inhibitor develop a dry cough which goes away only with stopping the medication, compared to only 2% of those who start an ARB. There is also a 1 in 1000 risk for developing angioedema from an ACE inhibitor, and while the risk is not known for sure for ARBs, it is likely much lower (approximately 1 in 10,000). You also point out the significantly lower cost of ACE inhibitors; however this cost difference will likely decrease in the future with generic ARBs such as losartan. Based on the proven efficacy, cost difference, and acceptably low risk for side effects, she decides to try the ACE inhibitor lisinopril first, with a plan to switch to an ARB if she notices any side effects from this medication.
Electronic supplementary material
(DOC 303 kb)
Acknowledgements
Disclaimer
This project was funded under Contract No. Contract No. 290-02-0025 from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsements by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Funders
This project was funded under Contract No. Contract No. 290-02-0025 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services. AHRQ commented on the planned methods and draft report, but did not participate in the search of the literature, data abstraction, or evaluation of individual studies. The first author is funded by a HSR&D career development award from the Department of Veterans Affairs.
Prior presentations
This work was presented at the Academy Health Annual Research Meeting in Seattle, WA on June 14th, 2011.
Conflict of Interest
None disclosed.
References
- 1.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335(8693):827–838. doi: 10.1016/0140-6736(90)90944-Z. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies.[Erratum appears in Lancet. 2003 Mar 22;361(9362):1060] Lancet. 2002;360(9349):1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366(9502):2026–2033. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- 4.Matchar DB, McCrory DC, Orlando LA, et al. Comparative Effectiveneness of Angiotensin-Converting Enzyme Inhibitors (ACEIs) and Angiotensin II Receptor Antagonists (ARBs) for Treating Essential Hypertension. Comparative Effectiveness Review No. 10. (Prepared by Duke Evidence-based Practice Center under Contract No. 290-02-0025.) Rockville, MD: Agency for Healthcare Research and Quality. November 2007. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed October 31, 2010. [PubMed]
- 5.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148(1):16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 6.Sanders GD, Coeytaux R, Dolor RJ, et al. Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin II Receptor Antagonists (ARBs), and Direct Renin Inhibitors for Treating Essential Hypertension: An Update. Comparative Effectiveness Review No. 34. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025.) AHRQ Publication No. 11-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality. June 2011. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed October 31, 2011. [PubMed]
- 7.Anonymous. Undertaking systematic reviews of research on effectiveness: CRD's guidance for those carrying out or commissioning reviews. York, UK: NHS Centre for Reviews and Dissemination. 2001 Mar. Report No.: CRD Report No. 4 (2nd edition).
- 8.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21–35. doi: 10.1016/S0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 9.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions–Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol. 2010;63(5):513–523. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen K, Weinberger MH, Egan B, et al. Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: a 6-month, randomized, double-blind trial. J Hypertens. 2008;26(3):589–599. doi: 10.1097/HJH.0b013e3282f3ad9a. [DOI] [PubMed] [Google Scholar]
- 12.Andersen K, Weinberger MH, Constance CM, et al. Comparative effects of aliskiren-based and ramipril-based therapy on the renin system during long-term (6 months) treatment and withdrawal in patients with hypertension. JRAAS - J Renin-Angiotensin-Aldosterone Syst. 2009;10(3):157–167. doi: 10.1177/1470320309342407. [DOI] [PubMed] [Google Scholar]
