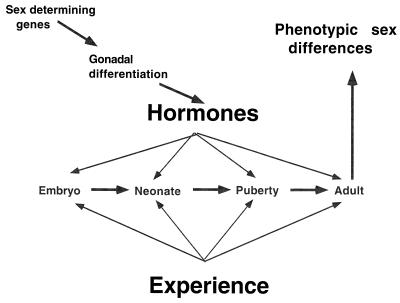
Sex differences in brain structure have been widely recognized since the pioneering studies of Raisman and Field (1). For the most part, brain sex differences are thought to arise in perinatal development through the actions of testosterone secreted by the developing testes, and these sex differences are believed to persist in the absence of gonadal hormones in adult life, very much like the basic plan of the male and female reproductive tracts, which are also developmentally determined. As shown in Fig. 1, the basic plan of brain and body sex differences is the result of a cascade of events beginning with the role of the sex-determining genes in sexual differentiation and continuing with the actions of hormones in embryonic, neonatal, peripubertal, and adult life. The emphasis on early developmental programming of brain structural sex differences was reinforced over several decades by the long-standing view that the brain is not capable of significant structural changes in adulthood. However, this view is changing, and in this issue of the Proceedings, Cooke et al. (2) describe a brain sex difference controlled entirely by circulating androgens. The postereodorsal nucleus of the medial amygdala is larger in male rats and females, but castration of adult males causes the volume of the nucleus to decrease to female levels within 4 weeks, whereas testosterone treatment of adult females for 4 weeks enlarges volume of this nucleus to male levels. Not only is the volume of the anatomical nucleus affected, but the individual cell soma areas are also increased in size by androgen, irrespective of genetic sex of the animal. These interesting findings are not as heretical as they might have seemed a few years ago, and they should be interpreted in light of new evidence for the structural plasticity of the adult brain at different levels of neuroanatomical and functional analysis in relation to the actions of circulating androgens, estrogens, and glucocorticoids. Moreover, as the authors point out, the findings of this new study also raise interesting issues pertaining to the interpretation of sex differences found in the human brain.
Figure 1.
Cascade of effects leading to phenotypic sex differences. Sex-determining genes promote the differentiation of the testes and testosterone secretion during embryonic, neonatal, peripubertal, and adult life masculinizes and defeminizes the brain. Estrogen actions in the female brain activate functions that have been allowed to develop in the absence of testosterone and testosterone given to a genetic female early in development produces an individual with many phenotypic characteristics of a genetic male, particularly regarding brain function and secondary sex characteristics. Experiences during the lifespan interact with the hormone actions to produce the final phenotype of the individual.
Adult Neuronal Plasticity in Sexually Dimorphic Systems.
Hormone actions in the adult brain affect not only neurochemistry but also structure of nerve cells in meaningful ways, and this realization constitutes a paradigm shift in our thinking about the plasticity of the adult brain. The first recognition of this came from studies on some of the recognized sexually dimorphic systems of the brain, in which adults treated with hormones showed sex-specific morphological changes. For example, androgen actions in the adult spinal nucleus of the bulbocavernosus (SNB), which innervates the penis, regulate both size and functional connectivity of these motor neurons, even though a significant part of the size of this nucleus is determined by developmental events (3, 4). Moreover, estrogen actions in the ventromedial nucleus of the adult female hypothalamus induce new synapses (5–7), and these effects are absent in adult-castrated males (8, 9) indicating, once again, an overriding importance of developmental actions of gonadal hormones, as if the very ability to show adult plasticity in response to hormone is programmed by early developmental actions of testosterone.
These two examples raise an issue that is important to understanding the implications of the Cooke article (2), namely, that in the ventromedial nucleus, in contrast to the SNB, overt size differences in the neuroanatomical nucleus are not the most salient feature of the sex difference. In the ventromedial nucleus of the hypothalamus, there are developmentally programmed sex differences in the pattern of synaptic connections on dendritic shafts and spines (10) and developmentally regulated sex differences in the inducibility of progesterone receptors (11). However, there are no major sex differences in estrogen induction of oxytocin receptors, but there is a sex difference in the effects of progesterone on these receptors (12). Thus, in the nucleus studied by Cooke et al. (2), the lack of a sex difference in cell body diameter or size of the neuroanatomical nucleus does not rule out the existence of developmentally programmed sex differences in neurochemistry or in fine-grained neuroanatomy of synaptic connections. At the same time, the findings of the Cooke et al. study fit very nicely into a growing literature showing effects of hormones on reversible neuroanatomical plasticity in the adult brain.
A New Appreciation of Hormonally Directed Adult Brain Plasticity.