- 13.Duprez DA, Munger MA, Botha J, et al. Aliskiren for geriatric lowering of systolic hypertension: A randomized controlled trial. J Hum Hypertens. 2010;24(9):600–608. doi: 10.1038/jhh.2009.107. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SD, Appelbaum E, Manning WJ, et al. Effect of the direct Renin inhibitor aliskiren, the Angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119(4):530–537. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- 15.Akat PB, Bapat TR, Murthy MB, et al. Comparison of the efficacy and tolerability of telmisartan and enalapril in patients of mild to moderate essential hypertension. Indian J Pharmacol. 2010;42(3):153–156. doi: 10.4103/0253-7613.66838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amerena J, Pappas S, Ouellet JP, et al. ABPM comparison of the anti-hypertensive profiles of telmisartan and enalapril in patients with mild-to-moderate essential hypertension. J Int Med Res. 2002;30(6):543–552. doi: 10.1177/147323000203000601. [DOI] [PubMed] [Google Scholar]
- 17.Argenziano L, Trimarco B. Effect of eprosartan and enalapril in the treatment of elderly hypertensive patients: subgroup analysis of a 26-week, double-blind, multicentre study. Eprosartan Multinational Study Group. Curr Med Res Opin. 1999;15(1):9–14. doi: 10.1185/03007999909115168. [DOI] [PubMed] [Google Scholar]
- 18.Breeze E, Rake EC, Donoghue MD, et al. Comparison of quality of life and cough on eprosartan and enalapril in people with moderate hypertension. J Hum Hypertens. 2001;15(12):857–862. doi: 10.1038/sj.jhh.1001282. [DOI] [PubMed] [Google Scholar]
- 19.Elliott WJ. Double-blind comparison of eprosartan and enalapril on cough and blood pressure in unselected hypertensive patients. Eprosartan Study Group. J Hum Hypertens. 1999;13(6):413–417. doi: 10.1038/sj.jhh.1000816. [DOI] [PubMed] [Google Scholar]
- 20.Gavras I, Gavras H. Effects of eprosartan versus enalapril in hypertensive patients on the renin-angiotensin-aldosterone system and safety parameters: results from a 26-week, double-blind, multicentre study. Eprosartan Multinational Study Group. Curr Med Res Opin. 1999;15(1):15–24. doi: 10.1185/03007999909115169. [DOI] [PubMed] [Google Scholar]
- 21.Levine B. Effect of eprosartan and enalapril in the treatment of black hypertensive patients: subgroup analysis of a 26-week, double-blind, multicentre study. Eprosartan Multinational Study Group. Curr Med Res Opin. 1999;15(1):25–32. doi: 10.1185/03007999909115170. [DOI] [PubMed] [Google Scholar]
- 22.Avanza ACJ, El Aouar LM, Mill JG. Reduction in left ventricular hypertrophy in hypertensive patients treated with enalapril, losartan or the combination of enalapril and losartan. Arq Bras Cardiol. 2000;74(2):103–117. [PubMed] [Google Scholar]
- 23.Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy.[erratum appears in N Engl J Med. 2005 Apr 21;352(16)1731] N Engl J Med. 2004;351(19):1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 24.Black HR, Graff A, Shute D, et al. Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: efficacy, tolerability and safety compared to an angiotensin-converting enzyme inhibitor, lisinopril. J Hum Hypertens. 1997;11(8):483–489. doi: 10.1038/sj.jhh.1000482. [DOI] [PubMed] [Google Scholar]
- 25.Celik T, Iyisoy A, Kursaklioglu H, et al. The comparative effects of telmisartan and ramipril on P-wave dispersion in hypertensive patients: a randomized clinical study. Clin Cardiol. 2005;28(6):298–302. doi: 10.1002/clc.4960280609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coca A, Calvo C, Garcia-Puig J, et al. A multicenter, randomized, double-blind comparison of the efficacy and safety of irbesartan and enalapril in adults with mild to moderate essential hypertension, as assessed by ambulatory blood pressure monitoring: the MAPAVEL Study (Monitorizacion Ambulatoria Presion Arterial APROVEL) Clin Ther. 2002;24(1):126–138. doi: 10.1016/S0149-2918(02)85010-X. [DOI] [PubMed] [Google Scholar]
- 27.Cuspidi C, Muiesan ML, Valagussa L, et al. Comparative effects of candesartan and enalapril on left ventricular hypertrophy in patients with essential hypertension: the candesartan assessment in the treatment of cardiac hypertrophy (CATCH) study. J Hypertens. 2002;20(11):2293–2300. doi: 10.1097/00004872-200211000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Rosa ML, Cardace P, Rossi M, et al. Comparative effects of chronic ACE inhibition and AT1 receptor blocked losartan on cardiac hypertrophy and renal function in hypertensive patients. J Hum Hypertens. 2002;16(2):133–140. doi: 10.1038/sj.jhh.1001305. [DOI] [PubMed] [Google Scholar]
- 29.Derosa G, Cicero AF, Ciccarelli L, et al. A randomized, double-blind, controlled, parallel-group comparison of perindopril and candesartan in hypertensive patients with type 2 diabetes mellitus. Clin Ther. 2003;25(7):2006–2021. doi: 10.1016/S0149-2918(03)80201-1. [DOI] [PubMed] [Google Scholar]
- 30.Deyneli O, Yavuz D, Velioglu A, et al. Effects of ACE inhibition and angiotension II receptor blockade on glomerular basement membrane protein excretion and change selectivity in type 2 diabetic patients. JRAAS - J Renin-Angiotensin-Aldosterone Syst. 2006;7(2):98–103. doi: 10.3317/jraas.2006.016. [DOI] [PubMed] [Google Scholar]
- 31.Eguchi K, Kario K, Shimada K. Comparison of candesartan with lisinopril on ambulatory blood pressure and morning surge in patients with systemic hypertension. Am J Cardiol. 2003;92(5):621–624. doi: 10.1016/S0002-9149(03)00739-2. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Campo L, Grande MT, Diego J, et al. Effect of different antihypertensive treatments on Ras, MAPK and Akt activation in hypertension and diabetes. Clin Sci. 2009;116(2):165–173. doi: 10.1042/CS20080119. [DOI] [PubMed] [Google Scholar]
- 33.Fogari R, Mugellini A, Zoppi A, et al. Losartan and perindopril effects on plasma plasminogen activator inhibitor-1 and fibrinogen in hypertensive type 2 diabetic patients. Am J Hypertens. 2002;15(4 Pt 1):316–320. doi: 10.1016/S0895-7061(01)02340-8. [DOI] [PubMed] [Google Scholar]
- 34.Fogari R, Mugellini A, Zoppi A, et al. Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension. Eur J Clin Pharmacol. 2004;59(12):863–868. doi: 10.1007/s00228-003-0717-9. [DOI] [PubMed] [Google Scholar]
- 35.Fogari R, Zoppi A, Preti P, et al. Differential effects of ACE-inhibition and angiotensin II antagonism on fibrinolysis and insulin sensitivity in hypertensive postmenopausal women. Am J Hypertens. 2001;14(9 Pt 1):921–926. doi: 10.1016/S0895-7061(01)02140-9. [DOI] [PubMed] [Google Scholar]
- 36.Fogari R, Mugellini A, Zoppi A, et al. Effect of telmisartan/hydrochlorothiazide vs lisinopril/hydrochlorothiazide combination on ambulatory blood pressure and cognitive function in elderly hypertensive patients. J Hum Hypertens. 2006;20(3):177–185. doi: 10.1038/sj.jhh.1001964. [DOI] [PubMed] [Google Scholar]
- 37.Fogari R, Derosa G, Ferrari I, et al. Effect of valsartan and ramipril on atrial fibrillation recurrence and P-wave dispersion in hypertensive patients with recurrent symptomatic lone atrial fibrillation. Am J Hypertens. 2008;21(9):1034–1039. doi: 10.1038/ajh.2008.217. [DOI] [PubMed] [Google Scholar]
- 38.Formosa V, Bellomo A, Iori A, et al. The treatment of hypertension with telmisartan in the sphere of circadian rhythm in metabolic syndrome in the elderly. Arch Gerontol Geriatr. 2009;49(Suppl 1):95–101. doi: 10.1016/j.archger.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Franke H. Antihypertensive effects of candesartan cilexetil, enalapril and placebo. J Hum Hypertens. 1997;11(Suppl 2):S61–S62. [PubMed] [Google Scholar]
- 40.Ghiadoni L, Magagna A, Versari D, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41(6):1281–1286. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 41.Guntekin U, Gunes Y, Tuncer M, et al. Comparison of the effects of quinapril and irbesartan on P-wave dispersion in hypertensive patients. Adv Ther. 2008;25(8):775–786. doi: 10.1007/s12325-008-0083-1. [DOI] [PubMed] [Google Scholar]
- 42.Hasford J, Mimran A, Simons WR. A population-based European cohort study of persistence in newly diagnosed hypertensive patients. J Hum Hypertens. 2002;16(8):569–575. doi: 10.1038/sj.jhh.1001451. [DOI] [PubMed] [Google Scholar]
- 43.Hermida RC, Ayala DE, Khder Y, et al. Ambulatory blood pressure-lowering effects of valsartan and enalapril after a missed dose in previously untreated patients with hypertension: a prospective, randomized, open-label, blinded end-point trial. Clin Ther. 2008;30(1):108–120. doi: 10.1016/j.clinthera.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Hosohata K, Saito S, Asayama K, et al. Progress report on The Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP) study: status at February 2004. Clin Exp Hypertens (New York) 2007;29(1):69–81. doi: 10.1080/10641960601096976. [DOI] [PubMed] [Google Scholar]