Estrogen induction of synapse formation is not confined to the hypothalamus, but it also occurs in the adult female hippocampus and shows a cyclic variation with the estrous cycle of the female rat (13, 14) and a dependency on excitatory amino acids and N-methyl-d-aspartate (NMDA) receptors as well as circulating estrogens (15, 16). Here, again, the adult male is refractory to the synapse-inducing effects of testosterone unless testosterone actions on sexual differentiation are prevented at birth (17). The dependency of this process on excitatory amino acids and NMDA receptors indicates that the hormone is not acting alone but in collaboration with ongoing excitatory synaptic transmission.
We now know that the hippocampus is also the site of other types of structural plasticity in the adult brain, and these also involve concurrent actions of hormones in concert with excitatory amino acids and NMDA receptors. The dentate gyrus of the hippocampal formation is capable of neurogenesis (18), and neuronal formation and survival are increased by an enriched environment (19) and by specific learning (20) as well as by voluntary exercise (21). Stress, on the other hand, decreases dentate gyrus neurogenesis (22, 23), and likely mediators include glucocorticoids (24) and excitatory amino acids (18).
Besides the dentate gyrus, pyramidal neurons in the CA3 field of the hippocampus, which receive innervation from the dentate gyrus via the mossy fiber input, show stress-induced remodeling that is also mediated by circulating glucocorticoids and excitatory amino acids acting via NMDA receptors (25–27). It is not clear yet the extent to which these two forms of plasticity are accompanied by a sex difference, although one study has shown for the stress-induced reorganization of dendrites in the CA3 region that females are much less responsive than males (28), and there are sex differences in hippocampal morphology (29–31) and function in terms of the strategies used by male and female rats in spatial learning (32).
Implications for Study of Human Brain Sex Differences.
Thus, sex differences are lurking in many places in the brain both outside of and within the traditional “reproductive” centers, and there are other brain areas that are targets of reproductive hormones for which we do not yet know about the existence of sex differences (33). The medial posterior dorsal nucleus of the amygdala studied by Cooke et al. (2) is one of those brain regions implicated in reproductive behavior, in particular chemosensory investigation and sexual arousal using pheromones and other olfactory cues as signals, and this nucleus is richly endowed with both androgen and estrogen receptors.
As noted by Cooke et al., there are hypothalamic nuclei that have been reported to differ in size between male and female rodents, and possibly homologous nuclei in humans are also known to differ in size between males and females. There are also some indications that such nuclei may differ in size between homosexual and heterosexual males (34, 35) and between transsexual and heterosexual males (36). Notable among these are hypothalamic nuclei for which sex differences are absent at birth, develop at around 4 years of age, and persist until around 50–60 years of age, where there is a decrease in volume at somewhat different rates in both sexes (37).
What Cooke et al. (2) suggest at the end of their article is that their findings “support the possibility that sexual dimorphisms of the human brain are due solely to circulating steroids in adulthood.” This is undoubtedly an overstatement of a valuable point. Reversible hormone actions may well explain, at least in part, the age-dependent changes in sex differences of human hypothalamic nuclei (37), and such reversible effects certainly must be taken into account as a factor in relation to the differences reported between transsexual and heterosexual males. However, morphological sex differences in the human brain are likely to reflect an interaction between developmental influences, experience, and hormone actions on the mature brain (38), and this may be particularly true outside of the hypothalamus and amygdala, where sex differences have been reported in the size of the corpus callosum (39) and anterior commissure (40) and in the cellularity of the posterior temporal cortex of the planum temporale (41). Although it is not known whether these structures differ in size as a function of androgen or estrogen levels in adulthood, the sex differences in cellularity of the planum temporale involved an 11% larger density of neurons in several cortical layers of females, with no overlap between males and females (41). It is difficult to conceive how such a sex difference could arise by other than a developmental process, unless there is neurogenesis in the adult temporal cortex regulated by circulating hormones.
In the case of the corpus callosum, the sex differences that have been described are so complex as to involve an interplay between genes, hormones, and experience and possibly both developmental and adult plasticity. In the studies of handedness and sex differences in the corpus callosum, handedness was a factor in callosal size in males but not in females; moreover, callosal size decreased with chronological age in males but not in females (42). Thus, there are likely to be permanent structural features of human brain sex differences in addition to effects of hormones on adult neuronal plasticity. The scheme in Fig. 1 highlights the fact that not only do hormones act throughout the lifespan of the individual but also that experiences, which can also change brain structure as well as function (43), interact across the lifespan with a progressively changing neural substrate (38).