- 45.Karlberg BE, Lins LE, Hermansson K. Efficacy and safety of telmisartan, a selective AT1 receptor antagonist, compared with enalapril in elderly patients with primary hypertension. TEES Study Group. J Hypertens. 1999;17(2):293–302. doi: 10.1097/00004872-199917020-00015. [DOI] [PubMed] [Google Scholar]
- 46.Kavgaci H, Sahin A, Ersoz H, et al. The effects of losartan and fosinopril in hypertensive type 2 diabetic patients. Diabetes Res Clin Pract. 2002;58(1):19–25. doi: 10.1016/S0168-8227(02)00102-X. [DOI] [PubMed] [Google Scholar]
- 47.Kloner RA, Neutel J, Roth EM, et al. Blood pressure control with amlodipine add-on therapy in patients with hypertension and diabetes: results of the Amlodipine Diabetic Hypertension Efficacy Response Evaluation Trial. Ann Pharmacother. 2008;42(11):1552–1562. doi: 10.1345/aph.1L076. [DOI] [PubMed] [Google Scholar]
- 48.Koylan N, Acarturk E, Canberk A, et al. Effect of irbesartan monotherapy compared with ACE inhibitors and calcium-channel blockers on patient compliance in essential hypertension patients: a multicenter, open-labeled, three-armed study. Blood Press Suppl. 2005;1:23–31. doi: 10.1080/08038020510040649. [DOI] [PubMed] [Google Scholar]
- 49.Lacourciere Y, Belanger A, Godin C, et al. Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int. 2000;58(2):762–769. doi: 10.1046/j.1523-1755.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- 50.Lacourciere Y, Neutel JM, Davidai G, et al. A multicenter, 14-week study of telmisartan and ramipril in patients with mild-to-moderate hypertension using ambulatory blood pressure monitoring. Am J Hypertens. 2006;19(1):104–112. doi: 10.1016/j.amjhyper.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Larochelle P, Flack JM, Marbury TC, et al. Effects and tolerability of irbesartan versus enalapril in patients with severe hypertension. Irbesartan Multicenter Investigators. Am J Cardiol. 1997;80(12):1613–1615. doi: 10.1016/S0002-9149(97)00784-4. [DOI] [PubMed] [Google Scholar]
- 52.Malacco E, Santonastaso M, Vari NA, et al. Comparison of valsartan 160 mg with lisinopril 20 mg, given as monotherapy or in combination with a diuretic, for the treatment of hypertension: the Blood Pressure Reduction and Tolerability of Valsartan in Comparison with Lisinopril (PREVAIL) study.[erratum appears in Clin Ther. 2004 Jul;26(7):1185] Clin Ther. 2004;26(6):855–865. doi: 10.1016/S0149-2918(04)90129-4. [DOI] [PubMed] [Google Scholar]
- 53.Malacco E, Omboni S, Volpe M, et al. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: The ESPORT study. J Hypertens. 2010;28(11):2342–2350. doi: 10.1097/HJH.0b013e32833e116b. [DOI] [PubMed] [Google Scholar]
- 54.Mallion JM, Bradstreet DC, Makris L, et al. Antihypertensive efficacy and tolerability of once daily losartan potassium compared with captopril in patients with mild to moderate essential hypertension. J Hypertens, Suppl. 1995;13(1):S35–S41. doi: 10.1097/00004872-199507001-00005. [DOI] [PubMed] [Google Scholar]
- 55.Malmqvist K, Kahan T, Dahl M. Angiotensin II type 1 (AT1) receptor blockade in hypertensive women: benefits of candesartan cilexetil versus enalapril or hydrochlorothiazide. Am J Hypertens. 2000;13(5 Pt 1):504–511. doi: 10.1016/S0895-7061(99)00264-2. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda H, Hayashi K, Saruta T. Distinct time courses of renal protective action of angiotensin receptor antagonists and ACE inhibitors in chronic renal disease. J Hum Hypertens. 2003;17(4):271–276. doi: 10.1038/sj.jhh.1001543. [DOI] [PubMed] [Google Scholar]
- 57.McInnes GT, O'Kane KP, Istad H, et al. Comparison of the AT1-receptor blocker, candesartan cilexetil, and the ACE inhibitor, lisinopril, in fixed combination with low dose hydrochlorothiazide in hypertensive patients. J Hum Hypertens. 2000;14(4):263–269. doi: 10.1038/sj.jhh.1000997. [DOI] [PubMed] [Google Scholar]
- 58.Menne J, Farsang C, Deak L, et al. Valsartan in combination with lisinopril versus the respective high dose monotherapies in hypertensive patients with microalbuminuria: the VALERIA trial. J Hypertens. 2008;26(9):1860–1867. doi: 10.1097/HJH.0b013e32830508aa. [DOI] [PubMed] [Google Scholar]
- 59.Mimran A, Ruilope L, Kerwin L, et al. A randomised, double-blind comparison of the angiotensin II receptor antagonist, irbesartan, with the full dose range of enalapril for the treatment of mild-to-moderate hypertension. J Hum Hypertens. 1998;12(3):203–208. doi: 10.1038/sj.jhh.1000591. [DOI] [PubMed] [Google Scholar]
- 60.Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321(7274):1440–1444. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naidoo DP, Sareli P, Marin F, et al. Increased efficacy and tolerability with losartan plus hydrochlorothiazide in patients with uncontrolled hypertension and therapy-related symptoms receiving two monotherapies. Adv Ther. 1999;16(5):187–199. [PubMed] [Google Scholar]
- 62.Nakamura T, Kawachi K, Saito Y, et al. Effects of ARB or ACE-inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Hear J. 2009;50(4):501–512. doi: 10.1536/ihj.50.501. [DOI] [PubMed] [Google Scholar]
- 63.Neutel JM, Frishman WH, Oparil S, et al. Comparison of telmisartan with lisinopril in patients with mild-to-moderate hypertension. Am J Ther. 1999;6(3):161–166. doi: 10.1097/00045391-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen S, Dollerup J, Nielsen B, et al. Losartan reduces albuminuria in patients with essential hypertension. An enalapril controlled 3 months study. Nephrol Dial Transplant. 1997;12(Suppl 2):19–23. [PubMed] [Google Scholar]
- 65.Tikkanen I, Omvik P, Jensen HA. Comparison of the angiotensin II antagonist losartan with the angiotensin converting enzyme inhibitor enalapril in patients with essential hypertension. J Hypertens. 1995;13(11):1343–1351. doi: 10.1097/00004872-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 66.Onal IK, Altun B, Onal ED, et al. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. 2009;20(4):369–372. doi: 10.1016/j.ejim.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Rabbia F, Silke B, Carra R, et al. Heart rate variability and baroreflex sensitivity during fosinopril, irbesartan and atenolol therapy in hypertension. Clin Drug Investig. 2004;24(11):651–659. doi: 10.2165/00044011-200424110-00004. [DOI] [PubMed] [Google Scholar]
- 68.Ragot S, Ezzaher A, Meunier A, et al. Comparison of trough effect of telmisartan vs perindopril using self blood pressure measurement: EVERESTE study. J Hum Hypertens. 2002;16(12):865–873. doi: 10.1038/sj.jhh.1001494. [DOI] [PubMed] [Google Scholar]
- 69.Rajzer M, Klocek M, Kawecka-Jaszcz K. Effect of amlodipine, quinapril, and losartan on pulse wave velocity and plasma collagen markers in patients with mild-to-moderate arterial hypertension. Am J Hypertens. 2003;16(6):439–444. doi: 10.1016/S0895-7061(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 70.Rehman A, Ismail SB, Naing L, et al. Reduction in arterial stiffness with angiotensin II antagonism and converting enzyme inhibition. A comparative study among Malayhypertensive subjects with a known genetic profile. Am J Hypertens. 2007;20(2):184–189. doi: 10.1016/j.amjhyper.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 71.Robles NR, Angulo E, Grois J, et al. Comparative effects of fosinopril and irbesartan on hematopoiesis in essential hypertensives. Ren Fail. 2004;26(4):399–404. doi: 10.1081/JDI-120039824. [DOI] [PubMed] [Google Scholar]
- 72.Roca-Cusachs A, Oigman W, Lepe L, et al. A randomized, double-blind comparison of the antihypertensive efficacy and safety of once-daily losartan compared to twice-daily captopril in mild to moderate essential hypertension. Acta Cardiol. 1997;52(6):495–506. [PubMed] [Google Scholar]
- 73.Rosei EA, Rizzoni D, Muiesan ML, et al. Effects of candesartan cilexetil and enalapril on inflammatory markers of atherosclerosis in hypertensive patients with non-insulin-dependent diabetes mellitus. J Hypertens. 2005;23(2):435–444. doi: 10.1097/00004872-200502000-00027. [DOI] [PubMed] [Google Scholar]
- 74.Ruff D, Gazdick LP, Berman R, et al. Comparative effects of combination drug therapy regimens commencing with either losartan potassium, an angiotensin II receptor antagonist, or enalapril maleate for the treatment of severe hypertension. J Hypertens. 1996;14(2):263–270. doi: 10.1097/00004872-199602000-00017. [DOI] [PubMed] [Google Scholar]