In conclusion, the study by Cooke et al. highlights the dynamic nature of the mature nervous system in its ability to change reversibly in response to circulating hormones. Thus, when a sex difference is reported in the structure of the brain, the role of adult hormone secretion must be considered along with the developmental actions of gonadal hormones.
Footnotes
A commentary on this article begins on page 7538.
References
- 1.Raisman G, Field P. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
- 2.Cooke B M, Tabibnia G, Breedlove S M. Proc Natl Acad Sci USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurz E M, Sengelaub D R, Arnold A P. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto A, Arai Y, Urano A, Hyodo S. Neurosci Lett. 1991;131:159–161. doi: 10.1016/0304-3940(91)90603-q. [DOI] [PubMed] [Google Scholar]
- 5.Carrer H, Aoki A. Brain Res. 1982;240:221–233. doi: 10.1016/0006-8993(82)90218-9. [DOI] [PubMed] [Google Scholar]
- 6.Frankfurt M, Gould E, Woolley C, McEwen B S. Neuroendocrinology. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- 7.Frankfurt M, McEwen B S. NeuroReport. 1991;2:380–382. doi: 10.1097/00001756-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Frankfurt M, McEwen B S. Neuroendocrinology. 1991;54:653–657. doi: 10.1159/000125975. [DOI] [PubMed] [Google Scholar]
- 9.Segarra A, McEwen B S. Neuroendocrinology. 1991;54:365–372. doi: 10.1159/000125915. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto A, Arai Y. Neuroendocrinology. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- 11.Parsons B, Rainbow T, McEwen B S. Endocrinology. 1984;115:1412–1417. doi: 10.1210/endo-115-4-1412. [DOI] [PubMed] [Google Scholar]
- 12.Coirini H, Johnson A, Schumacher M, McEwen B S. Neuroendocrinology. 1992;55:269–275. doi: 10.1159/000126125. [DOI] [PubMed] [Google Scholar]
- 13.Woolley C, McEwen B S. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould E, Woolley C, Frankfurt M, McEwen B S. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolley C, McEwen B S. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolley C S, Weiland N G, McEwen B S, Schwartzkroin P A. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis C, McEwen B S, Frankfurt M. Dev Brain Res. 1995;87:91–95. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- 18.Cameron H A, McEwen B S, Gould E. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;586:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 20.Gould E, Beylin A, Tanapat P, Reeves A, Shors T J. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 21.van Praag H, Kempermann G, Gage F H. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 22.Gould E, McEwen B S, Tanapat P, Galea L A M, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron H A, Gould E. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe Y, Gould E, McEwen B S. Brain Res. 1992;588:341–344. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 26.Magarinos A M, McEwen B S. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 27.Magarinos A M, McEwen B S, Flugge G, Fuchs E. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galea L A M, McEwen B S, Tanapat P, Deak T, Spencer R L, Dhabhar F S. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 29.Juraska J M. Psychoneuroendocrinology. 1991;16:105–119. doi: 10.1016/0306-4530(91)90073-3. [DOI] [PubMed] [Google Scholar]
- 30.Gould E, Westlind-Danielsson A, Frankfurt M, McEwen B S. J Neurosci. 1990;10:996–1003. doi: 10.1523/JNEUROSCI.10-03-00996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roof R L. Brain Res. 1993;610:148–151. doi: 10.1016/0006-8993(93)91228-k. [DOI] [PubMed] [Google Scholar]
- 32.Williams C L, Meck W H. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- 33.McEwen, B. S. & Alves, S. H. (1999) Endocrine Rev., in press. [DOI] [PubMed]
- 34.LeVay S. Science. 1991;253:1036–1038. [Google Scholar]
- 35.Swaab D, Hofman M. Brain Res. 1990;537:141–148. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J-N, Hofman M A, Gooren L J, Swaab D F. Nature (London) 1995;378:68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- 37.Swaab D F, Gooren L J G, Hofman M A. Prog Brain Res. 1992;93:205–219. doi: 10.1016/s0079-6123(08)64573-2. [DOI] [PubMed] [Google Scholar]
- 38.Goy R W. In: The Neurosciences: Second Study Program. Schmitt F O, editor. New York: Rockefeller Univ. Press; 1970. pp. 196–206. [Google Scholar]
- 39.Allen L S, Richey M F, Chai Y M, Gorski R A. J Neurosci. 1991;11:933–942. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen L, Gorski R. J Comp Neurol. 1991;312:97–104. doi: 10.1002/cne.903120108. [DOI] [PubMed] [Google Scholar]
- 41.Witelson S F, Glezer I I, Kigar D L. J Neurosci. 1995;15:3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witelson S. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 43.Greenough W T, Bailey C H. Trends Neurosci. 1988;11:142–147. [Google Scholar]