- 75.Ruilope L, Jager B, Prichard B. Eprosartan versus enalapril in elderly patients with hypertension: a double-blind, randomized trial. Blood Press. 2001;10(4):223–229. doi: 10.1080/08037050152669747. [DOI] [PubMed] [Google Scholar]
- 76.Saito S, Asayama K, Ohkubo T, et al. The second progress report on the Hypertension Objective treatment based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP) study. Blood Press Monit. 2004;9(5):243–247. doi: 10.1097/00126097-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez RA, Masnatta LD, Pesiney C, et al. Telmisartan improves insulin resistance in high renin nonmodulating salt-sensitive hypertensives. J Hypertens. 2008;26(12):2393–2398. doi: 10.1097/HJH.0b013e328312677e. [DOI] [PubMed] [Google Scholar]
- 78.Scaglione R, Argano C, Chiara T, et al. Effect of dual blockade of renin-angiotensin system on TGFbeta1 and left ventricular structure and function in hypertensive patients. J Hum Hypertens. 2007;21(4):307–315. doi: 10.1038/sj.jhh.1002161. [DOI] [PubMed] [Google Scholar]
- 79.Schieffer B, Bunte C, Witte J, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44(2):362–368. doi: 10.1016/j.jacc.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 80.Schram MT, Ittersum FJ, Spoelstra-de Man A, et al. Aggressive antihypertensive therapy based on hydrochlorothiazide, candesartan or lisinopril as initial choice in hypertensive type II diabetic individuals: effects on albumin excretion, endothelial function and inflammation in a double-blind, randomized clinical trial. J Hum Hypertens. 2005;19(6):429–437. doi: 10.1038/sj.jhh.1001812. [DOI] [PubMed] [Google Scholar]
- 81.Sengul AM, Altuntas Y, Kurklu A, et al. Beneficial effect of lisinopril plus telmisartan in patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Res Clin Pract. 2006;71(2):210–219. doi: 10.1016/j.diabres.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Shand BI. Haemorheological effects of losartan and enalapril in patients with renal parenchymal disease and hypertension. J Hum Hypertens. 2000;14(5):305–309. doi: 10.1038/sj.jhh.1001011. [DOI] [PubMed] [Google Scholar]
- 83.Shand BI, Lynn KL. A comparative study of losartan and enalapril on erythropoiesis and renal function in hypertensive patients with renal parenchymal disease. Clin Nephrol. 2000;54(5):427–428. [PubMed] [Google Scholar]
- 84.Shibasaki Y, Masaki H, Nishiue T, et al. Angiotensin II type 1 receptor antagonist, losartan, causes regression of left ventricular hypertrophy in end-stage renal disease. Nephron. 2002;90(3):256–261. doi: 10.1159/000049060. [DOI] [PubMed] [Google Scholar]
- 85.Sonoda M, Aoyagi T, Takenaka K, et al. A one-year study of the antiatherosclerotic effect of the angiotensin-II receptor blocker losartan in hypertensive patients. A comparison with angiotension-converting enzyme inhibitors. Int Hear J. 2008;49(1):95–103. doi: 10.1536/ihj.49.95. [DOI] [PubMed] [Google Scholar]
- 86.Souza-Barbosa LA, Ferreira-Melo SE, Ubaid-Girioli S, et al. Endothelial vascular function in hypertensive patients after renin-angiotensin system blockade. J Clin Hypertens. 2006;8(11):803–809. doi: 10.1111/j.1524-6175.2006.05663.x. [DOI] [PubMed] [Google Scholar]
- 87.Spinar J, Vitovec J, Soucek M, et al. CORD: COmparsion of Recommended Doses of ACE inhibitors and angiotensin II receptor blockers. Vnitrni Lekarstvi. 2009;55(5):481–488. [PubMed] [Google Scholar]
- 88.Spoelstra-de Man AM, Ittersum FJ, Schram MT, et al. Aggressive antihypertensive strategies based on hydrochlorothiazide, candesartan or lisinopril decrease left ventricular mass and improve arterial compliance in patients with type II diabetes mellitus and hypertension. J Hum Hypertens. 2006;20(8):599–611. doi: 10.1038/sj.jhh.1002025. [DOI] [PubMed] [Google Scholar]
- 89.Tedesco MA, Natale F, Calabro R. Effects of monotherapy and combination therapy on blood pressure control and target organ damage: a randomized prospective intervention study in a large population of hypertensive patients. J Clin Hypertens. 2006;8(9):634–641. doi: 10.1111/j.1524-6175.2006.05504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Townsend R, Haggert B, Liss C, et al. Efficacy and tolerability of losartan versus enalapril alone or in combination with hydrochlorothiazide in patients with essential hypertension. Clin Ther. 1995;17(5):911–923. doi: 10.1016/0149-2918(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 91.Uchiyama-Tanaka Y, Mori Y, Kishimoto N, et al. Comparison of the effects of quinapril and losartan on carotid artery intima-media thickness in patients with mild-to-moderate arterial hypertension. Kidney & Blood Press Res. 2005;28(2):111–116. doi: 10.1159/000084254. [DOI] [PubMed] [Google Scholar]
- 92.Verdecchia P, Schillaci G, Reboldi GP, et al. Long-term effects of losartan and enalapril, alone or with a diuretic, on ambulatory blood pressure and cardiac performance in hypertension: a case-control study. Blood Press Monit. 2000;5(3):187–193. doi: 10.1097/00126097-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 93.Veronesi M, Cicero AF, Prandin MG, et al. A prospective evaluation of persistence on antihypertensive treatment with different antihypertensive drugs in clinical practice. Vasc Health & Risk Manag. 2007;3(6):999–1005. [PMC free article] [PubMed] [Google Scholar]
- 94.Williams B, Gosse P, Lowe L, et al. The prospective, randomized investigation of the safety and efficacy of telmisartan versus ramipril using ambulatory blood pressure monitoring (PRISMA I) J Hypertens. 2006;24(1):193–200. doi: 10.1097/01.hjh.0000194364.11516.ab. [DOI] [PubMed] [Google Scholar]
- 95.Xu D, Liu J, Ji C, et al. Effects of telmisartan on hypertensive patients with dyslipidemia and insulin resistance. J of Geriatr Cardiol. 2007;4(3):149–152. [Google Scholar]
- 96.Yilmaz MI, Sonmez A, Caglar K, et al. Effect of antihypertensive agents on plasma adiponectin levels in hypertensive patients with metabolic syndrome. Nephrology. 2007;12(2):147–153. doi: 10.1111/j.1440-1797.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhu S, Liu Y, Wang L, et al. Transforming growth factor-(beta)(1) is associated with kidney damage in patients with essential hypertension: Renoprotective effect of ACE inhibitor and/or angiotensin II receptor blocker. Nephrol Dial Transplant. 2008;23(9):2841–2846. doi: 10.1093/ndt/gfn159. [DOI] [PubMed] [Google Scholar]
- 98.Mazzaglia G, Mantovani LG, Sturkenboom MC, et al. Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens. 2005;23(11):2093–2100. doi: 10.1097/01.hjh.0000186832.41125.8a. [DOI] [PubMed] [Google Scholar]
- 99.Cotter J, Oliveira P, Cunha P, et al. Different patterns of one-year evolution of microalbuminuria in hypertensive patients treated with different inhibitors of the renin-angiotensin system. Rev Port Cardiol. 2008;27(11):1395–1404. [PubMed] [Google Scholar]
- 100.Delea TE, Taneja C, Moynahan A, et al. Valsartan versus lisinopril or extended-release metoprolol in preventing cardiovascular and renal events in patients with hypertension. Am J Health Syst Pharm. 2007;64(11):1187–1196. doi: 10.2146/ajhp060380. [DOI] [PubMed] [Google Scholar]
- 101.Malde B, Regalado J, Greenberger PA. Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ann Allergy Asthma Immunol. 2007;98(1):57–63. doi: 10.1016/S1081-1206(10)60860-5. [DOI] [PubMed] [Google Scholar]
- 102.Ozturk S, Sar F, Bengi-Bozkurt O, et al. Study of ACEI versus ARB in managing hypertensive overt diabetic nephropathy: long-term analysis. Kidney Blood Press Res. 2009;32(4):268–275. doi: 10.1159/000239765. [DOI] [PubMed] [Google Scholar]
- 103.Sato A, Tabata M, Hayashi K, et al. Effects of the angiotensin II type 1 receptor antagonist candesartan, compared with angiotensin-converting enzyme inhibitors, on the urinary excretion of albumin and type IV collagen in patients with diabetic nephropathy. Clin Exp Nephrol. 2003;7(3):215–220. doi: 10.1007/s10157-003-0227-1. [DOI] [PubMed] [Google Scholar]
- 104.Gregoire JP, Moisan J, Guibert R, et al. Tolerability of antihypertensive drugs in a community-based setting. Clin Ther. 2001;23(5):715–726. doi: 10.1016/S0149-2918(01)80021-7. [DOI] [PubMed] [Google Scholar]
- 105.Mackay FJ, Pearce GL, Mann RD. Cough and angiotensin II receptor antagonists: cause or confounding? Br J Clin Pharmacol. 1999;47(1):111–114. doi: 10.1046/j.1365-2125.1999.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bloom BS. Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther. 1998;20(4):671–681. doi: 10.1016/S0149-2918(98)80130-6. [DOI] [PubMed] [Google Scholar]
- 107.Bourgault C, Senecal M, Brisson M, et al. Persistence and discontinuation patterns of antihypertensive therapy among newly treated patients: a population-based study. J Hum Hypertens. 2005;19(8):607–613. doi: 10.1038/sj.jhh.1001873. [DOI] [PubMed] [Google Scholar]
- 108.Burke TA, Sturkenboom MC, Lu SE, et al. Discontinuation of antihypertensive drugs among newly diagnosed hypertensive patients in UK general practice. J Hypertens. 2006;24(6):1193–1200. doi: 10.1097/01.hjh.0000226211.95936.f5. [DOI] [PubMed] [Google Scholar]
- 109.Conlin PR, Gerth WC, Fox J, et al. Four-year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other artihypertensive drug classes. Clin Ther. 2001;23(12):1999–2010. doi: 10.1016/S0149-2918(01)80152-1. [DOI] [PubMed] [Google Scholar]
- 110.Degli Esposti E, Sturani A, Martino M, et al. Long-term persistence with antihypertensive drugs in new patients. J Hum Hypertens. 2002;16(6):439–444. doi: 10.1038/sj.jhh.1001418. [DOI] [PubMed] [Google Scholar]
- 111.Degli Esposti L, Degli Esposti E, Valpiani G, et al. A retrospective, population-based analysis of persistence with antihypertensive drug therapy in primary care practice in Italy. Clin Ther. 2002;24(8):1347–1357. doi: 10.1016/S0149-2918(02)80039-X. [DOI] [PubMed] [Google Scholar]
- 112.Erkens JA, Panneman MM, Klungel OH, et al. Differences in antihypertensive drug persistence associated with drug class and gender: a PHARMO study. Pharmacoepidemiol Drug Saf. 2005;14(11):795–803. doi: 10.1002/pds.1156. [DOI] [PubMed] [Google Scholar]
- 113.Marentette MA, Gerth WC, Billings DK, et al. Antihypertensive persistence and drug class. Can J Cardiol. 2002;18(6):649–656. [PubMed] [Google Scholar]
- 114.Wogen J, Kreilick CA, Livornese RC, et al. Patient adherence with amlodipine, lisinopril, or valsartan therapy in a usual-care setting. J Managed Care Pharm. 2003;9(5):424–429. doi: 10.18553/jmcp.2003.9.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hasford J, Schroder-Bernhardi D, Rottenkolber M, et al. Persistence with antihypertensive treatments: results of a 3-year follow-up cohort study. Eur J Clin Pharmacol. 2007;63(11):1055–1061. doi: 10.1007/s00228-007-0340-2. [DOI] [PubMed] [Google Scholar]
- 116.Lachaine J, Petrella RJ, Merikle E, et al. Choices, persistence and adherence to antihypertensive agents: evidence from RAMQ data. Can J Cardiol. 2008;24(4):269–273. doi: 10.1016/S0828-282X(08)70175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel BV, Remigio-Baker RA, Mehta D, et al. Effects of initial antihypertensive drug class on patient persistence and compliance in a usual-care setting in the United States. J Clin Hypertens. 2007;9(9):692–700. doi: 10.1111/j.1524-6175.2007.07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simons LA, Ortiz M, Calcino G. Persistence with antihypertensive medication: Australia-wide experience, 2004–2006. Med J Aust. 2008;188(4):224–227. doi: 10.5694/j.1326-5377.2008.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 119.Meltzer D, Basu A, Conti R. The economics of comparative effectiveness studies: societal and private perspectives and their implications for prioritizing public investments in comparative effectiveness research. PharmacoEconomics. 2010;28(10):843–853. doi: 10.2165/11539400-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baker WL, Coleman CI, Kluger J, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors or angiotensin II-receptor blockers for ischemic heart disease. Ann Intern Med. 2009;151(12):861–871. doi: 10.7326/0003-4819-151-12-200912150-00162. [DOI] [PubMed] [Google Scholar]
- 121.Califf RM, McMurray JJ, Holman RR, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 122.Dream Trial Investigators. Bosch J, Yusuf S, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 123.Jong P, Demers C, McKelvie RS, et al. Angiotensin receptor blockers in heart failure: meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2002;39(3):463–470. doi: 10.1016/S0735-1097(01)01775-2. [DOI] [PubMed] [Google Scholar]
- 124.Balamuthusamy S, Srinivasan L, Verma M, et al. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. Am Heart J. 2008;155(5):791–805. doi: 10.1016/j.ahj.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 125.Sipahi I, Debanne SM, Rowland DY, et al. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11(7):627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 303 kb)